Abstract
ABSTRACT
Introduction: Tolosa-Hunt syndrome (THS) is a disorder related to inflammation of cavernous sinus and superior orbital fissure that usually presents with ophthalmoplegia and oculomotor nerve palsies. The etiology of the syndrome is unknown and the diagnosis is set by exclusion of other clinical conditions that manifest in a similar way. Intracranial aneurysms, such as intracavernous ones, should be included in the differential diagnosis as they can compress cranial nerves leading to similar clinical presentation.
Case report: We present a case of a 51-year-old woman who was referred to our clinic after 24-hour hospitalization in an ophthalmology clinic due to periorbital cellulitis in her right eye. A nose and paranasal sinuses computed tomography (CT) scan with contrast revealed sinusitis affecting the maxillary sinus, the ethmoidal cells and the sphenoid sinus on the right side. The patient underwent endoscopic sinus surgery on the right side, where middle meatal antrostomy, anterior and posterior ethmoidectomy and Draf I frontal sinus drainage were performed. There was an immediate improvement of the orbital edema and the neuro-opthalmologic examination of the right eye was normal. However, on the ninth postoperative day she presented headache, retro-orbital pain and diplopia of her left eye. In 24 hours, the symptoms progressed to ophthalmoplegia and ipsilateral palpebral ptosis. Intravenous treatment with corticosteroids was initiated under the suspicion of Tolosa-Hunt syndrome, without resolution of her symptoms. Emergency CT and CT-angiography scans revealed a possible intracavernous carotid artery aneurysm on the left side. The diagnosis was confirmed by a magnetic resonance imaging (MRI) scan and the patient was referred to neurosurgery department, where a cerebral angiography was performed and the giant intracavernous aneurysm was treated with guglielmi detachable coils.
Conclusion: Our case indicates that an intracavernous aneurysm can present with painful ophthalmoplegia in all directions, mimicking the Tolosa-Hunt syndrome. One must underline the importance of radiological examination focusing on the vascular structures, as it is essential for the differential diagnosis, defining the course of treatment applied in such cases.
Keywords: aneurysm, Tolosa-Hunt syndrome, opthalmoplegia.
List of abbreviations:
THS: Tolosa-Hunt syndrome; CS: cavernous sinus; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; CCA: cavernous carotid aneurysm; ICA: intracavernous aneurysm; ICHD: International Classification of Headache Disorders; GDC: guglielmi detachable coil
Introduction
Tolosa-Hunt syndrome (THS) is a disorder that is related to inflammation of the cavernous sinus (CS) as well as superior orbital fissure, although the exact etiology is unknown. Tolosa-Hunt syndrome has an estimated incidence of approximately one case per million per year. It affects any age group (10-80 years) and can present with headache, ophthalmoplegia, oculomotor nerve palsies or even loss of visual acuity. The duration of symptoms ranges from days to weeks and they can be ipsilateral or even contralateral. Moreover, recurrence of symptoms has been noted in some cases despite the initial remission.
Currently, the neuroimaging modalities that are performed for the diagnosis of THS include MRI and high-resolution CT scans. However, the review of the literature shows that CT scan is less sensitive than MRI scan. Other laboratory tests performed in cases of suspected THS are blood tests such as complete blood count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Tolosa-Hunt syndrome seems to be associated with leukocytosis and raised erythrocytes sedimentation rate. Usually, the treatment for THS involves intravenous corticosteroid administration. However, some studies have reported the efficacy of immunosuppression therapy such as cyclosporin or methotrexate, while even infliximab has been reported as a successful treatment to a patient with steroid-resistant THS.
Tolosa-Hunt syndrome is a diagnosis of exclusion, as other causes can mimic this condition. The differential diagnosis includes neoplastic conditions such as meningioma or chordoma, vascular conditions such as intracavernous artery or posterior artery aneurysms, and finally inflammatory conditions such as sinusitis, or sarcoidosis. Therefore, every patient should be thoroughly evaluated before the diagnosis is confirmed.
Cavernous carotid aneurysm (CCA) accounts for 2-9% of all internal carotid aneurysms. The causes for CCA include trauma, inflammation or idiopathic. Usually, patients are asymptomatic during the first stages of the aneurysm, but as it is enlarged they manifest diplopia, ptosis, ophthalmoplegia. Finally, one must underline the possibility of aneurysm rupture, that requires prompt medical intervention and in the majority of cases can be fatal.
In our study we present a patient suffering from an intracavernous aneurysm (ICA) mimicking THS.
Case Report
A 51-year-old woman was referred to our clinic after 24-hour hospitalization in an ophthalmology clinic due to a periorbital cellulitis of her right eye. The woman had a history of headache and fever for 10 days and three days prior to her admission a periorbital edema appeared on her right eye. Her clinical examination revealed periorbital edema, diplopia and a reduced visual acuity on her right eye. A CT scan was performed, which showed sinusitis involving mainly the maxillary, ethmoid and sphenoid sinuses on the right side. Consequently, the clinical manifestations were characterized as complications of sinusitis and the following day the patient underwent an endoscopic sinus surgery. A middle meatal antrostomy, anterior and posterior ethmoidectomy as well as frontal sinus drainage type DRAF I on the right side were performed. Part of the orbital lamina was removed in order to drain the orbital abscess leading to a successful orbital decompression. The patient was treated with intravenous antibiotics (sultamicillin, clindamycin) in combination with intravenous corticosteroids (dexamethasone). Postoperatively, the orbital edema was diminished and her visual acuity returned to normal. The intravenous corticosteroids were reduced. Her blood results improved steadily, the patient was asymptomatic and her neuro-ophthalmologic examination was normal.
On the ninth postoperative day, the woman suddenly complained about headache, retro-orbital pain on the left side and diplopia on her left eye, which in 24 hours escalated to ophthalmoplegia and ipsilateral palpebral ptosis. After a complete clinical neurologic examination, revealing paresis of the third, fifth and sixth cranial nerves, intravenous corticosteroid treatment was re-administered under the suspicion of a possible THS. No clinical improvement was noticed in spite of the corticosteroid treatment.
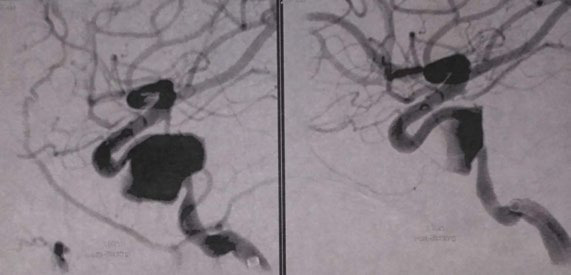
Emergency CT scan and CT angiography scan were performed, showing a possible intracavernous carotid artery aneurysm (ICA) on the left side. The diagnosis of intracavernous carotid artery aneurysm was confirmed by a magnetic resonance angiography (MRA) scan and the patient was referred to neurosurgery. Under their supervision, a cerebral angiography was performed and the giant intracavernous aneurysm was treated with Guglielmi detachable coils. The patient was symptom-free within two months postoperatively and a complete follow-up schedule has been planned for the future. Two years later, the patient remains symptom-free and her follow-up imaging examination is free of findings.
Discussion
Tolosa-Hunt syndrome is an inflammatory disorder of the cavernous sinus and superior orbital fissure. It was first described in 1954 by Dr. Eduardo Tolosa, a Spanish neurosurgeon. In 1961, Hunt et al also described similar cases. In 1966, Smith and Taxdal introduced the term Tolosa-Hunt syndrome for the first time. It is described as a painful ophthalmoplegia with unilateral periorbital headache. Its etiology remains unknown; it is referred to as idiopathic and from pathophysiological aspect it represents a non-specific granulomatous inflammatory disorder of the cavernous sinus. However, injuries, tumors or aneurysms could act as potential triggers of the syndrome. Dr. Eduardo Tolosa, who first reported the syndrome, described it as “a non-specific chronic inflammation of the septa and wall of the cavernous sinus with the proliferation of fibroblasts, an infiltration with lymphocytes and plasma cells.” Hunt et al also added that “such inflammatory changes, in a tight connective tissue, may exert pressure upon the penetrating nerves.”
Four cranial nerves, III, IV, V and VI, are involved. Pain is usually located peri-orbitally, but it can also be retro-orbital; it seems to be the presenting symptom before ophthalmoplegia appears which involves all three ocular-motor nerves in various combinations. The oculomotor nerve is the most commonly affected in 80% of THS cases, followed by the abducens nerve (70%), the ophthalmic branch of the trigeminal nerve (30%) and the trochlear nerve (29%). There can also be sympathetic or parasympathetic involvement causing pupillary abnormalities. In the literature, the involvement of the maxillary and mandibular branches of the trigeminal nerve, optical nerve and facial nerve have also been reported. The diagnosis of THS is basically set out of exclusion of other clinical entities with similar manifestations. It is suspected by the clinical presentation combined with negative neuroimaging scanning and good response to corticosteroid treatment. However, the responsiveness to treatment with steroids is no longer required to confirm the diagnosis of the syndrome. The third Edition of the International Classification of Headache Disorders (ICHD) has refined its diagnostic criteria to require the demonstration of granulomatous inflammation of the cavernous sinus, superior orbital fissure or orbit on magnetic resonance imaging or biopsy. Nevertheless, some case series in the literature underline that even the new diagnostic criteria are suboptimal for the diagnosis of THS, which remains a diagnosis of exclusion.
The proposed treatment for THS involves high doses of systemic corticosteroids, as it was first described; however, there are no specific data neither on the precise dose nor on the duration of treatment. Remission of symptoms is usually expected within the first 24 to 72 hours from the first dose of corticosteroids.
Except THS, there are many other diseases that can present with similar symptoms. The differential diagnosis of a painful ophthalmoplegia is extensive and must include neoplastic lesions such as pituitary adenomas, meningiomas, craniopharyngiomas, chordomas, etc., vascular disorders such as aneurysms and arterial malformations, inflammatory disorders such as orbital pseudotumors, sarcoidosis, giant cell arteritis, Wegener’s granulomatosis, bacterial, mycobacterial and fungal infections and other diseases such as ophthalmoplegic migraine, trauma or diabetic mononeuropathy. Whenever a patient presents with painful ophthalmoplegia, it is mandatory that he/she is fully evaluated in order to avoid misdiagnosis.
The cavernous sinus and the superior orbital fissure are the anatomic places where the ocular-motor nerves, the internal carotid artery and the ophthalmic branch of the trigeminal nerve co-exist. In THS the non-specific granulomatous inflammation can result in periarteritis of the cavernous sinus, resulting in compression and/or a stenosis of the cavernous portion of the internal carotid artery. Campbelli mentioned that leukocytes, lymphocytes and plasma cells can cause thickening of the vessel wall. However, a completely de novo aneurysm arising from the cavernous portion of the ICA is rather uncommon in THS. Zhou et al reported a case of an intracavernous carotid stenosis with a reversible dissecting aneurysm. A 49-year-old woman with oculomotor and abducens nerve palsy on the right side and retro-orbital pain was diagnosed with THS, and a CT angiography revealed a right intracavernous carotid dissecting aneurysm. After the administration of dexamethasone, not only the symptoms improved but also the follow-up CT scan showed a decrease in size of the dissecting aneurysm. On the other hand, Kambe et al reported a 58-year-old woman with right retro-orbital pain and abducens nerve palsy, whose CT angiography revealed stenosis of the left intracavernous ICA and aneurysms of bilateral ICAs. After prednisolone administration, the right C4 aneurysm decreased in size, while the left C3 aneurysm remained unchanged. The MRI showed an increase in the size of the hypophysis and a biopsy was performed. The histopathologic examination revealed the occurrence of inflammatory cells. The unchanged left C3 aneurysm was treated with Guglielmi detachable coils. It remains unknown whether the aneurysms in those cases pre-existed the illness or the local vasculitis as a consequence of the inflammation led to their development. Takasuna et al reported for the first time a case with a steroid-resistant THS case with a de novo intracavernous aneurysm. A 53-year-old woman appeared with recurrent bilateral painful ophthalmoplegia accompanied with sphenoid sinusitis and pituitary abscess. In the end, she was diagnosed with a de novo ICA aneurysm as well.
Intracavernous aneurysms can cause nerve palsy due to a mass effect. Hahn et al reported that 61% of patients with giant intracavernous aneurysms suffered from retro-orbital pain due to the compression and ischemia that giant aneurysms caused. On the other hand, non-giant aneurysms can also cause retro-orbital pain. Lanzino et al proposed that the pain might be attributed to a form of vascular compression syndrome. They pointed out that the oculomotor nerve contains sensory ganglion cells and conveys afferent fibers entering the brain stem and reaching the spinal trigeminal nucleus.
Cavernous carotid aneurysms pose dilemmas in management. Symptomatic aneurysms require either endovascular or open surgery treatment. Unfortunately, these modalities carry their own risks with high morbidity and mortality rates. Treatment options include artery ligation or clip application or embolization of the artery involved with success rates up to 60-80%. In our case, we used Guglielmi detachable coil (GDC) to treat the aneurysm. The GDC was introduced in 1991 in the treatment of aneurysms, showing good effectiveness as it is minimally invasive, with bleeding and vasoconstriction being their main drawbacks.
Mycotic aneurysms occur more often on patients with human immunodeficiency virus infection or those receiving immunosuppressive therapy. Shimizu et al reported a case of intracranial mycotic aneurysm that ruptured after the patient was misdiagnosed with THS and received high dose steroid therapy for nine days. Irwin et al reported for the first time an extracranial mycotic aneurysm associated with THS located in the abdominal aorta of a 57-year-old patient.
In our case, we initially suspected THS on the left side because of the patient’s recent history of inflammation of her right sinuses with periorbital cellulitis on the right side. Such inflammation does not spread easily to the other side because cavernous sinus and sella turcica are covered by bone and dura matter. However, there have been reported cases with inflammation related to THS spreading to adjacent sites such as the hypophysis or the contralateral cavernous sinus. Our initial diagnosis proved wrong, since the patient did not show any improvement on her symptoms after receiving corticosteroid medication. Given its rarity, most patients with painful ophthalmoplegia will not be diagnosed with THS. However, the history of our patient led us to consider this rare diagnosis. Our patient had no history of any allergic disease that could have caused symptoms like intense sneezing after FESS, putting pressure on the intracranial structures, leading to a possible small intracranial rupture. She had no other risk factors for developing an aneurysm such as smoking, hypertension, alcohol consumption or a positive family history. The origin of the aneurysm – whether it preexisted the illness and was enlarged by inflammation or it was a new THS-related mycotic aneurysm – is still uncertain. It is possible that the aneurysm could have been caused directly by the infiltration of inflammation in the intracavernous internal carotid artery (ICA) or it may have been affected by the extent of the surgical procedure.
The molecular mechanisms underlying the genesis of aneurysms are complex. Although there has been extensive research, our comprehension of the processes that cause aneurysms to form, grow and rupture is still not fully understood. An essential characteristic of intracranial aneurysms is the collapse of the internal elastic lamina (IEL), which is a layer of connective tissue located beneath the endothelium and separates the intima from the media layer. Additional features may include an uneven luminal surface, myointimal hyperplasia, disorganization of the muscular media, hypocellularization and the invasion of inflammatory cells. Inflammatory cells are common in aneurysm tissues in both human and animal models. These cells, including macrophages, neutrophils, T cells and B cells among others, are thought to play an important role in the disease. Neutrophils and mast cells are thought to play an essential part in intracranial aneurysms. In our case, we assumed that the development of the aneurysm was directly associated with the patient’s inflammatory disease; however, it remains unknown if it was just an unfortunate coincidence.
The major limitations of our study are the retrospective study design and the inability to generalize findings. Inferring causality from an uncontrolled observation is not possible.
Conclussion
Our case underlines the importance of including intracavernous aneurysms in the differential diagnosis of painful ophthalmoplegia in all directions. Such manifestations should lead to THS diagnosis only after exclusion of all other clinical conditions via thorough radiological examination. It is of great importance that the radiological examination focuses on vascular structures, since intracavernous aneurysms can be life-threatening, depending on their size, so their early diagnosis is crucial for determining the treatment strategies.
Ethics approval and consent to participate: The patient provided informed consent to use her data.
Consent for publication: The patient whose data were used in this manuscript provided her informed consent for publication.
Conflict of interests: None declared.
Financial support: None declared.
Authors’ contribution: All authors have participated in (a) conception and design, (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
FIGURE 1.
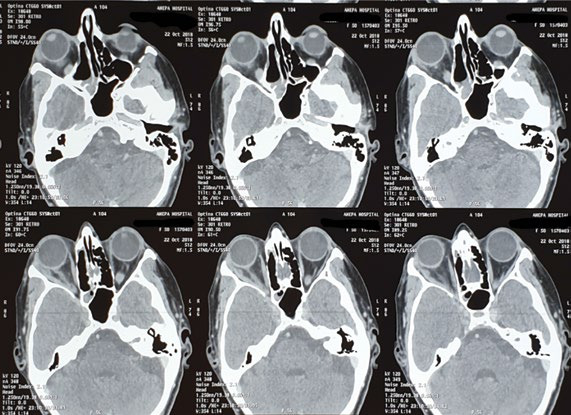
Computed tomography scan showing sinusitis and periorbital cellulitis on the right side
FIGURE 2.
Computed tomography scan showing sinusitis and periorbital cellulitis on the right side
FIGURE 3.
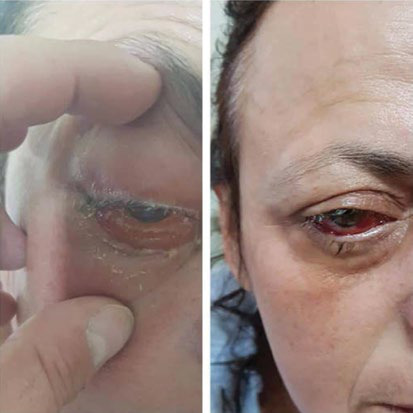
Periorbital celullitis preoperatively on the right eye and clinical improvement after the performance of endoscopic sinus surgery
FIGURE 4.
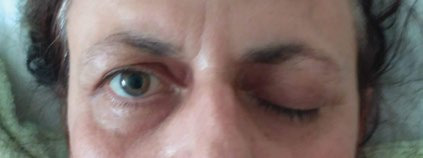
Ninth postoperative day: opthalmoplegia and palpebral ptosis on the left eye
FIGURE 5.
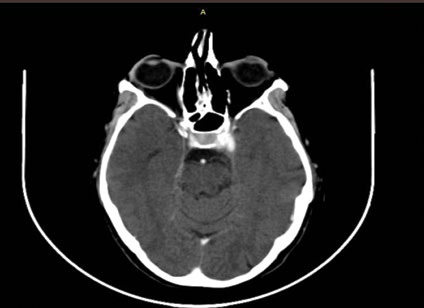
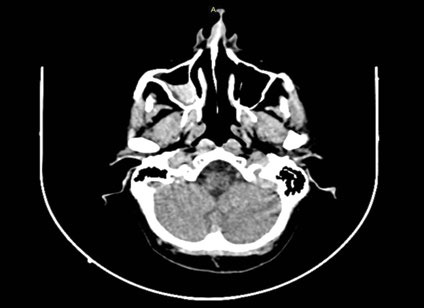
Computed tomography scan showing a possible intracavernous carotid artery aneurysm (ICA) on the left side
FIGURE 6.
Magnetic resonance angiography scan revealing a giant intracavernous carotid artery aneurysm on the left side
Contributor Information
Konstantina DINAKI, ENT resident, 1st Academic ORL Department, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Anastasia SARAFIDOU, Academic fellow, 1st Academic ORL Department, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Constantinos PAPADOPOULOS, Consultant, ORL Department General Hospital of Drama Hospital, Drama, Greece.
Stefanos TRIARIDIS, Professor, 1st Academic ORL Department, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Petros KARKOS, Professor, 1st Academic ORL Department, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
References
- 1.Schuknecht B, Sturm V, Huisman TAGM, Landau K. Tolosa-Hunt syndrome: MR imaging features in 15 patients with 20 episodes of painful ophthalmoplegia. Eur J Radiol. 2009;69:445–453. doi: 10.1016/j.ejrad.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Amrutkar CV BE. Tolosa-Hunt Syndrome. StatPearls [Internet] Treasure Isl StatPearls Publ 2024 Jan–PMID 29083745. Published online 2023.
- 3.Iaconetta G, Stella L, Esposito M, Cappabianca P. Tolosa-Hunt syndrome extending in the cerebello-pontine angle. Cephalalgia. 2005;25:746–750. doi: 10.1111/j.1468-2982.2005.00924.x. [DOI] [PubMed] [Google Scholar]
- 4.Yousem DM, Atlas SW, Grossman RI, et al. MR imaging of Tolosa-Hunt syndrome. Am J Roentgenol. 1990;154:167–170. doi: 10.2214/ajr.154.1.2104703. [DOI] [PubMed] [Google Scholar]
- 5.Kline LB, Hoyt WF. The Tolosa-Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:577–582. doi: 10.1136/jnnp.71.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannerz J. Recurrent Tolosa-Hunt syndrome: a report of ten new cases. Cephalalgia, 1999. [DOI] [PubMed]
- 7.Halabi T, Sawaya R. Successful Treatment of Tolosa-Hunt Syndrome after a Single Infusion of Infliximab. J Clin Neurol. 2018;14:126. doi: 10.3988/jcn.2018.14.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolosa E. Periarteritic lesions of the carotid siphon with the clinical features of a carotid infraclinoidal aneurysm. J Neurol Neurosurg Psychiatry. 1954;17:300–302. doi: 10.1136/jnnp.17.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt WE, Meagher JN, LeFever HE, Zeman W. Painful opthalmoplegia. Its relation to indolent inflammation of the carvernous sinus. Neurology. 1961;11:56–62. doi: 10.1212/wnl.11.1.56. [DOI] [PubMed] [Google Scholar]
- 10.Olesen J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 11.Mullen E, Rutland JW, Green MW, et al. Reappraising the Tolosa-Hunt Syndrome Diagnostic Criteria: A Case Series. Headache. 2020;60:259–264. doi: 10.1111/head.13692. [DOI] [PubMed] [Google Scholar]
- 12.Gladstone JP. An approach to the patient with painful ophthalmoplegia, with a focus on Tolosa-Hunt syndrome. Curr Pain Headache Rep. 2007;11:317–325. doi: 10.1007/s11916-007-0211-7. [DOI] [PubMed] [Google Scholar]
- 13.Campbell RJ, Okazaki H. Painful ophthalmoplegia (Tolosa-Hunt variant): autopsy findings in a patient with necrotizing intracavernous carotid vasculitis and inflammatory disease of the orbit. Mayo Clin Proc. 1987;62:520–526. doi: 10.1016/s0025-6196(12)65478-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Zhou G, Lu T, et al. Tolosa-Hunt syndrome with reversible dissection aneurysm. Neurol Sci. 2010;31:777–779. doi: 10.1007/s10072-010-0231-7. [DOI] [PubMed] [Google Scholar]
- 15.Kambe A, Tanaka Y, Numata H, et al. A case of Tolosa-Hunt syndrome affecting both the cavernous sinuses and the hypophysis, and associated with C3 and C4 aneurysms. Surg Neurol. 2006;65:304–307. doi: 10.1016/j.surneu.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Takasuna H, Sasaki R, Shiraishi M, et al. Steroid-resistant Tolosa-Hunt syndrome with a de novo intracavernous aneurysm: A case report. Surg Neurol Int, 2016. [DOI] [PMC free article] [PubMed]
- 17.Hahn CD, Nicolle DA, Lownie SP, Drake CG. Giant Cavernous Carotid Aneurysms. J Neuro-ophthalmology. 2001;20:253–258. [PubMed] [Google Scholar]
- 18.Lanzino G, Andreoli A, Tognetti F, et al. Orbital pain and unruptured carotid-posterior communicating artery aneurysms: the role of sensory fibers of the third cranial nerve. Acta Neurochir, (Wien) 1993. [DOI] [PubMed]
- 19.Ambekar S, Madhugiri V, Sharma M, et al. Evolution of management strategies for cavernous carotid aneurysms: a review. World Neurosurg. 2014;82:1077–1085. doi: 10.1016/j.wneu.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Suzuki N, Ottomo M. [A fungal aneurysm in a patient with presumed Tolosa-Hunt syndrome]. No Shinkei Geka. 1991;19:477–483. [PubMed] [Google Scholar]
- 21.Best IM, Bumpers HL. Mycotic aortic aneurysm in a patient with Tolosa-Hunt syndrome. South Med J. 2001;94:441–444. [PubMed] [Google Scholar]
- 22.Costache A, Berghi O, Cergan R, et al. Respiratory allergies: Salicaceae sensitization (Review). Exp Ther Med. 2021;21:609. doi: 10.3892/etm.2021.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Rui YN, Hagan JP, Kim DH. Intracranial Aneurysms: Pathology, Genetics, and Molecular Mechanisms. Neuromolecular Med. 2019;21:325. doi: 10.1007/s12017-019-08537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]


