Abstract
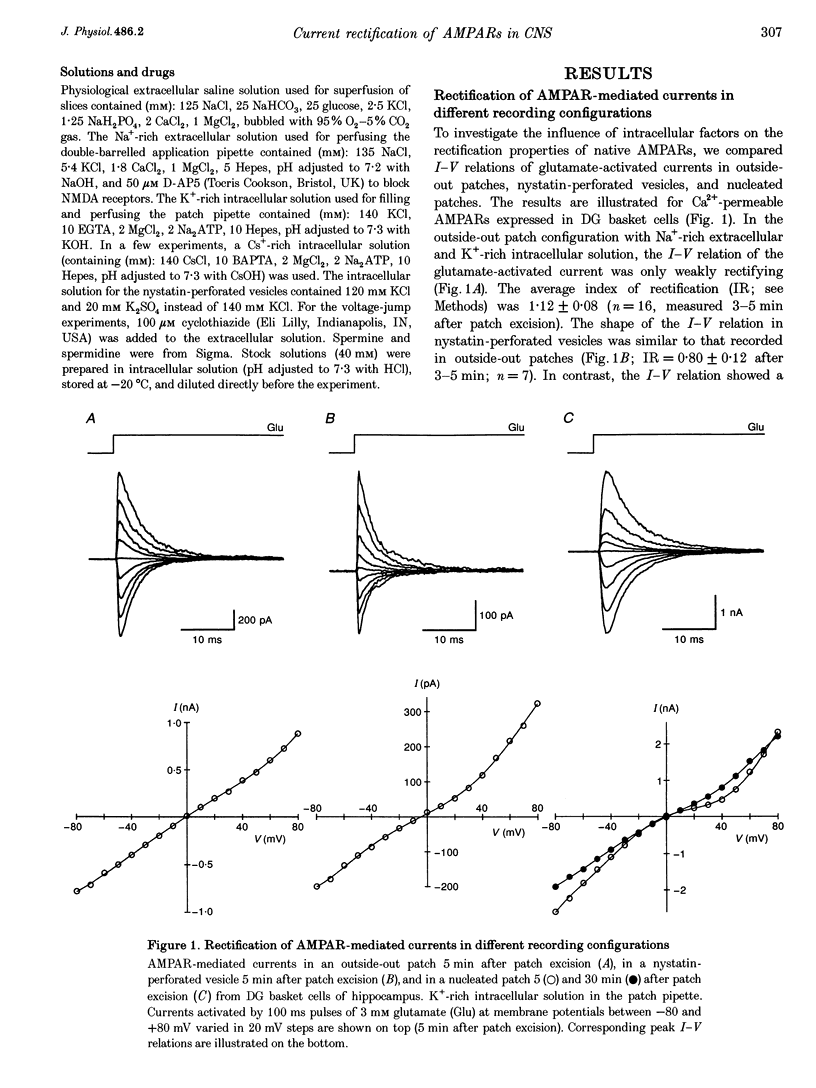
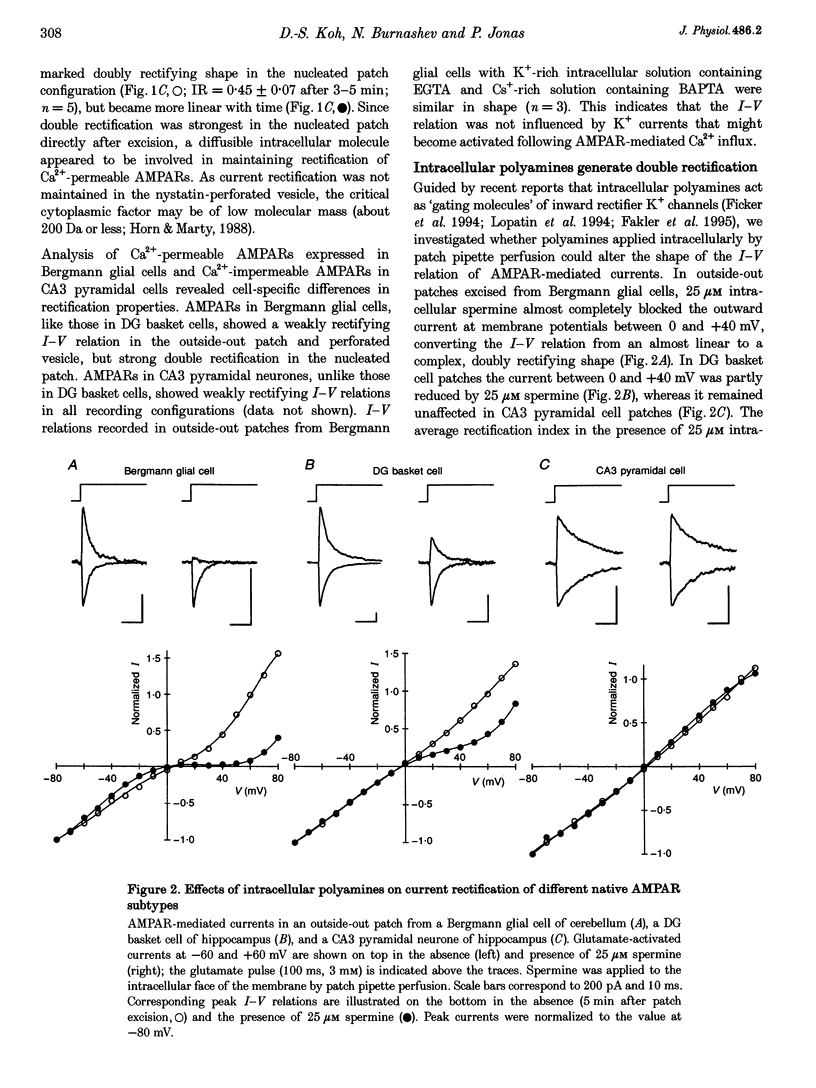
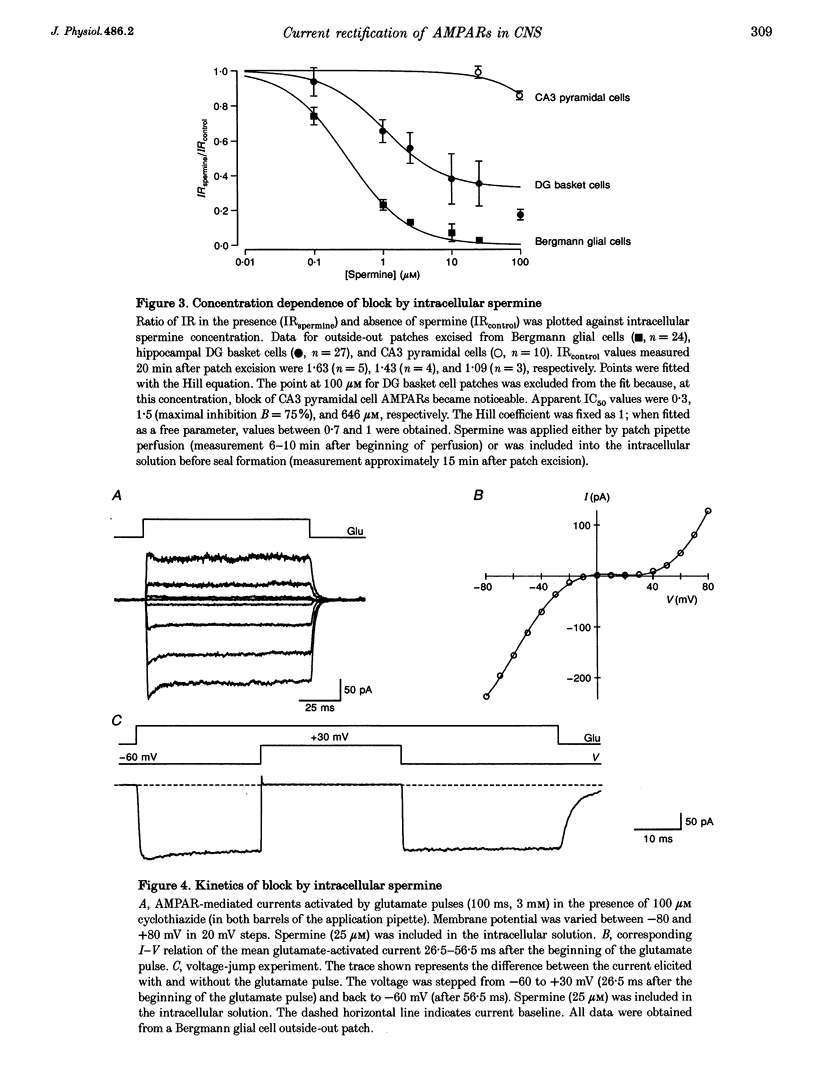
1. The influence of intracellular factors on current rectification of different subtypes of native alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate receptors (AMPARs) was studied in rat brain slices by combining fast application of glutamate with patch pipette perfusion. 2. The peak current-voltage (I-V) relation of the AMPARs expressed in Bergmann glial cells of cerebellum and dentate gyrus (DG) basket cells of hippocampus was weakly rectifying in outside-out patches and nystatin-perforated vesicles, but showed a doubly rectifying shape with a region of reduced slope between 0 and +40 mV in nucleated patches. The I-V relation of AMPARs expressed in hippocampal CA3 pyramidal neurones was linear in all recording configurations. 3. Intracellular application of 25 microM spermine, a naturally occurring polyamine, blocked outward currents in outside-out patches from Bergmann glial cells and DG basket cells in a voltage-dependent manner, generating I-V relations with a doubly rectifying shape which were similar to those recorded in nucleated patches. AMPARs in CA3 pyramidal cell patches were unaffected by 25 microM spermine. 4. The half-maximal blocking concentration of spermine at +40 mV was 0.3 microM in Bergmann glial cell patches and 1.5 microM in DG basket cell patches, whereas it was much higher (>> 100 microM) for CA3 pyramidal cell patches. Spermidine also affected current rectification, but with lower affinity. The block of outward current by polyamines following voltage jumps developed within < 0.5 ms. 5. We conclude that current rectification, rather than being an intrinsic property of the Ca(2+)-permeable AMPAR channel, is generated by polyamine block.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992 Jun 12;256(5063):1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Jonas P., Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J Physiol. 1992 Dec;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S., Raditsch M., Ruppersberg J. P., Jahn W., Monyer H., Schoepfer R., Witzemann V. Argiotoxin detects molecular differences in AMPA receptor channels. Neuron. 1993 Jun;10(6):1131–1140. doi: 10.1016/0896-6273(93)90061-u. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994 Jun;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Morales M., Ibarz J. M., Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci. 1994 Jul 1;6(7):1080–1088. doi: 10.1111/j.1460-9568.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J., Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol. 1993 Mar;462:373–392. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kusama-Eguchi K., Kobayashi H., Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991 Nov 5;266(31):20803–20809. [PubMed] [Google Scholar]



