Abstract
Changes over time in prevalence of glomerulopathies (GP) have been reported worldwide, but given the scarcity of data from Romania, we assessed the frequency of biopsy-proven GP over a 10-year period.
This single-centre retrospective study enrolled 1 254 adults with GP on native kidneys diagnosed between 01.01.2008 – 31.12.2017, whose cases were extracted from the kidney biopsy (KB) registry of the hospital. Those with repeated KB and insufficient tissue sample or missing data were all excluded. Demographic, clinical, laboratory and histological data were analyzed and compared between subjects who underwent KB in the first and last five years (2008-2012, n=355 vs. 2013-2017, n=899).
Even if nephrotic syndrome was the main reason for KB, its frequency decreased from one in two to one in three cases (p<0.001). During the second period, older subjects with lower glomerular filtration rate and proteinuria were found. Also, KB was prescribed for chronic kidney function decline three times more often (p<0.001), while acute nephritic syndrome almost doubled its prevalence among biopsies (p=0.005). IgA nephropathy and membranous nephropathy were similarly the two most frequent histological patterns in both time intervals. However, between 2013-2017, diabetic nephropathy was more commonly detected (12.3% vs. 4.8%, p<0.001), probably because more diabetics underwent KB, but also the crescentic glomerulonephritis showed higher prevalence over time (6.6% vs. 3.1%, p=0.02).
Subject to the limitations of our retrospective single-centre study, its findings suggested rather a change in the diagnostic approach than an actual different GP prevalence in adults, as KB were performed in patients with more advanced age, lower kidney function and less proteinuria.
Keywords::epidemiology, glomerulopathies, kidney biopsy, medical practice.
Introduction
Chronic kidney disease (CKD), a continuously growing health problem, with a global prevalence ranging from 8-9% in high-income populations to over 12% in low-and-middle income populations, recognizes glomerulopathies (GP) as one of its main causes (1, 2). Thus, in socio-economic developed countries, GP account for the third most frequent cause of renal replacement therapy (RRT) initiation (after diabetes mellitus and arterial hypertension/cardiovascular diseases), while in low- and middle-income settings they may be the main cause (3). In Romania, GP accounted for 15% of causes for RRT initiation in 2015 (4).
Moreover, GP represent a treatable cause of CKD, as therapeutic interventions can alter the natural course of the disease. Therefore, their correct diagnosis is of major interest and kidney biopsy (KB) is the best tool for this purpose. In addition, KB-based studies are useful for the description of granulopathy epidemiology. Previously published reports have shown both regional and temporal differences in the frequency of primary granulopathy histological patterns (5-9).
Since in Romania data are scarce and mainly come from the northeastern region of the country (10, 11), we investigate the prevalence of biopsy-proven GP and their changing trends over a 10-year period in a tertiary nephrology care centre which offers advanced specialized services for the major part of southeastern Romania.
Materials and methods
A retrospective, cross-sectional, single-center study was conducted in the oldest and largest nephrology hospital in Romania, which served (at least throughout the duration of the study) as a referral center for the 15 counties from the southeastern part of the country: Valcea, Dolj, Olt, Arges, Dambovita, Teleorman, Giurgiu, Calarasi, Ialomita, Constanta, Buzau, Prahova, Brasov, Ilfov and the Bucharest Municipality. According to the National Institute of Statistics, the resident population in these counties comprised over 8.5 million people (12). This number was used for the calculation of KB incidence rate, based on the assumption that the great majority of nephrological cases from this region were referred to our hospital.
Subjects
Patients with a diagnosis of primary or secondary glomerulopathy in their histological report, who underwent a native KB between 01.01.2008 and 31.12.2017, were enrolled. Patients under 18 years of age, those with kidney graft biopsy, results of a repeated biopsy on the same patient, cases with insufficient tissue sample for a complete pathological examination (<5 glomeruli) and those with incomplete histological or clinic and biological data were excluded. During the 10-year investigated period, KB were performed in 1 813 out of the 7 9921 hospitalizations in our tertiary referral nephrology center (including readmissions of the same patient). Of these, 1 254 cases (69.2%) met the criteria of the present study and were enrolled (Figure 1).
Subjects were divided into two groups according to the year when KB was performed: 355 patients from the first five years (2008-2012) in group 1 and 899 subjects from the last five years (2013-2017) in group 2. The characteristics and diagnoses were compared between these two groups.
The present study was carried out in compliance with the Helsinki Declaration and the rules of good clinical practice in trials with human subjects. Both at the time of hospital admission and before the KB procedure, each patient signed an informed written consent for the anonymous use of his/her personal data, including for research purposes, according to the internal procedures of the hospital and in accordance with the local regulations for patients’ rights. Our study was approved by the local Ethics Committee (No. 12/10.10.2018).
Studied variables
The patient’s medical records (both electronic and on paper) were the source for retrieving all investigated parameters from the time when KB was performed: demographic data (age, gender), past medical history [especially arterial hypertension (HTN) and diabetes mellitus], clinical data (presence of oedema, blood pressure), laboratory data [estimated glomerular filtration rate – eGFR based on serum creatinine according to MDRD formula, proteinuria expressed as urinary protein-to-creatinine ratio (PCR) and hematuria as both the percentage of subjects with dysmorphic hematuria and erythrocyte count per microliter in Stansfeld-Webb analysis, serum hemoglobin, serum albumin and total proteins, C-reactive protein, serum cholesterol and triglycerides] and histological data (histopathology pattern of the GP).
Kidney biopsies were performed percutaneously under ultrasound guidance using automated biopsy guns equipped with 16 Gauge needles. Tissue specimens were analyzed by the same pathologist in the local laboratory using all three methods (light microscopy, direct immunofluorescence and electron microscopy) in each case and provided all the KB results.
Also, the major nephrological syndrome that accounted for the KB indication was retroactively gathered. The following syndromes were considered: nephrotic syndrome (NS), defined as PCR >3 g/g and serum albumin <3.5 g/dL; acute nephritic syndrome (ANS), defined by the association of PCR 1-3.5 g/g with dysmorphic hematuria and commonly a recent decline in eGFR, in an acute setting; chronic nephritic syndrome (CNS), defined by the association of PCR 1-3.5 g/g with dysmorphic hematuria and stable eGFR; acute kidney injury (AKI), defined by sudden increase in serum creatinine over days, with/without oliguria; chronic kidney function decline (CKFD), defined as a persistently reduced eGFR below 60 mL/min for at least three months, without criteria for nephritic or nephrotic syndromes; and asymptomatic urinary abnormalities (AUA), defined as PCR <1 g/g with or without hematuria persistent for at least three months, with normal eGFR.
Statistical analysis
Numeric variables were expressed as mean with standard deviation (SD) or median with quartiles 1 and 3 (Q1;Q3), according to their distribution, and discrete variables – as percentages. Studied parameters from the time of KB were compared between subjects who had undergone the procedure during the first and last five years of the study period (2008-2012 and 2013-2017) using Student t test, Mann-Whitney U test or Chi-square test, as appropriate. Statistical significance was defined as p<0.05.
The annual incidence rate of KB was calculated as the ratio between total annual new cases of KB and the population of the southeastern Romania, as reported by NIS (12), and was expressed per million population (p.m.p./year).
The SPSS v26 software (SPSS, Inc., Chicago, IL) was used.
Results
Overall, subjects were mainly males (57%), middle-aged (50±15 years), with a high proportion of cardiovascular risk factors (59% HTN and 62% dyslipidemia). They had a moderate degree of kidney function decline as suggested by the median eGFR of 42 (21;65) mL/min, but high proteinuria since the PCR was 3 (1.1;6.2) g/g. Dysmorphic hematuria was found in 48% of subjects.
The total number of KB performed during 2013-2017 was significantly higher than in the previous period: 1 245 KB accounting for 2.5% of all hospital admissions in that time frame as compared to only 568 KB representing 1.8% of the total number of hospital admissions (p<0.001). This is indicative of both an increase in nephrological access to care, as well as an extension of KB indications. Of note, since the first biopsy performed in our hospital in 1995, the rate of KB was steadily increasing, so a near three-fold increase was recorded in the studied period, from approximately 11 p.m.p./year in 2008 to 34 p.m.p/year in 2017.
Subjects who underwent a KB during the second investigated period were older, more commonly hypertensive and diabetics, with a lower median eGFR and, accordingly, lower haemoglobin, but less proteinuria (Table 1). Conversely, the prevalence of oedema and dysmorphic hematuria was similar in both groups as well as parameters of the inflammatory and nutritional status (serum albumin and C-reactive protein) (Table 1). Thus, a change over time in the local medical practice regarding the indication for KB could be assumed, with its extension to elderly and patients with more co-morbidities and advanced CKD.
During both periods, NS represented the main reason for performing a KB, although a significant reduction in frequency, of over 17%, during 2013-2017 was observed. Moreover, the CKFD and ANS were strikely more frequently encountered during the second period (Table 2). Therefore, one might deduce that over the years indication for KB had shifted from the clinical overt symptoms (NS) to less obvious conditions (like stable, unexplained, increase in serum creatinine). On the other hand, the almost double proportion of patients with ANS among 2013-2017 could point out to an improvement in the ambulatory identification of this nephrological emergency and, consequently, to a timely manner referral.
The two most common histological patterns of GP were mesangioproliferative glomerulonephritis with immunoglobulin A deposition (IgA nephropathy – IgAN) and membranous nephropathy (MN) with no statistically significant difference between their prevalence from one period to the other (p=0.91 and p=0.24, respectively) (Figure 2).
The third most prevalent pattern of primary GP was minimal change disease (MCD) in both the investigated five-year intervals, but with a significant decrease in the later (p=0.004, Figure 2). The following recorded patterns were focal and segmental glomerulosclerosis (FSGS) and membrano-proliferative glomerulonephritis (MPGN), but with similar prevalence between the studied periods (p=0.31, and p=0.69). In contrast, crescentic glomerulonephritis (CGN) was more commonly seen among 2013-2017 (p=0.02). Similarly, a marked rise in prevalence, almost three times, was observed for diabetic nephropathy (p<0.0001, Figure 2), probably as a consequence of the higher number of patients with DM in whom KB was performed. Other secondary GP had comparable prevalence between the two time-frames, with lupus nephritis (p=0.75) and amyloidosis (p=0.07) each being found in almost one out of ten subjects, followed by endocapillary proliferative glomerulonephritis (mostly of post-infectious aetiology) (p=0.71) and hereditary glomerulopathies (Alport syndrome, Fabry disease and thin glomerular basement membrane disease) (p=0.50) (Figure 2).
Discussion
The findings of the current study cover a gap in the knowledge of glomerulopathy epidemiology and KB practice in the southeastern part of Romania. Previous data on the subject gathered in our country originated from northeastern (Moldova) and Western (Banat) regions (10, 11, 13). These studies analyzed three different intervals of time and had smaller sample sizes than ours [1995-2004, n=635 (13), 2005-2010, n=239 (10) and 2011-2019, n=442 (11)]. A major difference between the present findings and the reports from the other two regions of Romania consist in the prevalence of MPGN, which was the least frequent encountered pattern in our cohort in both the investigated periods (<5%), but was reported as the commonest type in all three reports from Moldova and Banat, albeit with a decreasing trend over time (from 38% in the first decade to 29% and 24%, respectively, thereafter (10, 11, 13)). The high prevalence of MPGN was explained by the authors as a result of its close relationship with the poor socio-economic status and the high prevalence of infection-related glomerulonephritis, either viral B and C hepatitis or streptococcal infections, possible due to low sanitation levels (10). However, it should be emphasized that the transmission electron microscopy analysis (TEM) was not largely available in the previous reports where histological diagnosis was based only on light microscopy and immunofluorescence (IF) (10), a fact which could hampered the accuracy of lesions identification, since TEM is of paramount importance in the diagnosis of some GP like MCD and MPGN (14).
The current study found IgAN as the most frequent histological diagnosis in adults during both studied intervals, in accordance with the biopsy registry reports from other countries in the Central and Western Europe (15-17). The second most common primary glomerulopathy was membranous nephropathy, in line with the Italian study (17), also without significant variations in time. As a matter of fact, according to worldwide reports, IgAN is the most prevalent primary glomerulonephritis in the adult population amongst Western countries but especially in Asian populations (5), while MN is the main cause of nephrotic syndrome in non-diabetic adults (7), data that support our findings.
Regarding the changing prevalence during the ten-year investigated period, we observed steady percentages for the most patterns of primary and secondary GP, except for MCD and CGN. The former displayed a decrease in prevalence, similar to an analysis from the United States, where a decreasing prevalence of MN was also found, in favour of increasing FSGS (18). However, the US study had a large proportion of African-American population (38%), which may be the reason for a higher prevalence of FSGS compared to MN (18). In contrast, more cases of CGN are observed in the second analyzed period in our centre, similar to the trend reported elsewhere (19, 20), and this finding aligns with the marked increase in acute nephritic syndrome as a KB reason. The nephrotic syndrome, followed by chronic nephritic syndrome, was the main indication to perform KB in our analysis, a practice pattern that is also encountered in the majority of KB registries (11, 20). Of note, in the same time-frame, more KB were performed to clarify the cause of an unexplained chronic decline in kidney function, possibly signifying a change in medical practice in the sense of extending the biopsy indication to less clinically obvious cases (with less proteinuria).
Supplementary arguments in favour of a shifting in practice resides from the observation of a steady growing in the number of KB, as well as the different characteristics of patients from the second investigated period: older, with lower kidney function and higher proportion of diabetes mellitus. Similar trends were reported by other studies (15-17, 21). Also, the marked increase in the prevalence of diabetic nephropathy in the later five years of the current research, when it surpassed lupus nephritis as the commonest secondary GP, could be inferred as an expression of advances in KB indications, through considering more diabetics as proper candidates for the histological diagnosis of kidney involvement. This change in practice is probably dictated by a growing body of evidence suggesting that diabetic kidney disease can be underlined by multiple glomerular disease patterns besides diabetic glomerulosclerosis (22).
The present study has several limitations, usually shared by all retrospective registry-based designs. Data were analyzed retrospectively and originated from a single centre, which predisposed to missing information in some cases and impeded the ability to generalize the results to the entire Romanian population. However, the strengths must be also highlighted, since the data came from the eldest nephrology centre in the country, with a large addressability from a wide geographical area, which routinely uses all three methods of kidney sample analysis (including electron microscopy).
CONCLUSIONS
Across the studied period we encountered a paradigm shift in kidney biopsy practice: more elderly and diabetic patients, with more severe decline in kidney function and lower proteinuria had to undergo a kidney biopsy. Nephrotic syndrome was the main indication for KB in our centre. IgAN and MN have remained the most common types of primary glomerulopathies, whereas DN and lupus nephritis have emerged as the leading causes of secondary GP. In addition, an increase in the prevalence of crescentic glomerulonephritis seems to exist, but this trend should be proven in future studies on a longer period.
Compliance with ethics requirements:
The authors declare that all procedures and experiments used in this study comply with the ethical standards in the Helsinki Declaration of 1975, as revised in 2008(5), as well as the national law. Informed consent was obtained from all patients included in the present study.
Conflicts of interest: none declared.
Financial support: none declared.
FIGURE 1.
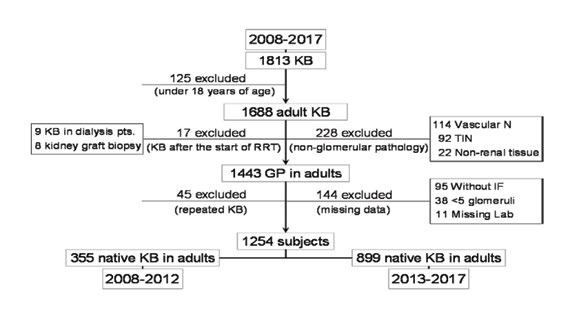
Study flow chart (GP: glomerulopathies; IF: immunofluorescence; KB: kidney biopsy; Lab: laboratory data; N: nephropathies; pts.: patients; RRT: renal replacement therapy; TIN: tubulo-interstitial nephropathies)
TABLE 1.
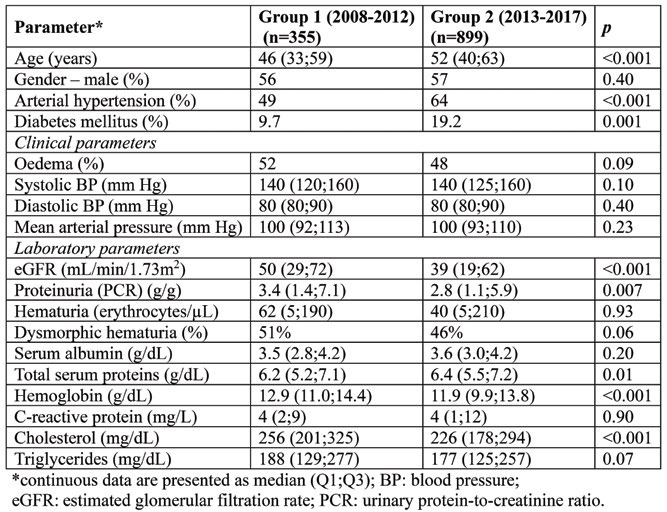
General characteristics of subjects from the two studied groups at the time of kidney biopsy
TABLE 2.
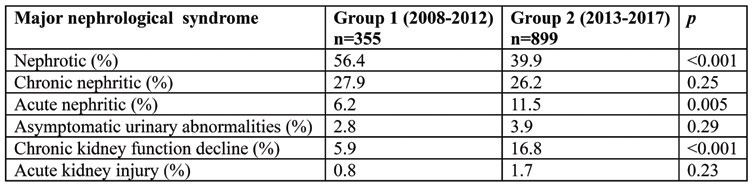
The kidney biopsy indication in the two studied groups
FIGURE 2.
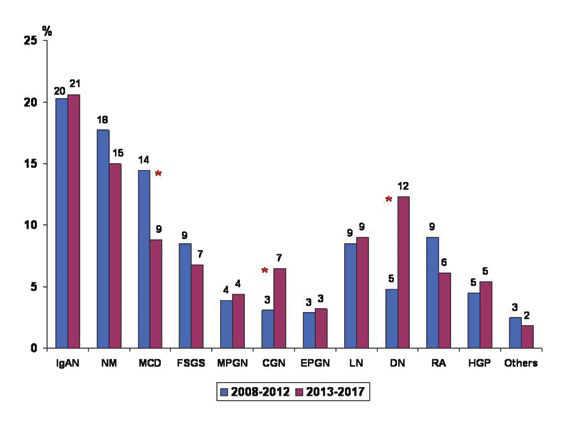
Variations in prevalence of glomerulopathies during the studied period (*: p<0,05; CGN: crescentic glomerulonephritis; DN: diabetic nephropathy; EPGN: diffuse endocapillary proliferative glomerulonephritis; FSGS: focal and segmental glomerulosclerosis; HGP: hereditary glomerulopathies; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; MN: membranous nephropathy; MPGN: membranoproliferative glomerulonephritis; Others = monoclonal immunoglobulin deposition disease, fibrillary glomerulonephritis, thrombotic microangiopathy; RA: renal amyloidosis).
Contributor Information
Nicolae PANA, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Diaverum Morarilor” Nephrology and Dialysis Medical Centre, Bucharest, Romania.
Laura CHIOTAN, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Otilia CIUREA, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania.
Nicoleta PETRE, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania.
Dana DUMITRU, “Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania.
Cristina CAPUSA, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania.
References
- 1.Kovesdy CP. Epidemiology of chronic kidney disease: An update 2022. Kidney Int Suppl. 2011;12:7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha V, Al-Ghamdi SMG, Li G, et al. Global economic burden associated with chronic kidney disease: A pragmatic review of medical costs for the Inside CKD Research Programme. Adv Ther. 2023;40:4405–4420. doi: 10.1007/s12325-023-02608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, Ke R, Teixeira W, et al. Global, Regional, and National Burden of CKD due to Glomerulonephritis from 1990 to 2019. Clin J Am Soc Nephrol. 2023;18:60–71. doi: 10.2215/CJN.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamellou E, Seikrit C, Tang SCW, et al. IgA nephropathy. Nat Rev Dis Primers. 2023;9:67. doi: 10.1038/s41572-023-00476-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Barratt J. Is IgA nephropathy the same disease in different parts of the world? Semin Immunopathol. 2021;43:707–715. doi: 10.1007/s00281-021-00884-7. [DOI] [PubMed] [Google Scholar]
- 7.Ronco P, Beck L. Membranous nephropathy. Nat Rev Dis Primers. 2021;7:69. doi: 10.1038/s41572-021-00303-z. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483–487. doi: 10.2215/CJN.00710805. [DOI] [PubMed] [Google Scholar]
- 9.Hanko JB, Mullan RN, O'Rourke DM, et al. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24:3050–3054. doi: 10.1093/ndt/gfp254. [DOI] [PubMed] [Google Scholar]
- 10.Volovăt C, Căruntu I, Costin C, et al. Changes in the histological spectrum of glomerular diseases in the past 16 years in the North-Eastern region of Romania. BMC Nephrology. 2013;14:148. doi: 10.1186/1471-2369-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covic A, Vlad CE, Căruntu ID, et al. Epidemiology of biopsy-proven glomerulonephritis in the past 25 years in the North-Eastern area of Romania. Int Urol Nephrol. 2022;54:365–376. doi: 10.1007/s11255-021-02881-z. [DOI] [PubMed] [Google Scholar]
- 13.Covic A, Schiller A, Volovat C, et al. Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419–424. doi: 10.1093/ndt/gfi207. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Haas M, Markowitz GS, et al. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J Am Soc Nephrol. 2016;27:1278–1287. doi: 10.1681/ASN.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz M, et al, on behalf of the Polish Society of Nephrology. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 2017. [DOI] [PubMed]
- 16.Maixnerova D, Jancova E, Skibova J, et al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994-2011. J Nephrol. 2015;28:39–49. doi: 10.1007/s40620-014-0090-z. [DOI] [PubMed] [Google Scholar]
- 17.Zaza G, Bernich P, Lupo A. 'Triveneto' Register of Renal Biopsies (TVRRB). Renal biopsy in chronic kidney disease: Lessons from a large Italian registry. Am J Nephrol. 2013;37:255–263. doi: 10.1159/000348566. [DOI] [PubMed] [Google Scholar]
- 18.O'Shaughnessy MM, Hogan SL, Poulton CJ, et al. Temporal and Demographic Trends in Glomerular Disease Epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsen AT, Karlsen C, Bakland G, et al. Increasing incidence and prevalence of ANCA-associated vasculitis in Northern Norway. Rheumatology (Oxford) 2020. [DOI] [PubMed]
- 20.Fiorentino M, Bolignano D, Tesar V, et al on behalf of the ERA-EDTA Immunonephrology Working Group. Renal Biopsy in 2015 – From Epidemiology to Evidence-Based Indications. Am J Nephrol. 2016;3:1–19. doi: 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- 21.Molnár A, Thomas MJ, Fintha A, et al. Kidney biopsy-based epidemiologic analysis shows growing biopsy rate among the elderly. Sci Rep. 2021;11:24479. doi: 10.1038/s41598-021-04274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma SG, Bomback AS, Radhakrishnan J, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–1724. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]


