Abstract
Amphotericin B deoxycholate (AMPH-B) is a polyene macrolide with antifungal activity. Liposomal AMPH-B (L-AMB) was developed to reduce side effects while maintaining antifungal activity. This study was aimed at evaluating and comparing the adverse event profiles of AMPH-B and L-AMB using a spontaneous reporting system.
We analyzed the adverse event reports of AMPH-B and L-AMB from the United States Food and Drug Administration Adverse Event Reporting System (FAERS). Case report counts of adverse events were generated according to the preferred terms of the Medical Dictionary for Regulatory Activities (MedDRA). Standardized MedDRA queries (SMQs) and system organ classes (SOCs) were used to compare the organ-specific adverse event profiles of AMPH-B and L-AMB. The reporting odds ratio and proportional reporting rate were used to detect pharmacovigilance signals.
The FAERS database contains 21,173,818 cases from January 2004 to March 2024. Adverse events were reported in 2438 cases receiving AMPH-B treatment and 3344 cases receiving L-AMB treatment, including 848 and 1591 cases receiving intravenous AMPH-B and L-AMB injections, respectively. The most frequently reported drug-related adverse event in the AMPH-B and L-AMB groups was hypokalemia. SOCs with statistically significant differences were “Inv” (laboratory tests), “Resp” (respiratory, thoracic, and mediastinal disorders), “Genrl” (general and systemic disorders and conditions at the site of administration), “Card” (cardiac disorders), and “Blood” (blood and lymphatic system disorders). No statistically significant difference was observed in the SMQ profile of adverse events in “Renal” (renal and urinary disorders) and “Hepat” (hepatobiliary disorders) between the L-AMB and AMPH-B formulations in this study.
Based on real-world data from FAERS, adverse event profiles of AMPH-B and L-AMB were compared. No statistically significant difference was observed in the SMQ profile of adverse events in the renal and hepatic SOCs between the L-AMB and AMPH-B formulations. Our results suggest that L-AMB is more tolerated by the kidneys than AMPH-B.
Keywords: ambisome, amphotericin b, fda adverse event reporting system, fungizone, liposomal amphotericin b
Introduction
Amphotericin B deoxycholate (AMPH-B) is a polyene macrolide with antifungal activity. It is used in an oral form to treat abnormal Candida growth in the gastrointestinal tract and in an injectable form to treat deep-seated dermatomycoses and fungal infections, such as Aspergillus, Candida, Cryptococcus, Mucor, and Coccidioides infections. For example, Fungizone® is an injectable AMPH-B formulation that has been used as the gold standard for the treatment of deep mycosis for more than 60 years since its launch in 1962. However, AMPH-B injections cause fever, chills, and many other serious side effects, including hypokalemia and renal dysfunction [1-3].
Recently, new drug delivery systems have been developed to increase the therapeutic efficacy of drugs and reduce their organ toxicity. Liposomalization is a technique used to develop such systems to either increase drug concentrations in tumor cells or decrease drug exposure in normal tissues. Liposomes are closed spherical vesicles consisting of multiple concentric bilayers of phospholipids, cholesterol, and other affinity materials mixed in specific proportions [4,5]. For example, liposomal amphotericin B (L-AMB) was developed to reduce serious side effects, while maintaining efficacy. The L-AMB Ambisome® showed the same pharmacological activity as AMPH-B in vitro against Aspergillus fumigatus, Aspergillus flavus, Candida albicans, Candida krusei, Candida lusitaniae, Candida parapsilosis, Candida tropicalis, Cryptococcus neoformans, and Blastomyces dermatitidis [4].
In an examination of the cytotoxicity of AMPH-B on human erythrocytes by measuring hemolytic potential, a difference of approximately 100-fold was observed between AMPH-B and L-AMB [6]. The cytotoxicity of L-AMB in some cell lines was also reported to be lower than that of AMPH-B [6]. Although different dosage forms of AMPH-B and L-AMB are expected to have different adverse event profiles, their descriptions in the package inserts are not quantitative [3,4], and few reports comprehensively evaluate the various adverse event profiles of different dosage forms.
L-AMB generally induces lesser adverse events than AMPH-B [4,5], with lowered risk of kidney damage [7]. However, the possible difference between the renal impairment profiles of L-AMB and AMPH-B has not been evaluated in diverse clinical settings. Although the advantage of L-AMB over AMPH-B is widely accepted, we believe that without this knowledge, there may be a risk of missing adverse events induced by L-AMB in routine practice. However, to our knowledge, few studies have compared the adverse event profiles of different AMPH-B formulations.
The United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) is the world’s largest spontaneous reporting system (SRS); it collects a wide variety of adverse drug events from clinicians and pharmaceutical companies and is recognized as a major pharmacovigilance tool that closely reflects clinical practice [8-10]. To our knowledge, few comprehensive studies have investigated the adverse events associated with AMPH-B and L-AMB using FAERS. Therefore, this study aimed to evaluate and compare the adverse event profiles of AMPH-B and L-AMB using data from the FAERS database.
Materials and methods
Ethical approval was not sought for this study because it was an observational study without any participants. All the results were obtained from data openly available online on the Food and Drug Administration website. All data from the FAERS database were fully anonymized by the regulatory authorities before we accessed them.
Data from the FAERS database were downloaded from the FDA website [11]. The FAERS database structure conforms to the international safety reporting guidelines drafted by the International Council on Harmonization (E2B). The FAERS database comprises seven data tables: patient demographic and administrative information (DEMO), drug/biological information (DRUG), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), drug therapy start and end dates (THER), and indications for use or diagnosis (INDI).
We created our dataset from the FAERS database using FileMaker Pro 18 Advanced software (FileMaker, Inc., Santa Clara, CA, USA), according to the ASCII Entity Relationship Diagram, which is publicly available on the FDA website. According to FDA recommendations, we removed duplicate reports from the same patient in the FAERS database from the analysis. Drugs reported in the case reports were classified into four categories according to the degree to which they were expected to contribute to adverse events: primary suspect (PS), secondary suspect (SS), concomitant (C), and interacting (I) drugs. Drugs recorded as PS were used for the analysis. Drugs in the FAERS database are registered voluntarily. They can be registered using generic names, brand names, or abbreviations. DrugBank (The Metabolomics Innovation Centre, Canada) is a reliable drug database that is used as a reference for pharmacovigilance analysis [12]. In this study, it was used as a source for batch conversion and drug name integration.
Case report counts of adverse events were generated according to the preferred terms (PTs) of the Medical Dictionary for Regulatory Activities (MedDRA) version 27.0. Standardized MedDRA queries (SMQs) are widely used to analyze SRS reports. They were constructed by the Maintenance and Support Services Organization and group PTs according to the levels associated with defined medical conditions. SMQ and system organ classes (SOCs) were used to compare the organ-specific adverse event profiles of AMPH-B and L-AMB. Pearson’s chi-square test was used to compare data between the two formulations. Data were considered statistically significant at p<0.05.
We calculated the reporting ratio and the reporting odds ratio (ROR) to study the influence of AMPH-B (Fungizone®) and L-AMB (Ambisome®) on adverse events [8-10,13]. A 2×2 contingency table was then created to identify drug combinations resulting in disproportionately adverse events. The “cases” were defined as patients reporting adverse events after AMPH-B and L-AMB use, and “non-cases” were defined as patients reporting all other events. ROR values were calculated as (a × d)/(b × c) and expressed as point estimates with 95% confidence intervals (CIs). A signal was defined as the lower limit of the 95% CI of the ROR being greater than one. Two or more cases are required to identify a signal [8,14].
All data analyses were performed using JMP Pro 16 software (JMP Statistical Discovery, Cary, NC, USA).
Results
The FAERS database contains 21,173,818 cases from January 2004 to March 2024. We analyzed 17,714,041 reports, excluding duplicate reports according to FDA recommendations. Adverse events of AMPH-B and L-AMB were reported in 2438 and 3344 cases, respectively; of these cases, 848 and 1591 cases received intravenous injections of AMPH-B and L-AMB, respectively. The most frequently reported adverse event related to AMPH-B was hypokalemia (excluding the PTs, “DRUG INEFFECTIVE” and “OFF LABEL USE”), followed by pyrexia and renal impairment. The most frequently reported adverse event related to L-AMB was hypokalemia (excluding the PTs, “DRUG INEFFECTIVE” and “OFF LABEL USE”), followed by renal impairment and dyspnea. No significant difference was observed in the expression profile of ROR signals between the two formulations of AMPH-B and L-AMB (Tables 1, 2).
Table 1. Number of reports and reporting odds ratios for amphotericin B formulations (AMPH-B).
* Confidence Interval
| Preferred term code | Preferred term | Total | Case (n) | Reporting odds ratio (95% CI*) |
| 10013709 | Drug ineffective | 1128032 | 224 | 5.3 (4.5–6.1) |
| – | Off label use | 650743 | 116 | 4.2 (3.4–5.1) |
| 10021015 | Hypokalemia | 39093 | 47 | 26.6 (19.8–35.6) |
| – | Death | 730753 | 38 | 1.1 (0.8–1.5) |
| 10037660 | Pyrexia | 299290 | 38 | 2.7 (2.0–3.8) |
| 10062237 | Renal impairment | 70466 | 30 | 9.2 (6.4–13.2) |
| – | Chills | 102188 | 29 | 6.1 (4.2–8.8) |
| 10043071 | Tachycardia | 76652 | 29 | 8.2 (5.6–11.8) |
| 10069339 | Acute kidney injury | 125590 | 28 | 4.8 (3.3–7.0) |
| 10005483 | Blood creatinine increased | 57873 | 25 | 9.3 (6.2–13.8) |
| – | Condition aggravated | 240861 | 23 | 2.0 (1.3–3.1) |
| 10013968 | Dyspnea | 486578 | 23 | 1.0 (0.7–1.5) |
| 10077361 | Multiple organ dysfunction syndrome | 20960 | 20 | 20.4 (13.1–31.8) |
| 10051792 | Infusion-related reaction | 52388 | 18 | 7.3 (4.6–11.7) |
| 10033661 | Pancytopenia | 47122 | 18 | 8.1 (5.1–13.0) |
| 10035664 | Pneumonia | 271408 | 17 | 1.3 (0.8–2.1) |
| 10007515 | Cardiac arrest | 73279 | 17 | 4.9 (3.0–8.0) |
| 10043089 | Tachypnea | 11352 | 17 | 31.9 (19.8–51.7) |
| 10038435 | Renal failure | 122048 | 17 | 2.9 (1.8–4.8) |
| 10033318 | Oxygen saturation decreased | 45423 | 16 | 7.5 (4.6–12.3) |
| – | Renal failure acute | 43092 | 16 | 7.9 (4.8–12.9) |
| 10038695 | Respiratory failure | 63850 | 16 | 5.3 (3.2–8.7) |
| 10040070 | Septic shock | 36043 | 14 | 8.2 (4.9–14.0) |
| 10020646 | Hyperkalemia | 29887 | 14 | 9.9 (5.9–16.9) |
| 10021097 | Hypotension | 173419 | 14 | 1.7 (1.0–2.9) |
| 10043554 | Thrombocytopenia | 94721 | 13 | 2.9 (1.7–5.0) |
| 10051118 | Drug ineffective for unapproved indication | 43837 | 13 | 6.3 (3.6–10.9) |
| 10029354 | Neutropenia | 112363 | 13 | 2.4 (1.4–4.2) |
| – | Disease progression | 98477 | 13 | 2.8 (1.6–4.8) |
| 10029155 | Nephropathy toxic | 8956 | 13 | 30.8 (17.8–53.3) |
| 10037844 | Rash | 358880 | 12 | 0.7 (0.4–1.2) |
| 10021027 | Hypomagnesemia | 11470 | 12 | 22.2 (12.5–39.2) |
| – | Headache | 541085 | 12 | 0.5 (0.3–0.8) |
| 10005724 | Blood potassium decreased | 26225 | 12 | 9.7 (5.5–17.1) |
| 10008479 | Chest pain | 163743 | 12 | 1.5 (0.9–2.7) |
| 10047700 | Vomiting | 396955 | 11 | 0.6 (0.3–1.0) |
| 10020772 | Hypertension | 182177 | 11 | 1.3 (0.7–2.3) |
| 10028813 | Nausea | 674394 | 11 | 0.3 (0.2–0.6) |
| 10020751 | Hypersensitivity | 159049 | 11 | 1.5 (0.8–2.6) |
| 10027417 | Metabolic acidosis | 26468 | 10 | 8.0 (4.3–14.9) |
| 10044565 | Tremor | 146245 | 9 | 1.3 (0.7–2.5) |
| 10038687 | Respiratory distress | 24185 | 9 | 7.8 (4.1–15.1) |
| 10040047 | Sepsis | 96944 | 9 | 1.9 (1.0–3.8) |
| 10017533 | Fungal infection | 28546 | 9 | 6.6 (3.4–12.8) |
| – | Drug interaction | 138348 | 9 | 1.4 (0.7–2.6) |
| 10027091 | Medication error | 47854 | 9 | 4.0 (2.1–7.6) |
| 10006473 | Bronchopulmonary aspergillosis | 6385 | 9 | 29.8 (15.4–57.5) |
| 10021143 | Hypoxia | 29665 | 9 | 6.4 (3.3–12.3) |
| 10003988 | Back pain | 202606 | 8 | 0.8 (0.4–1.7) |
| 10006482 | Bronchospasm | 12639 | 8 | 13.3 (6.7–26.8) |
| 10014387 | Electrocardiogram QT prolonged | 30412 | 8 | 5.5 (2.8–11.1) |
| 10074171 | Aspergillus infection | 5097 | 8 | 33.1 (16.5–66.5) |
| 10020642 | Hyperhidrosis | 113791 | 8 | 1.5 (0.7–3.0) |
| 10038537 | Renal tubular disorder | 2575 | 8 | 65.7 (32.7–132.0) |
| 10038428 | Renal disorder | 40451 | 8 | 4.2 (2.1–8.3) |
| 10003119 | Arrhythmia | 42283 | 8 | 4.0 (2.0–8.0) |
| 10000880 | Acute myeloid leukemia | 13325 | 8 | 12.7 (6.3–25.4) |
| 10065042 | Immune reconstitution inflammatory syndrome | 4357 | 8 | 38.8 (19.3–77.9) |
| 10065553 | Bone marrow failure | 19108 | 8 | 8.8 (4.4–17.7) |
| 10028098 | Mucormycosis | 2113 | 7 | 70.0 (33.2–147.5) |
| 10019663 | Hepatic failure | 26599 | 7 | 5.5 (2.6–11.6) |
| 10038540 | Renal tubular necrosis | 8395 | 7 | 17.6 (8.3–37.0) |
| 10051082 | Therapy non-responder | 33407 | 7 | 4.4 (2.1–9.3) |
| – | General physical health deterioration | 91284 | 7 | 1.6 (0.8–3.4) |
| – | Multi-organ failure | 17736 | 7 | 8.3 (3.9–17.5) |
| 10002198 | Anaphylactic reaction | 44463 | 7 | 3.3 (1.6–7.0) |
| 10071408 | Maternal exposure during pregnancy | 68553 | 7 | 2.1 (1.0–4.5) |
| 10029147 | Nephrogenic diabetes insipidus | 1008 | 7 | 147.3 (69.8–310.7) |
| 10011224 | Cough | 234271 | 7 | 0.6 (0.3–1.3) |
| 10005911 | Body temperature increased | 18329 | 7 | 8.0 (3.8–16.9) |
| 10001551 | Alanine aminotransferase increased | 54298 | 6 | 2.3 (1.0–5.2) |
| 10043414 | Therapeutic response decreased | 50145 | 6 | 2.5 (1.1–5.6) |
| 10048610 | Cardiotoxicity | 6896 | 6 | 18.3 (8.2–40.9) |
| 10040560 | Shock | 19115 | 6 | 6.6 (3.0–14.7) |
| 10002034 | Anemia | 167009 | 6 | 0.7 (0.3–1.7) |
| 10019717 | Hepatitis | 21811 | 6 | 5.8 (2.6–12.9) |
| 10007554 | Cardiac failure | 69628 | 6 | 1.8 (0.8–4.0) |
| 10016825 | Flushing | 91611 | 6 | 1.4 (0.6–3.1) |
| 10015150 | Erythema | 176486 | 6 | 0.7 (0.3–1.6) |
| 10028411 | Myalgia | 147005 | 6 | 0.9 (0.4–1.9) |
| 10012735 | Diarrhea | 537371 | 6 | 0.2 (0.1–0.5) |
| 10005851 | Blood urea increased | 15500 | 6 | 8.1 (3.6–18.2) |
| 10022611 | Interstitial lung disease | 40243 | 6 | 3.1 (1.4–7.0) |
| 10005364 | Blood bilirubin increased | 24125 | 6 | 5.2 (2.3–11.7) |
| 10033295 | Overdose | 196181 | 6 | 0.6 (0.3–1.4) |
| 10044223 | Toxic epidermal necrolysis | 12781 | 5 | 8.2 (3.4–19.8) |
| 10035528 | Platelet count decreased | 91823 | 5 | 1.1 (0.5–2.7) |
| 10024670 | Liver disorder | 37415 | 5 | 2.8 (1.2–6.8) |
| – | Malaise | 387511 | 5 | 0.3 (0.1–0.6) |
| 10027209 | Meningitis cryptococcal | 1367 | 5 | 77.1 (32.0–186.1) |
| 10002199 | Anaphylactic shock | 21099 | 5 | 5.0 (2.1–12.0) |
| 10007559 | Cardiac failure congestive | 76636 | 5 | 1.4 (0.6–3.3) |
| 10003481 | Aspartate aminotransferase increased | 47010 | 5 | 2.2 (0.9–5.4) |
| 10066901 | Treatment failure | 66328 | 5 | 1.6 (0.7–3.8) |
| 10024384 | Leukopenia | 42467 | 5 | 2.5 (1.0–5.9) |
| – | Gastrointestinal disorder | 67315 | 5 | 1.6 (0.6–3.7) |
| 10037087 | Pruritus | 294997 | 5 | 0.4 (0.1–0.8) |
| – | Disease recurrence | 39816 | 5 | 2.6 (1.1–6.3) |
| 10019670 | Hepatic function abnormal | 30864 | 5 | 3.4 (1.4–8.2) |
| 10005191 | Blister | 46659 | 5 | 2.2 (0.9–5.4) |
Table 2. Number of reports and reporting odds ratios for liposomal amphotericin B (L-AMB).
* Confidence Interval
| Preferred term code | Preferred term | Total (n) | Case (n) | Reporting odds ratio (95% CI*) |
| 10013709 | Drug ineffective | 1128032 | 311 | 3.6 (3.2–4.0) |
| – | Off label use | 650743 | 214 | 4.1 (3.5–4.7) |
| 10021015 | Hypokalemia | 39093 | 147 | 46.2 (39.0–54.8) |
| – | Death | 730753 | 75 | 1.1 (0.9–1.4) |
| 10062237 | Renal impairment | 70466 | 61 | 10.0 (7.7–12.9) |
| 10013968 | Dyspnea | 486578 | 59 | 1.4 (1.1–1.8) |
| 10005483 | Blood creatinine increased | 57873 | 58 | 11.6 (8.9–15.0) |
| 10037660 | Pyrexia | 299290 | 54 | 2.0 (1.6–2.7) |
| 10033661 | Pancytopenia | 47122 | 50 | 12.2 (9.2–16.1) |
| 10069339 | Acute kidney injury | 125590 | 43 | 3.9 (2.9–5.3) |
| 10003481 | Aspartate aminotransferase increased | 47010 | 42 | 10.2 (7.5–13.9) |
| 10038435 | Renal failure | 122048 | 38 | 3.5 (2.6–4.9) |
| 10001551 | Alanine aminotransferase increased | 54298 | 38 | 8.0 (5.8–11.0) |
| 10028813 | Nausea | 674394 | 37 | 0.6 (0.4–0.8) |
| 10035664 | Pneumonia | 271408 | 37 | 1.5 (1.1–2.1) |
| – | Condition aggravated | 240861 | 36 | 1.7 (1.2–2.3) |
| 10040047 | Sepsis | 96944 | 35 | 4.1 (2.9–5.7) |
| 10038695 | Respiratory failure | 63850 | 35 | 6.2 (4.4–8.7) |
| 10047700 | Vomiting | 396955 | 34 | 1.0 (0.7–1.3) |
| 10035528 | Platelet count decreased | 91823 | 34 | 4.2 (3.0–5.9) |
| 10005724 | Blood potassium decreased | 26225 | 32 | 13.9 (9.8–19.7) |
| – | Renal failure acute | 43092 | 32 | 8.4 (5.9–12.0) |
| 10037844 | Rash | 358880 | 31 | 1.0 (0.7–1.4) |
| 10021097 | Hypotension | 173419 | 30 | 1.9 (1.4–2.8) |
| 10051118 | Drug ineffective for unapproved indication | 43837 | 30 | 7.8 (5.4–11.1) |
| 10051792 | Infusion-related reaction | 52388 | 29 | 6.3 (4.3–9.0) |
| 10002034 | Anemia | 167009 | 29 | 2.0 (1.4–2.8) |
| 10007515 | Cardiac arrest | 73279 | 27 | 4.2 (2.8–6.1) |
| 10005364 | Blood bilirubin increased | 24125 | 26 | 12.2 (8.3–18.0) |
| 10003988 | Back pain | 202606 | 25 | 1.4 (0.9–2.0) |
| 10008479 | Chest pain | 163743 | 25 | 1.7 (1.2–2.5) |
| 10024690 | Liver function test abnormal | 27025 | 24 | 10.0 (6.7–15.0) |
| 10059570 | Blood alkaline phosphatase increased | 22746 | 23 | 11.4 (7.6–17.2) |
| – | Multi-organ failure | 17736 | 23 | 14.7 (9.7–22.1) |
| 10040560 | Shock | 19115 | 23 | 13.6 (9.0–20.5) |
| 10005851 | Blood urea increased | 15500 | 23 | 16.8 (11.1–25.3) |
| – | General physical health deterioration | 91284 | 23 | 2.8 (1.9–4.3) |
| 10012735 | Diarrhea | 537371 | 23 | 0.5 (0.3–0.7) |
| 10021027 | Hypomagnesemia | 11470 | 21 | 20.7 (13.4–31.8) |
| 10020646 | Hyperkalemia | 29887 | 21 | 7.9 (5.1–12.2) |
| 10007554 | Cardiac failure | 69628 | 21 | 3.4 (2.2–5.2) |
| 10040070 | Septic shock | 36043 | 21 | 6.6 (4.3–10.1) |
| 10013442 | Disseminated intravascular coagulation | 12728 | 21 | 18.6 (12.1–28.7) |
| 10019670 | Hepatic function abnormal | 30864 | 21 | 7.7 (5.0–11.8) |
| 10006473 | Bronchopulmonary aspergillosis | 6385 | 21 | 37.2 (24.2–57.3) |
| 10024670 | Liver disorder | 37415 | 20 | 6.0 (3.9–9.4) |
| – | Chills | 102188 | 20 | 2.2 (1.4–3.4) |
| 10038537 | Renal tubular disorder | 2575 | 20 | 88.2 (56.7–137.4) |
| 10018884 | Hemoglobin decreased | 90933 | 20 | 2.5 (1.6–3.8) |
| 10038428 | Renal disorder | 40451 | 19 | 5.3 (3.4–8.3) |
| 10002198 | Anaphylactic reaction | 44463 | 19 | 4.8 (3.1–7.6) |
| – | Drug interaction | 138348 | 18 | 1.5 (0.9–2.3) |
| 10029354 | Neutropenia | 112363 | 17 | 1.7 (1.0–2.7) |
| – | Headache | 541085 | 17 | 0.3 (0.2–0.6) |
| 10008635 | Cholestasis | 16115 | 16 | 11.2 (6.8–18.3) |
| – | Disease progression | 98477 | 16 | 1.8 (1.1–3.0) |
| 10043071 | Tachycardia | 76652 | 16 | 2.3 (1.4–3.8) |
| 10033318 | Oxygen saturation decreased | 45423 | 16 | 4.0 (2.4–6.5) |
| 10033647 | Pancreatitis acute | 18754 | 16 | 9.6 (5.9–15.7) |
| 10038687 | Respiratory distress | 24185 | 15 | 7.0 (4.2–11.6) |
| 10043554 | Thrombocytopenia | 94721 | 15 | 1.8 (1.1–2.9) |
| 10012373 | Depressed level of consciousness | 34709 | 15 | 4.8 (2.9–8.1) |
| 10077361 | Multiple organ dysfunction syndrome | 20960 | 15 | 8.0 (4.8–13.4) |
| – | Convulsion | 57452 | 15 | 2.9 (1.8–4.9) |
| 10005734 | Blood pressure decreased | 57383 | 14 | 2.7 (1.6–4.6) |
| 10047942 | White blood cell count decreased | 93344 | 14 | 1.7 (1.0–2.8) |
| 10029155 | Nephropathy toxic | 8956 | 14 | 17.6 (10.4–29.8) |
| 10017533 | Fungal infection | 28546 | 13 | 5.1 (3.0–8.8) |
| 10065553 | Bone marrow failure | 19108 | 13 | 7.6 (4.4–13.2) |
| 10000081 | Abdominal pain | 198738 | 13 | 0.7 (0.4–1.3) |
| – | Blood magnesium decreased | 7302 | 13 | 20.0 (11.6–34.6) |
| 10017693 | Gamma-glutamyltransferase increased | 20209 | 13 | 7.2 (4.2–12.5) |
| 10003119 | Arrhythmia | 42283 | 12 | 3.2 (1.8–5.6) |
| 10019075 | Hallucination, visual | 13307 | 12 | 10.1 (5.7–17.9) |
| 10035598 | Pleural effusion | 53488 | 12 | 2.5 (1.4–4.4) |
| 10016825 | Flushing | 91611 | 12 | 1.5 (0.8–2.6) |
| – | Malaise | 387511 | 12 | 0.3 (0.2–0.6) |
| 10002199 | Anaphylactic shock | 21099 | 12 | 6.4 (3.6–11.3) |
| 10022611 | Interstitial lung disease | 40243 | 12 | 3.3 (1.9–5.9) |
| 10007617 | Cardio-respiratory arrest | 38355 | 12 | 3.5 (2.0–6.2) |
| 10020642 | Hyperhidrosis | 113791 | 12 | 1.2 (0.7–2.1) |
| 10018838 | Hematocrit decreased | 18392 | 12 | 7.3 (4.1–12.9) |
| 10020772 | Hypertension | 182177 | 12 | 0.7 (0.4–1.3) |
| 10030095 | Edema | 45676 | 11 | 2.7 (1.5–4.9) |
| 10076573 | Wrong technique in the product usage process | 164412 | 11 | 0.7 (0.4–1.3) |
| 10065042 | Immune reconstitution inflammatory syndrome | 4357 | 11 | 28.4 (15.7–51.4) |
| 10015150 | Erythema | 176486 | 11 | 0.7 (0.4–1.3) |
| 10023126 | Jaundice | 24332 | 11 | 5.1 (2.8–9.2) |
| 10003549 | Asthenia | 323379 | 11 | 0.4 (0.2–0.7) |
| 10076476 | Product use in unapproved indication | 180756 | 11 | 0.7 (0.4–1.2) |
| 10038540 | Renal tubular necrosis | 8395 | 11 | 14.7 (8.1–26.6) |
| 10020751 | Hypersensitivity | 159049 | 10 | 0.7 (0.4–1.3) |
| 10038669 | Respiratory arrest | 25743 | 10 | 4.3 (2.3–8.1) |
| 10038535 | Renal tubular acidosis | 1767 | 10 | 63.8 (34.2–118.9) |
| 10020635 | Hyperglycemia | 31775 | 10 | 3.5 (1.9–6.6) |
| 10005470 | Blood creatine phosphokinase increased | 26696 | 10 | 4.2 (2.3–7.8) |
| 10029331 | Neuropathy peripheral | 77101 | 10 | 1.4 (0.8–2.7) |
| 10001052 | Acute respiratory distress syndrome | 15376 | 10 | 7.3 (3.9–13.6) |
| 10000880 | Acute myeloid leukemia | 13325 | 10 | 8.4 (4.5–15.7) |
| 10011224 | Cough | 234271 | 10 | 0.5 (0.3–0.9) |
| 10005725 | Blood potassium increased | 14240 | 10 | 7.9 (4.2–14.7) |
| 10076309 | Product use issue | 152105 | 10 | 0.7 (0.4–1.4) |
| – | Pain | 540553 | 10 | 0.2 (0.1–0.4) |
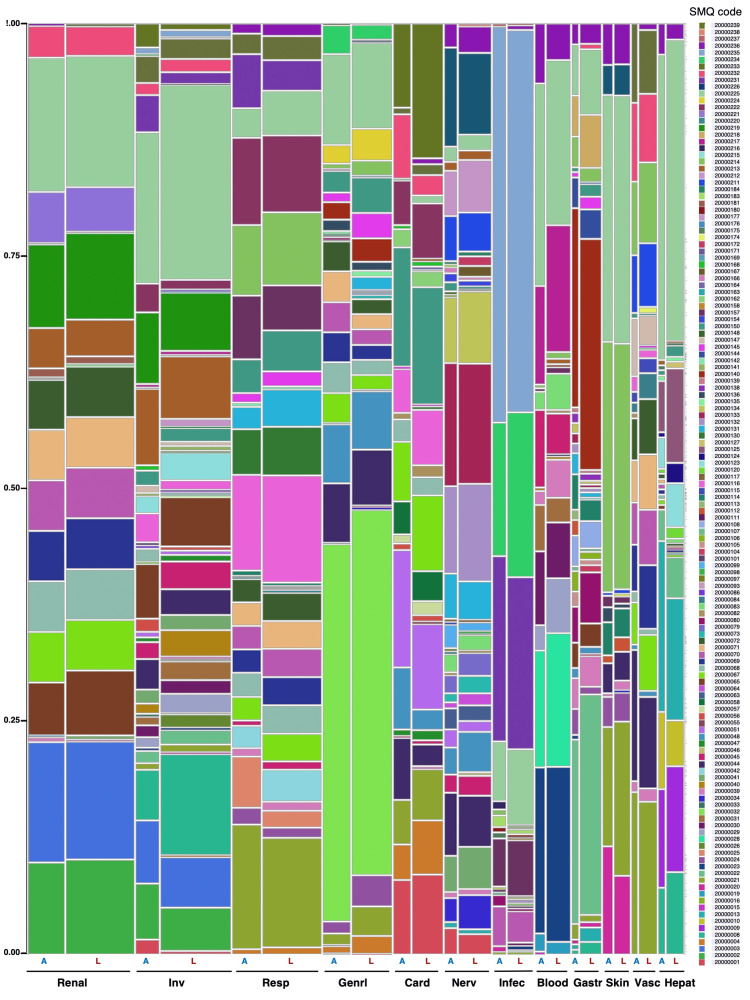
Adverse events were classified using SMQs, and the two formulations were compared by organ based on the SOC. The top 12 SOCs with p-values less than 5% are shown in Figure 1 and Table 3.
Table 3. List of standardized MedDRA* queries (SMQs) related to AMPH-B and L-AMB.
* the Medical Dictionary for Regulatory Activities
SIADH: Syndrome of inappropriate antidiuretic hormone secretion; AMPH-B: amphotericin B deoxycholate; L-AMB: liposomal amphotericin B deoxycholate
| Code | Standardized MedDRA* queries (SMQs) | Code | Standardized MedDRA* queries (SMQs) |
| 20000001 | Torsade de pointes/QT prolongation | 20000115 | Thrombophlebitis |
| 20000002 | Rhabdomyolysis/myopathy | 20000116 | Acute central respiratory depression |
| 20000003 | Acute renal failure | 20000117 | Psychosis and psychotic disorders |
| 20000004 | Cardiac failure | 20000120 | Infectious biliary disorders |
| 20000008 | Liver-related investigations, signs, and symptoms | 20000123 | Biliary system-related investigations, signs, and symptoms |
| 20000009 | Cholestasis and jaundice of hepatic origin | 20000124 | Gallbladder-related disorders |
| 20000010 | Hepatitis, non-infectious | 20000125 | Biliary tract disorders |
| 20000013 | Hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions | 20000127 | Gallstone-related disorders |
| 20000015 | Liver-related coagulation and bleeding disturbances | 20000130 | Pulmonary hypertension |
| 20000016 | Liver infections | 20000131 | Guillain-Barre syndrome |
| 20000019 | Hemolytic disorders | 20000132 | Noninfectious encephalitis |
| 20000020 | Severe cutaneous adverse reactions | 20000133 | Noninfectious encephalopathy/delirium |
| 20000021 | Anaphylactic reaction | 20000134 | Noninfectious meningitis |
| 20000022 | Acute pancreatitis | 20000135 | Accidents and injuries |
| 20000023 | Agranulocytosis | 20000136 | Extravasation events (injections, infusions and implants) |
| 20000024 | Angioedema | 20000138 | Gastrointestinal nonspecific inflammation |
| 20000025 | Asthma/bronchospasm | 20000139 | Gastrointestinal nonspecific dysfunction |
| 20000026 | Dyslipidemia | 20000140 | Gastrointestinal nonspecific symptoms and therapeutic procedures |
| 20000028 | Hematopoietic cytopenias affecting more than one type of blood cell | 20000141 | Hyponatremia/SIADH |
| 20000029 | Hematopoietic erythropenia | 20000142 | Hostility/aggression |
| 20000030 | Hematopoietic leukopenia | 20000144 | Ischemic colitis |
| 20000031 | Hematopoietic thrombocytopenia | 20000145 | Hemodynamic edema, effusions, and fluid overload |
| 20000032 | Lack of efficacy/effect | 20000147 | Hypertension |
| 20000033 | Lactic acidosis | 20000148 | Optic nerve disorders |
| 20000034 | Peripheral neuropathy | 20000150 | Cardiomyopathy |
| 20000039 | Hemorrhage terms (excl laboratory terms) | 20000154 | Demyelination |
| 20000040 | Hemorrhage laboratory terms | 20000157 | Eosinophilic pneumonia |
| 20000041 | Hyperglycemia/new onset diabetes mellitus | 20000158 | Retinal disorders |
| 20000042 | Interstitial lung disease | 20000162 | Cardiac arrhythmia terms, nonspecific |
| 20000044 | Neuroleptic malignant syndrome | 20000163 | Bradyarrhythmia terms, nonspecific |
| 20000045 | Systemic lupus erythematosus | 20000164 | Tachyarrhythmia terms, nonspecific |
| 20000046 | Taste and smell disorders | 20000166 | Conditions associated with central nervous system hemorrhages and cerebrovascular accidents |
| 20000047 | Myocardial infarction | 20000167 | Depression (excl suicide and self-injury) |
| 20000048 | Anticholinergic syndrome | 20000168 | Other ischemic heart disease |
| 20000051 | Arrhythmia-related investigations, signs and symptoms | 20000169 | Premalignant disorders, general conditions and other site-specific disorders |
| 20000055 | Disorders of sinus node function | 20000171 | Hearing impairment |
| 20000056 | Conduction defects | 20000172 | Vestibular disorders |
| 20000057 | Supraventricular tachyarrhythmias | 20000174 | Vasculitis |
| 20000058 | Ventricular tachyarrhythmias | 20000175 | Conjunctival disorders |
| 20000063 | Ischemic central nervous system vascular conditions | 20000176 | Lacrimal disorders |
| 20000064 | Hemorrhagic central nervous system vascular conditions | 20000177 | Lipodystrophy |
| 20000065 | Retroperitoneal fibrosis | 20000180 | Osteonecrosis |
| 20000067 | Shock-associated circulatory or cardiac conditions (excl torsade de pointes) | 20000181 | Renovascular disorders |
| 20000068 | Torsade de pointes, shock-associated conditions | 20000183 | Ocular infections |
| 20000069 | Hypovolemic shock conditions | 20000184 | Ocular motility disorders |
| 20000070 | Toxic-septic shock conditions | 20000211 | Hypotonic-hyporesponsive episode |
| 20000071 | Anaphylactic/anaphylactoid shock conditions | 20000212 | Generalized convulsive seizures following immunization |
| 20000072 | Hypoglycemic and neurogenic shock conditions | 20000213 | Chronic kidney disease |
| 20000073 | Dementia | 20000214 | Hypersensitivity |
| 20000079 | Convulsions | 20000215 | Malignant lymphomas |
| 20000080 | Pseudomembranous colitis | 20000216 | Arthritis |
| 20000082 | Embolic and thrombotic events, arterial | 20000217 | Myelodysplastic syndrome |
| 20000083 | Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous | 20000218 | Noninfectious diarrhea |
| 20000084 | Embolic and thrombotic events, venous | 20000219 | Tumor lysis syndrome |
| 20000086 | Blood premalignant disorders | 20000220 | Proteinuria |
| 20000093 | Malignancy-related therapeutic and diagnostic procedures | 20000221 | Tubulointerstitial diseases |
| 20000097 | Dyskinesia | 20000222 | Respiratory failure |
| 20000098 | Dystonia | 20000224 | Medication errors |
| 20000099 | Parkinson-like events | 20000225 | Drug reaction with eosinophilia and systemic symptoms syndrome |
| 20000101 | Drug abuse and dependence | 20000226 | Hypoglycemia |
| 20000104 | Gastrointestinal perforation, ulcer, hemorrhage, obstruction non-specific findings/procedures | 20000231 | Infective pneumonia |
| 20000105 | Gastrointestinal obstruction | 20000232 | Dehydration |
| 20000106 | Gastrointestinal ulceration | 20000233 | Hypokalemia |
| 20000107 | Gastrointestinal perforation | 20000234 | Sepsis |
| 20000108 | Gastrointestinal hemorrhage | 20000235 | Opportunistic infections |
| 20000111 | Oropharyngeal infections | 20000236 | Immune-mediated/autoimmune disorders |
| 20000112 | Oropharyngeal allergic conditions | 20000237 | COVID-19 |
| 20000113 | Gingival disorders | 20000238 | Sexual dysfunction |
| 20000114 | Oropharyngeal conditions (excl neoplasms, infections and allergies) | 20000239 | Noninfectious myocarditis/pericarditis |
Figure 1. The organ-specific adverse event profiles of AMPH-B and L-AMB based on standardized MedDRA queries (SMQs).
The plot is divided into rectangles where each vertical length represents the proportion of each level of the Y variable within each level of the X variable
AMPH-B: Amphotericin B deoxycholate; L-AMB: liposomal amphotericin B deoxycholate
The results of the Pearson’s chi-square test indicate that p-values less than 5% were obtained for the following SOCs: “Inv” < 0.0001, “Resp” = 0.0090, “Genrl” < 0.0001, “Card” < 0.0001, and “Blood” = 0.0427. No statistically significant difference was observed in the SMQ profile of adverse events in “Renal (p = 0.9302),” ”Nerv (nervous system disorder) (p = 0.6775),” “Infec (infections and infestations) (p = 0.4707),” and “Hepat (p = 0.5828)” of SOCs between the L-AMB and AMPH-B formulations (Table 4).
Table 4. Comparison of SMQ profiles of amphotericin B and liposomal amphotericin B in each SOC classification.
* p < 0.05
SMQ: Standardized MedDRA query; SOC: system organ class
| System organ class | Abbreviation | Total (n) | Amphotericin B (AMPH-B, n) | Liposomal Amphotericin B (L-AMB, n) | p-value |
| Renal and urinary disorders | Renal | 2412 | 848 | 1564 | 0.9302 |
| Investigations | Inv | 2153 | 548 | 1605 | <0.0001* |
| Respiratory, thoracic and mediastinal disorders | Resp | 2029 | 671 | 1358 | 0.0090 * |
| General disorders and administration site conditions | Genrl | 1566 | 646 | 920 | <0.0001* |
| Cardiac disorders | Card | 1142 | 421 | 721 | <0.0001* |
| Nervous system disorders | Nerv | 1075 | 310 | 765 | 0.6775 |
| Infections and infestations | Infec | 943 | 327 | 616 | 0.4707 |
| Blood and lymphatic system disorders | Blood | 827 | 263 | 564 | 0.0427 * |
| Gastrointestinal disorders | Gastr | 693 | 182 | 511 | 0.1105 |
| Skin and subcutaneous tissue disorders | Skin | 636 | 249 | 387 | 0.9233 |
| Vascular disorders | Vasc | 614 | 177 | 437 | 0.8023 |
| Hepatobiliary disorders | Hepat | 585 | 180 | 405 | 0.5828 |
| Metabolism and nutrition disorders | Metab | 521 | 160 | 361 | 0.0703 |
| Psychiatric disorders | Psych | 369 | 104 | 265 | 0.8963 |
| Immune system disorders | Immun | 259 | 91 | 168 | 0.9434 |
| Injury, poisoning and procedural complications | Inj&P | 241 | 90 | 151 | 0.0875 |
| Musculoskeletal and connective tissue disorders | Musc | 177 | 67 | 110 | 0.3746 |
| Eye disorders | Eye | 166 | 35 | 131 | 0.9554 |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Neopl | 129 | 45 | 84 | 0.2326 |
| Surgical and medical procedures | Surg | 54 | 21 | 33 | 0.0548 |
| Ear and labyrinth disorders | Ear | 25 | 5 | 20 | 0.0303 * |
| Pregnancy, puerperium and perinatal conditions | Preg | 13 | 8 | 5 | 0.7092 |
| Congenital, familial and genetic disorders | Cong | 10 | 2 | 8 | 0.5982 |
| Product issues | Prod | 5 | 1 | 4 | 0.0821 |
| Reproductive system and breast disorders | Repro | 2 | 2 | 0 | - |
| Social circumstances | SocCI | 1 | 1 | 0 | - |
Discussion
AMPH-B has severe side effects, such as renal dysfunction, hypokalemia, fever during intravenous administration, chills, nausea, and vomiting [1-5]. Therefore, the patient’s condition should be constantly monitored after drug administration, and the dosage should be adjusted according to the severity of the side effects. L-AMB is a liposomal formulation that was developed with relatively fewer side effects and the same antifungal activity as AMPH-B. L-AMB reduces renal dysfunction by encapsulating AMPH-B within liposomes, which limits leakage from capillaries and transfer to tissue cells while facilitating transfer to infected foci with increased vascular permeability.
The incidence of hypertension, hypotension, tachycardia, hypoxemia, hypokalemia, and various events associated with impaired renal function is lower with L-AMB than with AMPH-B [4]. Furthermore, acute side effects, such as chills and fever, are reduced by half upon intravenous injection of L-AMB compared to conventional AMPH-B preparation [5]. A meta-analysis revealed that L-AMB reduced nephrotoxicity [7]. L-AMB has been shown to be significantly less toxic than amphotericin B deoxycholate; however, adverse events may still occur [15]. According to the package insert statement, adverse events associated with L-AMB include renal dysfunction, hypokalemia, and hypomagnesemia [3,4]. The fever and hypokalemia reported in previous studies and in the package inserts should be noted, as they were also observed in reports from the FAERS database.
Interestingly, no statistically significant difference was observed in the SMQ profile of adverse events in the renal SOC between the L-AMB and AMPH-B formulations in this study. We consider this result in conjunction with previous studies showing a lower risk of renal impairment with L-AMB than with AMPH-B. L-AMB is approved for use at higher doses than AMPH-B [3,4,16]. Therefore, the absence of significant differences in SMQ profiles when L-AMB was administered at higher doses than AMPH-B suggests that L-AMB is better tolerated by the kidney than AMPH-B. These results may reassure clinicians about the safety of L-AMB in relation to renal function.
According to our study findings, SOCs with statistically significant differences were “Inv,” “Resp,” “Genrl,” “Card,” and “Blood.” In the “Inv” SOC, L-AMB had a higher percentage of reports of liver-related investigations, signs, and symptoms (SMQ: 20000008) and drug reaction with eosinophilia and systemic symptoms syndrome (SMQ: 20000225) than AMPH-B. Further speculations on the reasons for this higher percentage is difficult. Hypokalemia is a well-known adverse event of AMPH-B [1-3]. The SOCs of AMPH-B and L-AMB were compared in the present study, but there was no marked difference in incidence in the rate of hypokalemia (SMQ: 20000233) with the SOCs of either formulation (data not shown).
Despite the broad-spectrum bactericidal activity of AMPH-B, its clinical use has been affected by limitations such as parenteral administration, injection-related reactions, acute and chronic toxicity, and dose limitations. L-AMB can be used for longer periods and at higher doses than AMPH-B, thus representing a major advance in the treatment of invasive fungal infections [1]. This study compares adverse event reports during intravenous administration of L-AMB (Ambisome®, n=1591) and AMPH-B (Fungizone®, n=848). Adverse events of other dosage forms such as amphotericin B lipid complex (ABLC, Abelcet® [17]) and amphotericin B colloidal dispersion (ABCD, Amphocil® [18] and Amphotec® [19]) have been reported in the FAERS. ABCD, which contains uniform disk-shaped particles, was discontinued in 2011 because of its high rate of infusion-related events [1,20]. Adverse events of Abelcet®, Amphocil®, and Amphotec® were reported in six cases, 10 cases, and one case, respectively; the route of administration was entered into the database as intravenous in 6, 7, and 0 of these cases, respectively. Therefore, ABLC and ABCD were excluded from the analysis in this study.
Current lipid-based formulations of amphotericin B such as ABLC and L-AMB show better tolerability and toxicity profiles than AMPH-B but are not without side effects. Lipid-based formulations of amphotericin B have different pharmacological properties compared to AMPH-B [1]. L-AMB and ABLC can be administered at high doses, and their efficacy and toxicity vary by formulation. The guidelines of the Infectious Diseases Society of America state that L-AMB and ABLC have the same spectrum of activity as AMPH-B but different pharmacologic characteristics and frequency of adverse events [21]. ABLC and L-AMB have characteristic pharmacokinetic profiles (Cmax, volume of distribution, and elimination half-life) that determine their efficacy and toxicity, respectively [1], which may influence the final therapeutic outcome. In the future, when enough adverse event reports of ABLC are accumulated in clinical practice, it may be possible to make detailed comparisons with the adverse events of other formulations.
Amphotericin B is the drug of choice for treating many serious fungal infections because it has the widest spectrum of action of all known antifungal agents and the lowest potential for resistance [9]. Recently, an increase in the minimum inhibitory concentration of amphotericin B against Aspergillus species was reported. To clarify the global epidemic trends of amphotericin B resistance in clinical Aspergillus isolates, the minimum inhibitory concentration of amphotericin B from 2010 to 2020 was systematically evaluated [22]. The results indicated that amphotericin B resistance is more prevalent in Aspergillus terreus and Aspergillus flavus isolates. Some differences were observed in the prevalence of amphotericin B resistance in Aspergillus species in various regions. In the present study, we did not analyze the resistance trends by district for the United States, Asia, and Europe. Future analysis should consider the differences in amphotericin B resistance by region.
This study has several limitations. SRSs are subject to over-reporting, under-reporting, missing data, exclusion of healthy individuals, length of the post-launch period of the drug, and presence of confounding factors. Cases reported in the FAERS database do not always contain sufficient information regarding patient background, such as comorbid conditions and concomitant drug administration, to allow for proper evaluation. For example, invasive fungal infection is a life-threatening complication that occurs after allogeneic hematopoietic cell transplantation, with Candida and Aspergillus being the major causative organisms. Patients with primary agranulocytosis or acquired agranulocytosis (e.g., due to toxicity) who develop invasive fungal infections have different characteristics, history, and outcomes compared to other patient groups. In studies involving adverse spontaneous report databases such as FAERS, it is often difficult to obtain accurate patient backgrounds, and no widely accepted method for statistically adjusting for covariates exists. Therefore, invasive fungal infections can be analyzed only after a sufficient number of cases have been accumulated. Further epidemiologic studies may be needed to confirm the results of the present study; these issues must be fully considered when analyzing drug safety using FAERS data. It would be interesting to investigate the relationship between dose and adverse event occurrence. Although FAERS has a dose entry, it was not considered in this study because many reports contained missing or inaccurate dose calculations. A more detailed analysis focusing on these factors will be the subject of future research.
Conclusions
Based on real-world data from FAERS, the adverse event profiles of AMPH-B and L-AMB were generated and compared. The SMQ profile of adverse events in renal SOC showed no statistically significant difference between the L-AMB and AMPH-B formulations. Our results suggest that L-AMB is more tolerated by the kidneys than AMPH-B.
Acknowledgments
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement
The authors disclose the receipt of the following financial support for the research, authorship, and/or publication of this article. This research was partially supported by JSPS KAKENHI,Grant Numbers 23H05264, 21K11100, and 21K06646. The funders played no role in the study design, data collection and analysis, decision to publish this article, or article preparation.
Disclosures
Human subjects: All authors have confirmed that this study did not involve human participants or tissue.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: The authors disclose the receipt of the following financial support for the research, authorship, and/or publication of this article. This research was partially supported by JSPS KAKENHI,Grant Numbers 23H05264, 21K11100, and 21K06646. The funders played no role in the study design, data collection and analysis, decision to publish this article, or article preparation.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Mitsuhiro Nakamura, Mika Maezawa, Kazuhiro Iguchi, Kohei Tahara, Hirofumi Tamaki, Yuka Nokura
Acquisition, analysis, or interpretation of data: Mitsuhiro Nakamura, Koumi Miyasaka, Mika Maezawa, Sakiko Hirofuji, Moe Yamashita, Nanaka Ichihara, Tomofumi Yamazaki, Kana Sugishita, Satoshi Nakao, Yuka Nokura
Drafting of the manuscript: Mitsuhiro Nakamura, Koumi Miyasaka, Mika Maezawa, Sakiko Hirofuji, Moe Yamashita, Nanaka Ichihara, Tomofumi Yamazaki, Kana Sugishita, Satoshi Nakao, Yuka Nokura
Critical review of the manuscript for important intellectual content: Mitsuhiro Nakamura, Koumi Miyasaka, Mika Maezawa, Kazuhiro Iguchi, Kohei Tahara, Hirofumi Tamaki, Satoshi Nakao, Yuka Nokura
References
- 1.Sixty years of amphotericin B: an overview of the main antifungal agent used to treat invasive fungal infections. Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F. Infect Dis Ther. 2021;10:115–147. doi: 10.1007/s40121-020-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liposomal amphotericin B (Article in Japanese) Fukasawa M. Nihon Ishinkin Gakkai Zasshi. 2005;46:229–231. doi: 10.3314/jjmm.46.229. [DOI] [PubMed] [Google Scholar]
- 3.E.R. Squibb & Sons, L.L.C L.L.C. FUNGIZONE - amphotericin b injection, powder, lyophilized, for solution. [ Sep; 2024 ]. 2009. https://tapermd.com/bbw/Fungizone.pdf https://tapermd.com/bbw/Fungizone.pdf
- 4.AmBisome® (amphotericin B) liposome for injection. [ Sep; 2024 ];https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050740s016lbl.pdf 2008 2008:50740. [Google Scholar]
- 5.Liposomal amphotericin B (AmBisome(®)): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Stone NR, Bicanic T, Salim R, Hope W. Drugs. 2016;76:485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Development, characterization, efficacy and mode of action of Ambisome, a unilamellar liposomal formulation of amphotericin B. Adler-Moore JP, Proffitt RT. J Liposome Res. 1993;3:429–450. [Google Scholar]
- 7.Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. Petersen GH, Alzghari SK, Chee W, Sankari SS, La-Beck NM. J Control Release. 2016;232:255–264. doi: 10.1016/j.jconrel.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 8.A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. Pharmacoepidemiol Drug Saf. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 9.Evaluation of pregabalin-induced adverse events related to falls using the FDA adverse event reporting system and Japanese Adverse Drug Event Report databases. Mukai R, Hasegawa S, Umetsu R, et al. J Clin Pharm Ther. 2019;44:285–291. doi: 10.1111/jcpt.12790. [DOI] [PubMed] [Google Scholar]
- 10.Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. Fukuda A, Tahara K, Hane Y, et al. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0185654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Adminisitation. FDA Adverse Event Reporting System (FAERS) Public Dashboard. [ Sep; 2024 ]. 2024. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard
- 12.DRUGBANK online. [ Sep; 2024 ]. 2024. https://go.drugbank.com/ https://go.drugbank.com/
- 13.Quantitative signal detection using spontaneous ADR reporting. Bate A, Evans SJ. Pharmacoepidemiol Drug Saf. 2009;18:427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 14.Poluzzi E, Raschi E, Piccinni C, et al. Data Mining Applications in Engineering and Medicine. IntechOpen; 2012. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS) pp. 265–302. [Google Scholar]
- 15.Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Mistro S, Maciel IM, de Menezes RG, Maia ZP, Schooley RT, Badaró R. Clin. Infect. Dis. 2012;54:1774–1777. doi: 10.1093/cid/cis290. [DOI] [PubMed] [Google Scholar]
- 16.Clinical pharmacokinetics, pharmacodynamics, safety and efficacy of liposomal amphotericin B. Groll AH, Rijnders BJ, Walsh TJ, Adler-Moore J, Lewis RE, Brüggemann RJ. Clin Infect Dis. 2019;68:0–74. doi: 10.1093/cid/ciz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liposome Company, Inc. Abelcet® (Amphotericin B lipid complex) [ Oct; 2024 ]. 1991. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=57891 https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=57891
- 18.Overview of amphotericin B colloidal dispersion (amphocil) Stevens DA. J Infect. 1994;28:45–49. doi: 10.1016/s0163-4453(94)95971-4. [DOI] [PubMed] [Google Scholar]
- 19.InterMune, Inc. Amphotec® (amphotericin B injection, lipid complex) [ Oct; 2024 ]. 2006. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=9b8ea543-1de8-472f-9666-34f99ca2f183&type=display https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=9b8ea543-1de8-472f-9666-34f99ca2f183&type=display
- 20.Amphotericin B formulations: a comparative review of efficacy and toxicity. Hamill RJ. Drugs. 2013;73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 21.Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Patterson TF, Thompson GR 3rd, Denning DW, et al. Clin Infect Dis. 2016;63:0. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trends in the Prevalence of Amphotericin B-Resistance (AmBR) among clinical isolates of Aspergillus species. Fakhim H, Badali H, Dannaoui E, et al. J Mycol Med. 2022;32:101310. doi: 10.1016/j.mycmed.2022.101310. [DOI] [PubMed] [Google Scholar]