Abstract
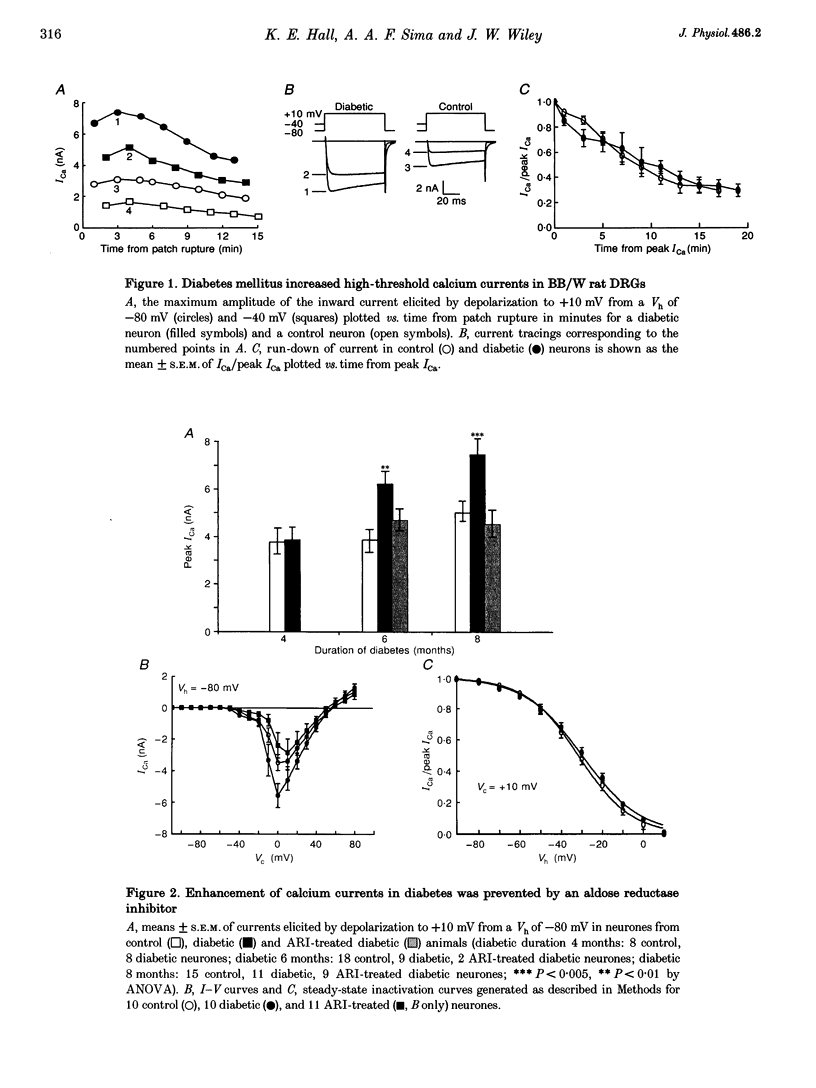
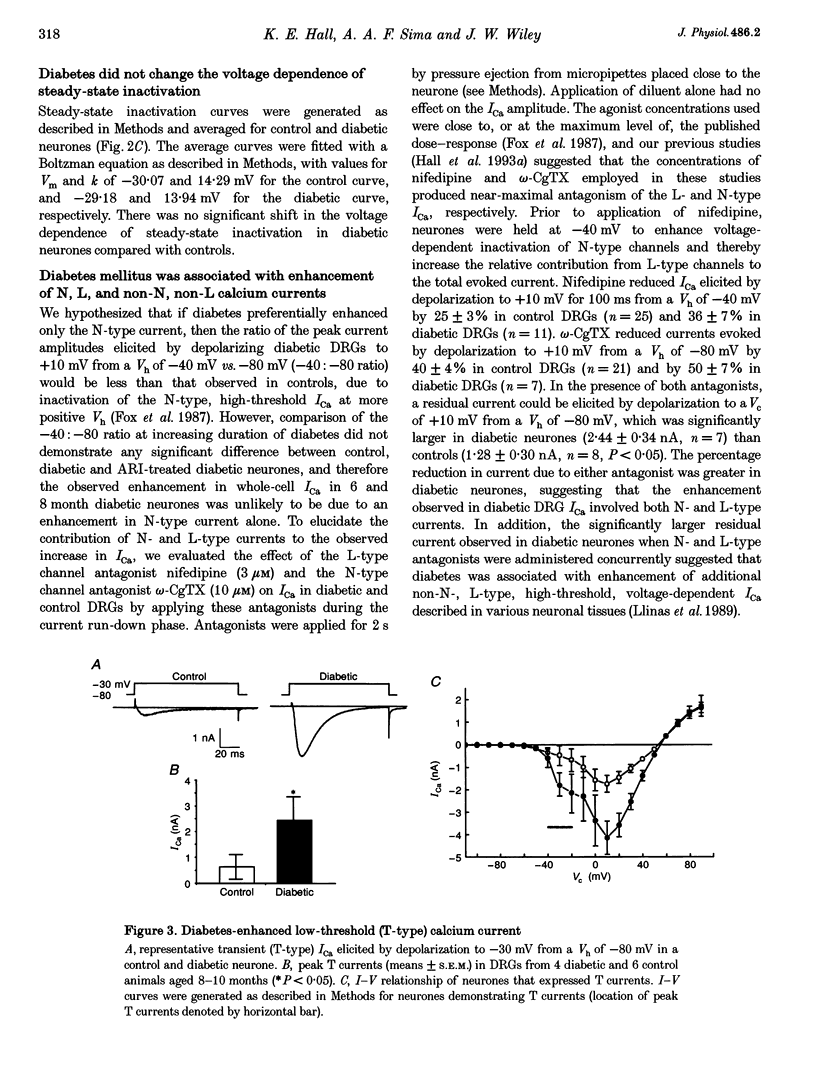
1. Whole-cell, high-threshold, voltage-dependent calcium currents (ICa) were enhanced in acutely dissociated, capsaicin-sensitive dorsal root ganglion neurones from diabetic Bio Bred/Worchester (BB/W) rats, compared with those from age-matched, non-diabetic controls. The magnitude of the enhancement increased with the duration of diabetes, and reached significance at diabetic durations of 6 months (diabetic: 6.3 +/- 0.4 nA; current density (CD), 157 +/- 12 pA pF-1; means +/- S.E.M., n = 9, P < 0.01; control: 3.9 +/- 0.6 nA; CD, 116 +/- 11 pA pF-1; n = 18) and 8 months (diabetic: 7.6 +/- 0.4 nA; CD, 177 +/- 25 pA pF-1; n = 11, P < 0.005; control: 5.1 +/- 0.5 nA; CD, 111 +/- 26 pA pF-1; n = 15). Low-threshold, voltage-dependent ICa were also enhanced in neurones from animals diabetic for 8 months (diabetic: 2.5 +/- 0.7 nA, n = 4, P < 0.05; control: 0.7 +/- 0.5 nA, n = 6). 2. The ICa enhancement was prevented by long-term treatment of diabetic animals with an aldose reductase inhibitor (ARI; peak ICa at 6 months: 4.41 +/- 0.48 nA, n = 2; at 8 months: 4.32 +/- 0.60 nA, n = 9). 3. The ICa enhancement was not due to a shift in the voltage dependence of either the current-voltage relationship or steady-state inactivation. 4. The L channel antagonist nifedipine and preferential N channel antagonist omega-conotoxin GVIA (omega-CgTX) caused a greater inhibition of high-threshold ICa in diabetic neurones compared with controls (nifedipine: control: 25 +/- 3%, n = 26; diabetic: 36 +/- 7%, n = 11; omega-CgTX: control: 40 +/- 4%, n = 21; diabetic: 50 +/- 7%, n = 7). Diabetic neurones also demonstrated a significantly greater residual current (2.44 +/- 0.34 nA, n = 7) in the presence of both antagonists vs. controls (1.28 +/- 0.30 nA, n = 8, P < 0.05), suggesting that N-, L- and additional non-N-, non-L-type high-threshold ICa were enhanced.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F., Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Neuropathy-functional abnormalities in the BB rat. Metabolism. 1983 Jul;32(7 Suppl 1):112–117. doi: 10.1016/s0026-0495(83)80023-7. [DOI] [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. K., Bray G. M., Aguayo A. J., Rasminsky M. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp Neurol. 1976 Oct;53(1):285–288. doi: 10.1016/0014-4886(76)90299-5. [DOI] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Doupnik C. A., Pun R. Y. Cyclic AMP-dependent phosphorylation modifies the gating properties of L-type Ca2+ channels in bovine adrenal chromaffin cells. Pflugers Arch. 1992 Jan;420(1):61–71. doi: 10.1007/BF00378642. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Green R. J., King R. H., Thomas P. K., Baron D. N. Sodium-potassium-ATPase activity in the dorsal root ganglia of rats with streptozotocin-induced diabetes. Diabetologia. 1985 Feb;28(2):104–107. doi: 10.1007/BF00279925. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes. 1988 Jun;37(6):688–693. doi: 10.2337/diab.37.6.688. [DOI] [PubMed] [Google Scholar]
- Gross R. A., MacDonald R. L. Activators of protein kinase C selectively enhance inactivation of a calcium current component of cultured sensory neurons in a pertussis toxin-sensitive manner. J Neurophysiol. 1989 Jun;61(6):1259–1269. doi: 10.1152/jn.1989.61.6.1259. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Wiley J. W., Ryan-Jastrow T., Macdonald R. L. Regulation by GTP and its stable thiol derivatives of calcium current components in rat nodose ganglion neurons. Mol Pharmacol. 1990 Apr;37(4):546–553. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Inoguchi T., Battan R., Handler E., Sportsman J. R., Heath W., King G. L. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti-Berggren L., Larsson O., Rorsman P., Ammälä C., Bokvist K., Wåhlander K., Nicotera P., Dypbukt J., Orrenius S., Hallberg A. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993 Jul 2;261(5117):86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Mechanisms of increased responses of the aorta to alpha-adrenoceptor agonists in streptozotocin-induced diabetic rats. J Pharmacobiodyn. 1988 Oct;11(10):707–713. doi: 10.1248/bpb1978.11.707. [DOI] [PubMed] [Google Scholar]
- Kappelle A. C., Biessels G., Bravenboer B., van Buren T., Traber J., de Wildt D. J., Gispen W. H. Beneficial effect of the Ca2+ antagonist, nimodipine, on existing diabetic neuropathy in the BB/Wor rat. Br J Pharmacol. 1994 Mar;111(3):887–893. doi: 10.1111/j.1476-5381.1994.tb14821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Rushovich E. H., Thomas T. P., Ueda T., Agranoff B. W., Greene D. A. Diminished specific activity of cytosolic protein kinase C in sciatic nerve of streptozocin-induced diabetic rats and its correction by dietary myo-inositol. Diabetes. 1991 Nov;40(11):1545–1554. doi: 10.2337/diab.40.11.1545. [DOI] [PubMed] [Google Scholar]
- Levy J., Gavin J. R., 3rd, Sowers J. R. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med. 1994 Mar;96(3):260–273. doi: 10.1016/0002-9343(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Nobe S., Aomine M., Arita M., Ito S., Takaki R. Chronic diabetes mellitus prolongs action potential duration of rat ventricular muscles: circumstantial evidence for impaired Ca2+ channel. Cardiovasc Res. 1990 May;24(5):381–389. doi: 10.1093/cvr/24.5.381. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Bril V., Nathaniel V., McEwen T. A., Brown M. B., Lattimer S. A., Greene D. A. Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. N Engl J Med. 1988 Sep 1;319(9):548–555. doi: 10.1056/NEJM198809013190905. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Brismar T. Reversible diabetic nerve dysfunction: structural correlates to electrophysiological abnormalities. Ann Neurol. 1985 Jul;18(1):21–29. doi: 10.1002/ana.410180105. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Prashar A., Zhang W. X., Chakrabarti S., Greene D. A. Preventive effect of long-term aldose reductase inhibition (ponalrestat) on nerve conduction and sural nerve structure in the spontaneously diabetic Bio-Breeding rat. J Clin Invest. 1990 May;85(5):1410–1420. doi: 10.1172/JCI114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima A. A. The development and structural characterization of the neuropathies in the spontaneously diabetic BB Wistar rat. Metabolism. 1983 Jul;32(7 Suppl 1):106–111. doi: 10.1016/s0026-0495(83)80022-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kashiwagi A., Ogawa T., Abe N., Asahina T., Ikebuchi M., Takagi Y., Shigeta Y. Effect of verapamil on cardiac protein kinase C activity in diabetic rats. Eur J Pharmacol. 1991 Aug 6;200(2-3):353–356. doi: 10.1016/0014-2999(91)90595-h. [DOI] [PubMed] [Google Scholar]
- Tang E. Y., Parker P. J., Beattie J., Houslay M. D. Diabetes induces selective alterations in the expression of protein kinase C isoforms in hepatocytes. FEBS Lett. 1993 Jul 12;326(1-3):117–123. doi: 10.1016/0014-5793(93)81774-t. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Tsien R. W. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993 Feb;10(2):127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. A myo-inositol pool utilized for phosphatidylinositol synthesis is depleted in sciatic nerve from rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9818–9822. doi: 10.1073/pnas.87.24.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]