Abstract
BACKGROUND
Elevated soluble stimulating factor 2 (sST2) level is observed in cardiovascular diseases, such as heart failure and acute coronary syndrome, which reflects myocardial fibrosis and hypertrophy, indicating adverse clinical outcomes. However, the association between sST2 and hypertensive heart disease are less understood. This study aimed to determine the relationship of sST2 with left ventricular hypertrophy (LVH) and geometric remodeling in essential hypertension (EH).
METHODS
We enrolled 483 patients (aged 18–80 years; 51.35% female). sST2 measurements and echocardiographic analyses were performed.
RESULTS
Stepwise multiple linear regression analysis showed significant associations among sST2, left ventricular (LV) mass, and LV mass index. The prevalence of LVH and concentric hypertrophy (CH) increased with higher sST2 grade levels (P for trend < 0.05). Logistic regression analysis suggested that the highest tertile of sST2 was significantly associated with increased LVH risk, compared with the lowest tertile (multivariate-adjusted odds ratio [OR] of highest group: 6.61; P < 0.001). Similar results were observed in the left ventricular geometric remodeling; the highest tertile of sST2 was significantly associated with increased CH risk (multivariate-adjusted OR of highest group: 5.80; P < 0.001). The receiver operating characteristic analysis results revealed that sST2 had potential predictive value for LVH (area under the curve [AUC]: 0.752, 95% confidence interval [CI]: 0.704–0.800) and CH (AUC: 0.750, 95% CI: 0.699–0.802) in patients with EH.
CONCLUSIONS
High sST2 level is strongly related to LVH and CH in patients with EH and can be used as a biomarker for the diagnosis and risk assessment of hypertensive heart disease.
CLINICAL TRIALS REGISTRATION
Trial Number ChiCTR2400082764
Keywords: concentric hypertrophy, essential hypertension, left ventricular hypertrophy, sST2
Essential hypertension (EH) is a common cardiovascular disease (CVD) risk factor associated with adverse cardiovascular outcomes.1,2 Prolonged EH can lead to an increased load on the left ventricle (LV), which may subsequently induce the development of left ventricular hypertrophy (LVH).3 LVH manifests as a complex series of myocardial adaptations characterized by augmented thickness of ventricular walls, heightened myocardial mass, and structural remodeling of the myocardium. These mechanisms give rise to distinct geometric patterns in the LV, such as concentric remodeling (CR), eccentric hypertrophy, and concentric hypertrophy (CH).4,5 LVH constitutes a distinct risk factor that autonomously contributes to both morbidity and mortality associated with CVD.3Several studies have established a link between abnormalities in LV geometry and CVD risk, with CH being the strongest predictor of increased risk of cardiovascular events.6–8 Notably, LVH serves as a pivotal prognostic indicator that significantly influences the clinical outcomes of individuals afflicted with EH.9,10 It serves as a robust independent prognosticator of cardiovascular occurrence both within the general population and among individuals with hypertension.11,12 LVH often goes undiagnosed owing to its early asymptomatic presentation. Consequently, the utilization of biomarker assessment models concerning LVH and LV geometric remodeling in individuals with EH aids cardiologists in identifying risk factors aimed at impeding the onset or advancement of LVH, mitigating the frequency of cardiovascular incidents, and ameliorating the prognosis of patients with hypertension.13,14
LVH pathophysiology is intricate and its biochemical pathways remain unclear, posing challenges in interpreting biomarker patterns and their alterations in the realms of diagnosis and prognosis. Several biochemical markers such as sST2, cardiotrophin-1, soluble urokinase-type plasminogen activator receptor, and matrix metalloproteinase-3 have been investigated.15–17 Although the predictive value of these biomarkers is limited in terms of clinical applicability, there is relatively little research on the use of sST2 as a biomarker for CVD and its application in hypertensive heart disease. Prior investigations have demonstrated sST2’s capacity to differentiate between the various stages of hypertensive heart disease. sST2 has high sensitivity and specificity for identifying hypertension with LVH in patients with hypertensive heart failure.18–20
Previous studies have observed elevated levels of sST2 in patients with hypertensive LVH. However, the precise relationship between sST2 levels and the specific patterns of left ventricular geometric remodeling in LVH remains obscure. In this study, we investigated the relationship between sST2 and LV echocardiographic parameters, pathological LV geometric patterns in patients with EH, as well as the diagnostic value of sST2 in LVH and geometric remodeling.
METHODS
Patients
This retrospective study was conducted in Guangzhou, China, at the Guangdong Provincial Hospital of Chinese Medicine, spanning the period from January 2021 to December 2023. The cohort comprised 483 inpatients, including 235 men and 248 women, aged 18–80 years. This study adhered to the ethical guidelines outlined in the Helsinki Declaration of 1975. The study was approved by the Ethical Committee of Guangdong Provincial Hospital of Chinese Medicine (ethical approval number: ZE2024-088-01). As this was a retrospective observational study without any intervention, the requirement for informed consent was waived by the Ethics Committee.
The study’s inclusion criteria were as follows: (1) hypertension diagnosed according to the diagnostic criteria delineated in the 2023 ESH guidelines for the management of arterial hypertension; (2) availability of demographic, laboratory, and echocardiography data to ensure the fidelity of the research data; and (3) participant age between 18 and 80 years. The exclusion criteria were as follows: (1) secondary hypertension; (2) instances of acute heart failure, acute coronary syndrome, and previous myocardial infarction; (3) ejection fraction (EF) < 50%; (4) presence of myocarditis, cardiomyopathy, valvular heart disease (aortic valve stenosis, moderate to severe mitral valve regurgitation or stenosis), congenital heart disease, pericardial disease, or pulmonary hypertension; (5) concurrent acute and chronic infections, chronic obstructive pulmonary disease, severe hypohepatia and renal insufficiency, aminotransferase level > 3 times the upper limit of normal, and estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m²; (6) existence of immunological diseases, malignant tumors, thyroid diseases, or anemia.
Clinical data collection and laboratory measurements
Demographic characteristics included age, sex, body mass index (BMI), history of diabetes, and hyperlipidemia. BMI was calculated as weight (kg)/[height (m)]2. The laboratory characteristics included creatinine (Cr), estimate glomerular filtration rate (eGFR), low-density lipoprotein (LDL-C), total cholesterol (TC), triglycerides (TG), non-high-density lipoprotein cholesterol (non-HDL-C), high-density lipoprotein cholesterol (HDL-C), uric acid (UA), glucose(Glu), glycosylated hemoglobin, type A1C (HbA1c), sST2, and N-terminal pro b-type natriuretic peptide (NT-pro-BNP). Hypertension characteristics include systolic blood pressure (SBP), diastolic blood pressure(DBP), mean arterial pressure(MAP), pulse pressure, hypertension grade, and duration. SBP and DBP were measured by cuff sphygmomanometer (oscillometric method).
sST2 examination method
On the morning of admission, a total of 5 ml elbow vein blood was collected from fasting patients. The blood samples were set under conditions of 4 °C for 1 hour and subsequently centrifuged 1,000 g for 15 minutes. The blood serum was separated and stored at –80 °C until the test. The sST2 level was determined using an ELISA kit (Joinstar Biotechnology Co, Ltd, Hangzhou, China). The normal range of sST2 levels was <35 ng/ml.
Echocardiogram
A comprehensive two-dimensional echocardiography examination was performed on the patient in the left lateral decubitus position. The examination was conducted using an S5-1 color Doppler ultrasound array probe, with a frequency range of 1.0–5.0 MHz, on a Philips EPIQ 7C echocardiographic machine. According to the guidelines of American Society of Echocardiography, the following cardiac dimensions and functional metrics were measured: left atrial size (LA), right ventricular diameter (RVD), left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular posterior wall thickness (LVPWT), interventricular septal thickness (IVST), relative wall thickness (RWT), ejection fraction (EF), and fractional shortening (FS). Left ventricular mass (LVM) was computed using the Devereux formula: LVM(g) = 0.8 × 1.04 × [(LVEDD + IVST + LVPWT)³ − LVEDD³] + 0.6. The left ventricular mass index (LVMI) was determined by adjusting the LVM for height as follows: LVMI (g/m²) = LVM(g)/body surface area (m2). LVH was defined as LVMI ≥ 95 g/m² in women and ≥115 g/m² in men. RWT was determined using the formula [2 × LVPWT/LVEDD]. A split value of 0.42 for RWT was applied for both sexes, facilitating the categorization of LVM augmentation into either concentric (RWT > 0.42) or eccentric (RWT ≤ 0.42) hypertrophy. This criterion also enables the recognition of CR, characterized by a normal LVM but RWT > 0.42. Based on the LVMI and RWT, left ventricular geometric remodeling was divided into 4 categories. Patients presenting with a normal LVMI were categorized into either normal geometry (RWT ≤ 0.42) or CR (RWT > 0.42). Similarly, patients with LVH were categorized into one of the remaining 2 categories, eccentric hypertrophy (RWT ≤ 0.42) or CH (RWT > 0.42).
Statistical analysis
Patient data were collected and analyzed based on the sST2 levels (tertiles). The clinical and anthropometric characteristics of the study participants were presented as percentages for categorical data and as means with standard deviations for continuous data. χ2 test was utilized to compare sST2 groups for categorical data. If the data did not follow a normal distribution for differences between multiple groups, the Kruskal–Wallis (H) test for multiple samples was used. Pearson’s correlation analysis was used to analyze the correlations between sST2, NT-proBNP, HbA1c, BMI, age, SBP, hypertension grade and duration, and echocardiographic parameters. Important confounders were controlled for in the different models, including age, sex, BMI, diabetes, HbA1c, Cr, TG, SBP, pulse pressure, and hypertension grade and duration. Furthermore, a multivariate logistic regression analysis was performed to assess the association between sST2 levels (ng/ml) and pathological patterns of LV geometry, namely CR, CH, and eccentric hypertrophy. An assessment of the predictive efficacy of sST2 in LVH and geometric remodeling in patients with EH was conducted by creating a receiver operating characteristic (ROC) curve. Parameters such as area under the curve (AUC), specificity, and sensitivity were calculated. The optimal cutoff value on the ROC curve was determined using Youden’s index, which seeks to maximize the distance from the diagonal line representing chance discrimination. A P value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (version 25.0).
RESULTS
Clinical characteristics of the study participants
Table 1 provides an overview of the characteristics of the 483 individuals diagnosed with EH. The median age of the patients in the three tertiles was 66, 65, and 66 years, with interquartile ranges of 58–71, 57–72, and 59–73 years, respectively. Significant differences in the prevalence of diabetes were observed among tertiles 1, 2, and 3. Notably, tertile 3 displayed a greater prevalence of diabetes than did tertiles 1 and 2. Moreover, there were significant differences among the 3 tertiles in terms of median HbA1c and median sST2 levels.
Table 1.
Characteristics of the participants in the data sets
| Overall, N = 483 |
Tertile 1 <12.6 ng/ml n = 161 |
Tertile 2 12.6–18.7 ng/ml n = 162 |
Tertile 3 ≥18.8 ng/ml n = 160 |
P value | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, median (IQR) | 66 (58, 72) | 66 (58, 71) | 65 (57, 72) | 66 (59, 73) | 0.486 |
| Female, n (%) | 248(51.35%) | 87(54.04%) | 77 (47.53%) | 84 (52.50%) | 0.473 |
| BMI, median (IQR) | 25 (23.21, 27.26) | 24.73 (22.65, 27.04) | 24.93 (23.43, 26.85) | 25.21 (23.66, 27.70) | 0.078 |
| BMI, grade | 0.215 | ||||
| ≥28 | 89(18.43%) | 25 (15.53%) | 29 (17.90%) | 35 (21.88%) | |
| [24, 28) | 236(48.86%) | 71 (44.10%) | 85 (52.47%) | 80 (50.00%) | |
| [20, 24) | 155 (32.09%) | 64 (39.75%) | 47 (29.01%) | 44 (27.50%) | |
| <20 | 3(0.62%) | 1 (0.62%) | 1 (0.62%) | 1 (0.63%) | |
| Diabetes, n (%) | 200 (41.41%) | 55 (34.16%) | 58 (35.80%) | 87 (54.38%) | <0.001 |
| Hyperlipemia, n (%) | 267 (55.28%) | 94 (58.39%) | 86 (53.09%) | 87 (54.38%) | 0.608 |
| Laboratory characteristics | |||||
| Cr, median (IQR) ( mol/l) |
76(64, 92) | 76(65, 90) | 78.00 (64.25, 94.75) | 74.50 (63, 91) | 0.468 |
| eGFR, median (IQR) (ml/min/1.73 m2) | 83(68.34, 92.66) | 85.39 (64.62, 93.01) | 80.46 (70.29, 91.30) | 83.26 (68.42, 93.02) | 0.829 |
| LDL-C, median (IQR) (mmol/l) | 2.62(1.96, 3.35) | 2.52 (1.89, 3.25) | 2.65 (1.91, 3.35) | 2.71 (2.08, 3.41) | 0.296 |
| TC, median (IQR) (mmol/l) | 4.32(3.56, 5.26) | 4.23 (3.46, 4.96) | 4.36 (3.46, 5.34) | 4.42 (3.68, 5.35) | 0.152 |
| TG, median (IQR) (mmol/l) | 1.54(1.13, 2.22) | 1.47 (1.11, 2.13) | 1.56 (1.15, 2.23) | 1.61 (1.14, 2.28) | 0.321 |
| Non-HDL-C, median (IQR) (mmol/l) | 3.18 (2.40, 3.95) | 3.00 (2.32, 3.79) | 3.31 (2.30, 3.88) | 3.19 (2.56, 4.22) | 0.110 |
| HDL-C, median (IQR) (mmol/l) | 1.15 (0.96, 1.38) | 1.15 (0.95, 1.42) | 1.15 (0.99, 1.34) | 1.15 (0.96, 1.39) | 0.932 |
| UA, median (IQR) ( mol/l) | 359 (299, 431.5) | 345 (290, 416) | 361.5 (321, 433.5) | 362.5 (290, 439.25) | 0.421 |
| Glu, median (IQR) (mmol/l) | 6.88 (5.68, 9.10) | 6.74 (5.53, 8.82) | 6.88 (5.84, 9.16) | 6.92 (5.80, 9.77) | 0.360 |
| HbA1c, median (IQR) (mmol/l) | 6.00 (5.60, 6.70) | 5.90 (5.60, 6.50) | 5.90 (5.60, 6.58) | 6.30 (5.70, 7.00) | 0.005 |
| sST2, median (IQR) (ng/ml) | 15.30 (10.95, 20.40) | 9.30 (6.70, 10.90) | 15.30 (13.90, 17.28) | 22.80 (20.40, 26.43) | <0.001 |
| NT-proBNP, median (IQR) (pg/ml) | 62.60 (29.30, 142.45) | 62.90 (27.10, 142.80) | 55.50 (28.73, 117.65) | 69.60 (32.98, 199.83) | 0.156 |
| Hypertension characteristics | |||||
| SBP, median (IQR) (mmHg) | 139 (126, 155) | 138 (127, 154) | 136 (124.25, 153) | 145 (126, 159.25) | 0.181 |
| DBP, median (IQR) (mmHg) | 80 (74, 88) | 82 (75, 89) | 79 (72, 86.75) | 80 (74, 87) | 0.265 |
| MAP, median (IQR) (mmHg) | 100.67 (92.50, 109.17) | 101.33 (94.00, 108.67) | 99.00 (90.17, 109.08) | 101.83 (92.25, 109.75) | 0.312 |
| Pulse pressure, median (IQR) (mmHg) | 56 (46, 70) | 56 (46, 67) | 55 (47, 68.75) | 60 (45, 76) | 0.123 |
| Grade, n (%) | 0.089 | ||||
| I | 68 (14.08%) | 25 (15.53%) | 27 (16.67%) | 16 (10.00%) | |
| II | 188 (38.92%) | 68 (42.24%) | 65 (40.12%) | 55 (34.38%) | |
| III | 227 (47.00%) | 68 (42.24%) | 70 (43.21%) | 89 (55.63%) | |
| Duration, median (IQR) | 8 (3, 11) | 7 (3, 11) | 7.5 (3, 11) | 8.5 (4, 15) | 0.268 |
Abbreviations: BMI, body mass index; Cr, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GLU, glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MAP, mean arterial pressure; NT-proBNP, N-terminal pro brain-type natriuretic peptide; SBP, systolic blood pressure; sST2, soluble stimulating factor 2; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Echocardiography characteristics
Table 2 presents a comparison of echocardiographic parameters across different tertiles of sST2. When compared with tertile 1 and tertile 2, tertile 3 had a significant increase in LVM, LVMI, and LVH. Similarly, there was a discernible disparity among the 3 groups in terms of the LVPWT and IVST (P < 0.001). This examination showed a distinct proclivity toward more pronounced LV remodeling within tertile 3, emphasizing a substantial disparity when contrasted with the tertiles 1 and 2; in the patterns of LVH, the proportion of CH in tertile 3 (47.50%) was significantly higher than that in the tertiles 1 and 2 (13.04% and 18.52%, respectively, P < 0.001).
Table 2.
Echocardiography characteristics of the participants in the data sets
| Overall, N = 483 | Tertile 1 <12.6 ng/ml n = 161 |
Tertile 2 12.6-18.7 ng/ml n = 162 |
Tertile 3 ≥18.8 ng/ml n = 160 |
P value | |
|---|---|---|---|---|---|
| Echocardiography characteristics | |||||
| LA, median (IQR) (mm) | 33 (30, 36) | 33 (30, 35) | 33 (30, 35) | 34 (31, 37) | 0.030 |
| RVD, median (IQR) (mm) | 20 (18, 22) | 20 (18, 22) | 20 (18, 22) | 20 (19, 22) | 0.694 |
| LVEDD, median (IQR) (mm) | 46 (43, 48) | 45 (42, 48) | 45 (43, 48) | 47 (45, 49) | <0.001 |
| LVEDS, median (IQR) (mm) | 28 (26, 30) | 28 (26, 30) | 28 (26, 30) | 29 (27, 31) | 0.016 |
| LVPWT, median (IQR) (mm) | 11 (10, 11) | 10 (9, 11) | 11 (10, 11) | 11 (10, 12) | <0.001 |
| IVST, median (IQR) (mm) | 11 (10, 11.5) | 10 (10, 11) | 11 (10, 11) | 11 (10, 12) | <0.001 |
| EF, median (IQR) (%) | 69 (65, 72) | 69 (65, 72) | 69 (66, 72) | 68.5 (64, 72) | 0.439 |
| FS, median (IQR) (%) | 39 (35, 41) | 38 (36, 41) | 39 (36, 41) | 38.5 (35, 41) | 0.589 |
| RWT, median (IQR) | 0.46 (0.43, 0.50) | 0.45(0.41, 0.50) | 0.47(0.43, 0.50) | 0.47(0.43, 0.51) | 0.148 |
| LVM, median (IQR) (g) | 169.6 (145.64, 198.1) | 161.36 (140.09, 182.45) | 165.95 (145.64, 187.54) | 185.81 (162.65, 211.09) | <0.001 |
| LVMI, median (IQR) (g/m2.7) | 93.78 (83.03, 106.23) | 90.13 (80.08, 99.61) | 91.98 (82.84, 101.50) | 102.43 (89.66, 117.60) | <0.001 |
| LVH, n (%) | 148 (30.64%) | 25 (15.53%) | 38 (23.46%) | 85 (53.13%) | <0.001 |
| Patterns of LV hypertrophy | <0.001 | ||||
| Normal LV, n (%) | 97 (20.08%) | 47 (29.19%) | 28 (17.28%) | 22 (13.75%) | |
| Concentric remodeling, n (%) | 238 (49.28%) | 89 (55.28%) | 96 (59.26%) | 53 (33.13%) | |
| Eccentric hypertrophy, n (%) | 21 (4.35%) | 4 (2.48%) | 8 (4.94%) | 9 (5.63%) | |
| Concentric hypertrophy, n (%) | 127 (26.29%) | 21 (13.04%) | 30 (18.52%) | 76 (47.50%) | |
Abbreviations: EF, ejection fraction; FS, fractional shortening; IVST, interventricular septum thickness; LVH, left ventricular hypertrophy; LVM, left ventricular mass; LVMI, left ventricular mass index; LA, left atrial area; LVEDD, left ventricular end-diastolic diameter; LVEDS, left ventricular end-systolic diameter in systole; LVPWT, left ventricular posterior wall thickness; RVD, right ventricular diameter; RWT, relative wall thickness.
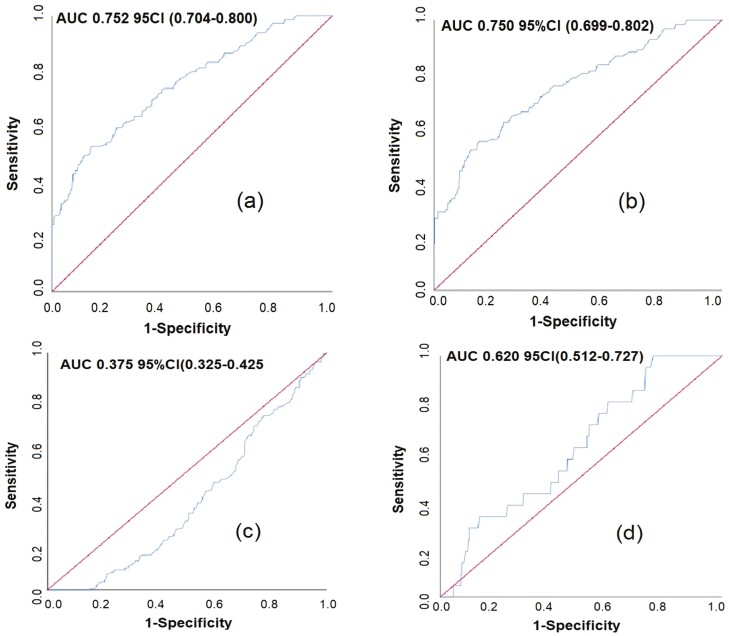
Evaluation of sST2 in identifying LVH and CH in patients with EH
As shown in Figure 1, sST2 demonstrated high specificity and sensitivity in identifying LVH and CH in patients with EH. The AUC of sST2 in identifying LVH (AUC: 0.752, 95% confidence interval [CI]: 0.704–0.800, sensitivity 52.7%, specificity 85.7%) and CH (AUC 0.750, 95% CI: 0.699–0.802, sensitivity 55.1%, specificity 84.3%) were significantly superior to that of sST2 in identifying CR (AUC 0.375, 95% CI: 0.325–0.425) and eccentric hypertrophy (AUC 0.620, 95% CI: 0.512–0.727).
Figure 1.
Receiver operating characteristic (ROC) of sST2 in identifying occurrence and patterns of left ventricular hypertrophy (LVH) in patients with essential hypertension (EH). Patterns of LVH were measured in 483 EH patients, including CH, CR, and eccentric hypertrophy. Statistics performed by ROC curve, results revealed that sST2 had potential predictive value for LVH (area under the curve [AUC]: 0.752, 95% confidence interval [CI]: 0.704–0.800) and CH (AUC: 0.750, 95% CI: 0.699–0.802) in patients with EH.
Figure 1: ROC of sST2 in identifying occurrence and patterns of LVH in EH patients. (a) ROC of sST2 in identifying occurrence of LVH in EH patients. (b) ROC of sST2 in identifying concentric hypertrophy of LVH in EH patients. (c) ROC of sST2 in identifying concentric remodeling of LVH in EH patients. (d) ROC of sST2 in identifying eccentric hypertrophy of LVH in EH patients. Abbreviations: AUC, the area under the ROC curve; CI, confidence interval; EH, essential hypertension; LVH, left ventricular hypertrophy; ROC, receiver operating characteristic curve; sST2, soluble stimulating factor 2.
Association between LVH and sST2 in patients with EH
Table 3 presents the odds ratio (OR) for sST2 among patients with LVH. The patients in the highest sST2 tertile had a significantly higher risk of LVH. This association persisted even after adjusting for potential confounding factors, including age, sex, BMI, diabetes, HbA1c, Cr, TG, SBP, pulse pressure, hypertension grade, and duration (OR = 6.61, 95% CI: 3.66–11.95; P < 0.001).
Table 3.
Odds ratio for left ventricular hypertrophy by sST2
| sST2(mg/ml) | Odds ratio(95% confidence interval) | ||||
|---|---|---|---|---|---|
| LVH, n% | Non-LVH, n% | Crude | Model 1 | Model 2 | |
| Tertile 1 (<12.6) |
25 (15.53%) |
136 (84.47%) |
Reference | Reference | Reference |
| Tertile 2 [12.6–18.7] |
38 (23.46%) |
124 (76.54%) |
1.67 (0.95–2.92) |
1.88 (1.05–3.36) |
1.83 (0.99–3.36) |
| Tertile 3 (≥ 18.8) |
85 (53.13%) |
75 (46.88%) |
6.17 (3.64–10.45) |
6.99 (3.99–12.27) |
6.61 (3.66–11.95) |
| P for trend | P < 0.001 | P < 0.001 | P < 0.001 | ||
| sST2 (per SD increase) | 3.71 (2.73–5.04) |
4.10 (2.94–5.74) |
4.20 (2.93–6.04) |
||
Model 1: adjusted for age, sex, BMI.
Model 2: multivariate stepwise logistic regression adjusted for age, sex, BMI, diabetes, HbA1c, creatinine, triglyceride, systolic pressure, pulse pressure, Hypertension Grade and Duration.
Abbreviations: LVH, left ventricular hypertrophy; SD, standard deviation; sST2, soluble stimulating factor 2.
Association between CH and sST2 in patients with EH
Table 4 summarizes the association between CH and sST2; those in the highest quartile of sST2 were significantly associated with increased CH risk. After adjusting for age, sex, BMI, diabetes mellitus, HbA1c, TG, SBP, pulse pressure, hypertension grade, and duration, the association remained significantly (OR = 5.80, 95% CI: 3.18–10.50; P < 0.001).
Table 4.
Odds ratio for concentric hypertrophy by sST2
| sST2(mg/ml) | Odds ratio(95% confidence interval) | ||||
|---|---|---|---|---|---|
| CH, n% | Non-CH, n% | Crude | Model 1 | Model 2 | |
| Tertile 1 (<12.6) |
21 (13.04%) |
140 (84.96%) |
Reference | Reference | Reference |
| Tertile 2 [12.6–18.7] |
30 (18.52%) |
132 (81.48%) |
1.52 (0.83–2.78) |
1.67 (0.89–3.12) |
1.58 (0.83–2.99) |
| Tertile 3 (≥ 18.8) |
76 (47.50%) |
84 (52.50%) |
6.03 (3.47-10.49) |
6.61 (3.70–11.84) |
5.80 (3.18–10.50) |
| P for trend | P < 0.001 | P < 0.001 | P < 0.001 | ||
| sST2 (per SD increase) | 3.52 (2.60–4.76) |
3.76 (2.71–5.21) |
3.59 (2.57–5.00) |
||
Model 1: adjusted for age, sex, and BMI.
Model 2: multivariate stepwise logistic regression adjusted for age, sex, BMI, diabetes, HbA1c,triglyceride, systolic pressure, pulse pressure, hypertension grade and duration.
Abbreviations: CH, concentric hypertrophy; SD, standard deviation; sST2,soluble stimulating factor 2.
Pearson correlation analysis between sST2 and echocardiographic characteristics of LVH
Pearson correlation analysis revealed that the positive relationships between sST2 and the interventricular septum, LVPWT, RWT, LVM, and LVMI remained significant after adjusting for significant covariates. These covariates included HbA1c, BMI, SBP, and grade for the interventricular septum; HbA1c, BMI, SBP, and grade for LVPWT; HbA1c, SBP, grade for RWT; NT-proBNP, BMI, and SBP for LVM; and NT-proBNP level, age, SBP, grade, and duration for LVMI (Table 5).
Table 5.
Pearson correlation analysis with echocardiographic characteristics of left ventricular hypertrophy as the dependent variable
| Variables | Interventricular septum | LV posterior wall thickness | Relative wall thickness | LV mass | LV mass index |
|---|---|---|---|---|---|
| sST2 | 0.321** | 0.315** | 0.146** | 0.361** | 0.397** |
| NT-proBNP | 0.186** | 0.208* | |||
| HbA1c | 0.165** | 0.156** | 0.123** | ||
| Age | 0.115* | ||||
| BMI | 0.265** | 0.250** | 0.305** | ||
| SBP | 0.131* | 0.144* | 0.112** | 0.123** | 0.183** |
| Grade | 0.164* | 0.160** | 0.133** | 0.176** | |
| Duration | 0.170** |
Abbreviations: BMI, body mass index; HbA1c, glycosylated hemoglobin; NT-proBNP, N-terminal pro brain-type natriuretic peptide; SBP, systolic blood pressure; sST2, soluble stimulating factor 2.
*P < 0.05.
**P < 0.001.
Logistic regression analysis relating sST2 level to LV geometric patterns
CR, eccentric hypertrophy, and CH were present in 49.28%, 4.35%, and 26.29% of study participants, respectively. Table 6 presents the association between sST2 as a continuous independent variable and abnormal LV geometric patterns derived from the logistic regression model. After multivariate adjustments for age, sex, and BMI, the association between sST2 and CH was significant (OR: 1.17, 95% CI: 1.12–1.21, P < 0.001) and was modulated by sex (OR: 3.81, 95% CI: 2.25–6.44, P < 0.001), hypertension grade (OR: 1.98, 95% CI: 1.35–2.92, P = 0.001), and eGFR (OR: 0.98, 95% CI: 0.97–1.00, P = 0.013). No significant association was found between sST2 levels and the risk of eccentric hypertrophy.
Table 6.
Logistic regression analysis relating soluble ST2 concentration to left ventricular geometric patterns
| LV geometric pattern predictors | Concentric hypertrophy | Eccentric hypertrophy | Concentric remodeling | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| sST2 | 1.17 (1.12–1.21) | <0.001 | 0.93 (0.90–0.95) | <0.001 | ||
| Female | 3.81 (2.25–6.44) | <0.001 | 0.37 (0.25–0.50) | <0.001 | ||
| Grade | 1.98 (1.35–2.92) | 0.001 | ||||
| eGFR | 0.98 (0.97–1.00) | 0.013 | ||||
| Hyperlipemia | 1.65 (1.12–2.43) | 0.012 | ||||
| Duration | 1.08 (1.02–1.13) | 0.004 | 0.97 (0.94–1.00) | 0.032 | ||
| SBP | 1.02 (1.00–1.04) | 0.05 | ||||
Abbreviations: CI, confidence interval; sST2, soluble stimulating factor 2; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; OR, odds ratio.
DISCUSSION
The LV is a key point for the end-organ damage induced by hypertension. LVH manifests as a complex series of myocardial adaptations. The development of LVH involves multiple biological mechanisms, including the hypertrophy and fibrosis processes of cardiac muscle cells.21 In clinical practice, electrocardiography serves as a prevalent screening method for LVH owing to its widespread availability and cost-effectiveness,22,23 as proposed in the Chinese hypertension guidelines.24 Various electrocardiographic criteria, such as the Sokolow–Lyon and Cornell criteria, have been used for LVH assessment.25 Nonetheless, the sensitivity of electrocardiography in detecting LVH is considered relatively low, especially among the Chinese and other East Asian populations.26 Echocardiography has shown superior sensitivity for LVH detection. However, this approach requires considerable professional knowledge and has low accessibility.6,27 According to the 2023 ESC/ESH guidelines, echocardiography is a secondary diagnostic tool for LVH. It is recommended, only if specific cardiac structures and functions affect treatment decisions.28 Therefore, biomarkers associated with LVH and CH are clinically important for risk stratification and prognosis in hypertension patients.
Previous studies have identified several biomarkers associated with an increased risk of LVH for patients with EH; however, these studies have not sufficiently explored the relationship between biomarkers and left ventricular geometric remodeling. The principal finding of this study is that sST2 may provide diagnosis and prediction value for LVH and CH in EH patients. The current study encompassed a cohort of 483 patients, of whom 30.64% were diagnosed with LVH. A significant association was observed between the prevalence of LVH and the levels of sST2. Notably, 53.13% of the patients within the highest tertile level of sST2 were LVH. Furthermore, in term of echocardiographic measures, higher ST2 levels were associated with an increase of the interventricular septum thickness, posterior LV wall thickness, and LVMI. These associations remained robust even after adjusting for a variety of confounders. These findings are consistent with previous research20 that found a positive relation between sST2 levels and cardiac mass in patients with EH. The main role of ST2 in hypertension is through the interleukin (IL)-33/ST2 signaling pathway. IL-33 and ST2 are members of the IL-1 cytokine family, and IL-33 acts as a dual-function pro-inflammatory and anti-inflammatory cytokine. It is mainly secreted by endothelial cells in the heart and interacts with the ST2L receptor to provide anti-hypertrophic and anti-fibrotic effects, protecting the heart.29 From a mechanistic standpoint, LVH causes a hypertrophic myocardium that experiences intricate alterations impacting both cardiomyocytes and the interstitium. Such alterations include focal or diffuse fibrosis, inflammation, edema, and fatty infiltration.3 Altered sST2 levels are correlated with myocardial stress and injury, and sST2 has become a potential prognostic biomarker of CVD.30
The results of the ROC analysis revealed that sST2 had potential predictive value for LVH and CH in patients with EH, with a cutoff value of 20.25 ng/ml. Logistic regression analysis suggested that the highest tertile of sST2 was significantly associated with increased LVH risk, compared with the lowest tertile (multivariate-adjusted OR of highest group: 6.61; P < 0.001). These findings are consistent with previous research by Ojj et al. that identified sST2 as an independent risk factor for LVH in patients with EH.31 Similarly, they reported that ROC curve analysis indicated that sST2 had good sensitivity and specificity for predicting LVH in patients with EH. However, this study did not further explore the relationship of sST2 in EH patients with LV geometric remodeling. Several geometric remodeling of LV, encompass normal geometry, CR, CH, and eccentric hypertrophy. Among them, CH has an important prognostic significance and is correlated with compromised cardiac systolic and diastolic function, along with an increased risk of CVD and all-cause mortality.32 A study by Guzik underscores the significance of CH as a strong prognostic indicator of outcomes.6 Notably, in patients with CH, elevated values of RWT and LVPWT in diastole correlate with an increased possibility of experiencing major adverse cardiovascular and cerebrovascular events. Previous studies have reported different results on the relationship between sST2 levels and LV geometric patterns. Zhang et al.33 demonstrated the highest sST2 levels in maintenance hemodialysis patients with LV CR and CH, whereas another study34 observed significantly high sST2 levels in EH patients with CH. In our study, we found a higher risk of CH in the highest sST2 tertile after adjusting for covariates (multivariate-adjusted OR of the highest group: 5.80; P < 0.001). Therefore, our findings further augment previous research by clearly demonstrating that sST2 is not only associated with LVH but also with CH. This is possibly because ST2 belongs to the IL-1 receptor family, which binds to its ligand IL-33 and participates in the inflammatory response and fibrosis process in heart disease. During LV remodeling, myocardial and endothelial cells release ST2 under mechanical stress and inflammatory stimulation, which serves as a marker of disease progression. These findings indicate that elevated serum soluble ST2 levels are associated with hypertensive LVH and left ventricular geometric remodeling, particularly CH. These associations may serve as indicators of the extent of cardiac injury. Moreover, the analysis reveals that being female and the severity of hypertension are significant risk factors for the development of concentric LVH. This could be attributed to the fact that a substantial proportion of the participants are postmenopausal women, who have reduced estrogen levels and, consequently, a diminished cardiovascular protective effect.
In conclusion, our retrospective study indicates that elevated sST2 level is related to LV structural changes, such as increased LVMI and IVST in patients with EH, supporting that sST2 may not only be used as a potential biomarker for the diagnosis and risk assessment of hypertensive heart disease but also as an important intervention target for reversing hypertensive LV remodeling. Owing to the study’s cross-sectional nature and inherent design limitations, clarifying the precise mechanism through which sST2 influences LVH and geometric remodeling remains beyond the study’s scope. Future research should consider conducting prospective cohort studies and relevant animal experiments to provide valuable insights into the relationship between sST2 and hypertensive heart disease.
Contributor Information
Xia Wang, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Shu-Jie Han, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Xiao-Li Wang, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Yun-Feng Xu, The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Hui-Cheng Wang, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Jiang-Yang Peng, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Guang-Ming Pan, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Ya-Hui Chen, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
Chuangchang Wang, Department of Cardiovascular, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China; The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China.
FUNDING
This work was supported by the Guangzhou Municipal Science and Technology Project (No. 202201020318).
CONFLICT OF INTEREST
The authors declared no conflict of interest..
Data Availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mahfoud F, Böhm M, Bongarth CM, Bosch R, Schmieder RE, Schunkert H, Stellbrink C, Trenkwalder P, Vonend O, Weil J, Kreutz R.. Kommentar zu den Leitlinien (2018) der Europäischen Gesellschaft für Kardiologie (ESC) und der Europäischen Gesellschaft für Hypertonie (ESH) für das Management der arteriellen Hypertonie. Internist (Berl) 2019; 60:424–430. [DOI] [PubMed] [Google Scholar]
- 2. Vallée A, Safar ME, Blacher J.. Hypertension artérielle permanente essentielle: définitions et revue hémodynamique, clinique et thérapeutique. La Presse Médicale 2019; 48:19–28. [DOI] [PubMed] [Google Scholar]
- 3. Bacharova L, Kollarova M, Bezak B, Bohm A.. Left ventricular hypertrophy and ventricular Tachyarrhythmia: the role of biomarkers. Int J Mol Sci 2023; 24:3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheng Y, Li M, Xu M, Zhang Y, Xu J, Huang Y, Li X, Yao G, Sui W, Zhang M, Zhang Y, Zhang C, Zhang Y, Zhang M.. Left ventricular and atrial remodelling in hypertensive patients using thresholds from international guidelines and EMINCA data. Eur Heart J – Cardiovasc Imaging 2022; 23:166–174. [DOI] [PubMed] [Google Scholar]
- 5. Tadic M, Cuspidi C, Saeed S, Lazic JS, Vukomanovic V, Grassi G, Sala C, Celic V.. The influence of left ventricular geometry on myocardial work in essential hypertension. J Hum Hypertens 2022; 36:524–530. [DOI] [PubMed] [Google Scholar]
- 6. Guzik BM, McCallum L, Zmudka K, Guzik TJ, Dominiczak AF, Padmanabhan S.. Echocardiography predictors of survival in hypertensive patients with left ventricular hypertrophy. Am J Hypertens 2021; 34:636–644. [DOI] [PubMed] [Google Scholar]
- 7. Yildiz M, Oktay AA, Stewart MH, Milani RV, Ventura HO, Lavie CJ.. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis 2020; 63:10–21. [DOI] [PubMed] [Google Scholar]
- 8. Aro AL, Reinier K, Phan D, Teodorescu C, Uy-Evanado A, Nichols GA, Gunson K, Jui J, Chugh SS.. Left-ventricular geometry and risk of sudden cardiac arrest in patients with preserved or moderately reduced left-ventricular ejection fraction. Europace 2017; 19:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Støylen A, Dalen H, Molmen HE.. Left ventricular longitudinal shortening: relation to stroke volume and ejection fraction in ageing, blood pressure, body size and gender in the HUNT3 study. Open Heart 2020; 7:e001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vriz O, Pirisi M, Habib E, Galzerano D, Fadel B, Antonini-Canterin F, Veldtman G, Bossone E.. Age related structural and functional changes in left ventricular performance in healthy subjects: a 2D echocardiographic study. Int J Cardiovasc Imaging 2019; 35:2037–2047. [DOI] [PubMed] [Google Scholar]
- 11. Miceli F, Presta V, Citoni B, Canichella F, Figliuzzi I, Ferrucci A, Volpe M, Tocci G.. Conventional and new electrocardiographic criteria for hypertension-mediated cardiac organ damage: a narrative review. J Clin Hypertens 2019; 21:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad MI, Mujtaba M, Anees MA, Li Y, Soliman EZ.. Interrelation between electrocardiographic left atrial abnormality, left ventricular hypertrophy, and mortality in participants with hypertension. Am J Cardiol 2019; 124:886–891. [DOI] [PubMed] [Google Scholar]
- 13. Wang CC, Liang LK, Luo SM, Wang HC, Wang XL, Cheng YH, Pan GM, Peng JY, Han SJ, Wang X.. Nomogram-based risk assessment model for left ventricular hypertrophy in patients with essential hypertension: incorporating clinical characteristics and biomarkers. J Clin Hypertens 2024; 26:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lønnebakken MT, Izzo R, Mancusi C, Gerdts E, Losi MA, Canciello G, Giugliano G, De Luca N, Trimarco B, de Simone G.. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc 2017; 6:e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carneros D, Santamaría EM, Larequi E, Vélez-Ortiz JM, Reboredo M, Mancheño U, Perugorria MJ, Navas P, Romero-Gómez M, Prieto J, Hervás-Stubbs S, Bustos M.. Cardiotrophin-1 is an anti-inflammatory cytokine and promotes IL-4–induced M2 macrophage polarization. FASEB J 2019; 33:7578–7587. [DOI] [PubMed] [Google Scholar]
- 16. Rasmussen LJH, Petersen JEV, Eugen-Olsen J.. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol 2021; 12:780641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan D, Kassiri Z.. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and its therapeutic implications in cardiovascular pathology. Front Physiol 2020; 11:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pascual-Figal D, Lax A, Asensio López MC.. Circulating ST2, from biomarker to pathogenic mediator. Rev Esp Cardiol 2023; 76:672–674. [DOI] [PubMed] [Google Scholar]
- 19. Huttin O, Kobayashi M, Ferreira JP, Coiro S, Bozec E, Selton-Suty C, Filipetti L, Lamiral Z, Rossignol P, Zannad F, Girerd N.. Circulating multimarker approach to identify patients with preclinical left ventricular remodelling and/or diastolic dysfunction. ESC Heart Failure 2021; 8:1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei P, Liu L, Wang X, Zong B, Liu X, Zhang M, Fu Q, Wang L, Cao B.. Expression of soluble ST2 in patients with essential hypertension and its relationship with left ventricular hypertrophy. ESC Heart Failure 2023; 10:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruilope LM, Schmieder RE.. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 2008; 21:500–508. [DOI] [PubMed] [Google Scholar]
- 22. Li G, Shi C, Li T, Ouyang N, Guo X, Chen Y, Li Z, Zhou Y, Yang H, Yu S, Sun G, Sun Y.. A nomogram integrating non-ECG factors with ECG to screen left ventricular hypertrophy among hypertensive patients from northern China. J Hypertens 2022; 40:264–273. [DOI] [PubMed] [Google Scholar]
- 23. Oikonomou E, Theofilis P, Mpahara A, Lazaros G, Niarchou P, Vogiatzi G, Tsalamandris S, Fountoulakis P, Christoforatou E, Mystakidou V, Anastasiou M, Goliopoulou A, Tousoulis D.. Diagnostic performance of electrocardiographic criteria in echocardiographic diagnosis of different patterns of left ventricular hypertrophy. Noninvasive Electrocardiol 2020; 25:e12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin R, Yin L, Li L, Silva-Nash J, Tan J, Pan Z, Zeng J, Yan LL.. Hypertension in China: burdens, guidelines and policy responses: a state-of-the-art review. J Hum Hypertens 2022; 36:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu M, Ge Z, Huang J, Shao X, Li J, Yang J.. Modified Cornell electrocardiographic criteria in the assessment of left ventricular hypertrophy geometry of patients with essential hypertension. J Clin Hypertens (Greenwich) 2020; 22:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang D, Xu JZ, Zhang W, Chen Y, Li J, An Y, Bian R, Wang JG.. Performance of electrocardiographic criteria for echocardiographically diagnosed left ventricular hypertrophy in Chinese hypertensive patients. Am J Hypertens 2020; 33:831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marcato JP, Senra Santos F, Gama Palone A, Lenci Marques G.. Evaluation of different criteria in the diagnosis of left ventricular hypertrophy by electrocardiogram in comparison with echocardiogram. Cureus 2022; 14:e26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, De Winter T, Elliott PM, Flather M, Garcia-Pavia P, Haugaa KH, Ingles J, Jurcut RO, Klaassen S, Limongelli G, Loeys B, Mogensen J, Olivotto I, Pantazis A, Sharma S, Van Tintelen JP, Ware JS, Kaski JP; ESC Scientific Document Group. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 2023; 44:3503–3626. [DOI] [PubMed] [Google Scholar]
- 29. Dudek M, Kałużna-Oleksy M, Migaj J, Sawczak F, Krysztofiak H, Lesiak M, Straburzyńska-Migaj E.. sST2 and heart failure—clinical utility and prognosis. J Clin Med 2023; 12:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR.. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017; 135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 31. Ojji DB, Opie LH, Lecour S, Lacerda L, Adeyemi OM, Sliwa K.. The effect of left ventricular remodelling on soluble ST2 in a cohort of hypertensive subjects. J Hum Hypertens 2014; 28:432–437. [DOI] [PubMed] [Google Scholar]
- 32. Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, Benjamin EJ, Vasan RS.. The natural history of left ventricular geometry in the community. JACC: Cardiovasc Imaging. 2014; 7:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Xie Y, Shen B, Nie Y, Cao X, Xiang F, Zou J.. Relationship between soluble ST2 and left ventricular geometry in maintenance hemodialysis patients. Blood Purif 2021; 50:84–92. [DOI] [PubMed] [Google Scholar]
- 34. Ojji DB, Opie LH, Lecour S, Lacerda L, Adeyemi O, Sliwa K.. Relationship between left ventricular geometry and soluble ST 2 in a cohort of hypertensive patients. J Clin Hypertens 2013; 15:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.