Key Points
Question
What is the efficacy associated with spinal cord stimulation (SCS) for chronic pain, including both conventional and novel forms of SCS, compared with conventional medical management (CMM)?
Findings
This systematic review and network meta-analysis included 13 randomized clinical trials and 1561 patients and found that both conventional and novel SCS were associated with superior efficacy vs CMM after 6 months of follow-up across 5 of 6 outcomes.
Meaning
This systematic review and network meta-analysis improved on prior evidence synthesis studies by including more up-to-date randomized clinical trial data and using more advanced analytical methods, supporting the effectiveness of SCS therapy.
This systematic review and network meta-analysis evaluates the efficacy associated with conventional and novel spinal cord stimulation therapies compared with conventional medical management.
Abstract
Importance
Chronic back and lower extremity pain is one of the leading causes of disability worldwide. Spinal cord stimulation (SCS) aims to improve symptoms and quality of life.
Objective
To evaluate the efficacy of SCS therapies compared with conventional medical management (CMM).
Data Sources
MEDLINE, Embase, and Cochrane Library were systematically searched from inception to September 2, 2022.
Study Selection
Selected studies were randomized clinical trials comparing SCS therapies with sham (placebo) and/or CMM or standard treatments for adults with chronic back or leg pain who had not previously used SCS.
Data Extraction and Synthesis
Evidence synthesis estimated odds ratios (ORs) and mean differences (MDs) and their associated credible intervals (CrI) through bayesian network meta-analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for network meta-analyses was followed.
Main Outcomes and Measures
The primary outcomes were pain-related end points, including pain intensity (measured by visual analog scale) and proportion of patients achieving at least 50% pain relief (responder rate) in the back or leg. Quality of life (measured by EQ-5D index score) and functional disability (measured by the Oswestry Disability Index score) were also considered.
Results
A total of 13 studies of 1561 patients were included in the network meta-analysis comparing conventional and novel SCS therapies with CMM across the 6 outcomes of interest at the 6-month follow-up. Both conventional and novel SCS therapies were associated with superior efficacy compared with CMM in responder rates in back (conventional SCS: OR, 3.00; 95% CrI, 1.49 to 6.72; novel SCS: OR, 8.76; 95% CrI, 3.84 to 22.31), pain intensity in back (conventional SCS: MD, −1.17; 95% CrI, −1.64 to −0.70; novel SCS: MD, −2.34; 95% CrI, −2.96 to −1.73), pain intensity in leg (conventional SCS: MD, −2.89; 95% CrI, −4.03 to −1.81; novel SCS: MD, −4.01; 95% CrI, −5.31 to −2.75), and EQ-5D index score (conventional SCS: MD, 0.15; 95% CrI, 0.09 to 0.21; novel SCS: MD, 0.17; 95% CrI, 0.13 to 0.21). For functional disability, conventional SCS was superior to CMM (MD, −7.10; 95% CrI, −10.91 to −3.36). No statistically significant differences were observed for other comparisons.
Conclusions and Relevance
This systematic review and network meta-analysis found that SCS therapies for treatment of chronic pain in back and/or lower extremities were associated with greater improvements in pain compared with CMM. These findings highlight the potential of SCS therapies as an effective and valuable option in chronic pain management.
Introduction
Chronic low back pain with or without lower limb pain is often difficult to treat, and patients experience a significant impact to their quality of life (QOL) and productivity.1 In the US alone, prevalence of chronic pain is estimated at 20.4% of adults, with 8.0% experiencing high-impact chronic pain.2 Globally, a survey performed by the World Health Organization estimated the cross-country mean proportion of the population reporting pain in the past 30 days to be 27.5%.3 The underlying chronic pain etiologies, such as persistent spinal pain syndrome type 1 and 2, complex regional pain syndrome type 1 and 2, and painful diabetic polyneuropathy, are difficult to treat with conventional medical management (CMM) options, such as analgesics, physical therapy, and cognitive behavioral therapy.
Spinal cord stimulation (SCS) is a widely accepted therapy for people with chronic pain who experience intractable pain despite at least 6 months of CMM. SCS therapy involves an implantable neurostimulator device that delivers electrical current to the spinal cord to provide pain control. Patients must first undergo a trial procedure to confirm clinical efficacy, typically at least a 50% improvement in pain intensity relative to baseline. Only if the trial proves successful is the permanent device implanted. Initial clinical practice was primarily focused on using tonic stimulation modes, defined as being in the 40 to 80 Hz range. In the last 10 to 15 years, novel modes of stimulation (eg, high-frequency stimulation, burst stimulation, closed loop, and differential target multiplexed) have been introduced, with clinical studies reporting better efficacy for novel SCS compared with tonic (or conventional) SCS. Benefits of SCS include the ability to test the therapy before permanent implant and the ability to reverse therapy by explanting the device. This provides an important alternative to pain management vs long-term opioid use or repeated spinal operations.
Despite its clinical success, there is still debate about aspects of SCS therapy. Systematic literature reviews (SLRs) and meta-analyses published by O’Connell et al4 in 2021 and Traeger et al5 in 2023 (both as Cochrane reviews) note that it is unclear whether SCS reduces pain intensity, disability, or medication use or improves function and QOL. Practicing SCS clinicians have published rebuttal articles citing several methodological flaws with the negative evidence synthesis articles, the primary critique pointing to the exclusion of many randomized clinical trials (RCTs) investigating novel SCS, which resulted in an incomplete and nonrepresentative set of SCS studies included in the 2 Cochrane reviews.6,7
The aim of this article is to evaluate the efficacy and safety associated with SCS, including modern RCTs that compared novel forms of SCS vs tonic stimulation, compared with sham (placebo) and/or CMM. In addition, this review extends the restrictive methodology of the 2 aforementioned Cochrane reviews4,5 by extending the analytical framework from simple meta-analysis to network meta-analysis (NMA). To our knowledge, this is the first meta-analysis of SCS therapies that incorporates both trials comparing treatment with CMM and more recent studies that compare novel forms of SCS with tonic or conventional SCS.
Methods
Identification of Studies
This systematic review and network meta-analysis is reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement extension for systematic reviews incorporating network meta-analysis (PRISMA-NWA).8 An SLR was conducted on literature published between January 1946 and September 2, 2022, to identify relevant RCTs and observational studies reporting efficacy and safety outcomes of SCS for chronic back and leg pain. The SLR was conducted in accordance with guidelines published by the Cochrane Collaboration and by the Centre for Reviews and Dissemination.9 A predefined search strategy was used to identify eligible studies. Eligibility criteria for study inclusion were developed using the Population, Intervention, Comparator, Outcomes, Study design (PICOS) statement. The population evaluated included adults (age ≥18 years) with chronic back and/or leg pain (failed back surgery syndrome [FBSS], also known as persistent spinal pain syndrome type 2; nonsurgical refractory back pain, also known as persistent spinal pain syndrome type 1; complex regional pain syndrome) or painful diabetic peripheral neuropathy. Details regarding the search strategy and the inclusion and exclusion criteria can be found in eTables 1 through 4 in Supplement 1.
The databases searched for the SLR included Medline (including Ovid MEDLINE Epub Ahead of Print, Medline In-Process and other nonindexed citations, Ovid MEDLINE Daily, Ovid MEDLINE, and Versions), Embase (1974 to current), and the Cochrane Library (including the central register of controlled trials and the database of systematic reviews). The methodological quality of the studies included from the systematic review was assessed using the criteria for methodological quality as specified by the revised Cochrane Collaboration tool for assessing risk of bias in RCTs.10
Feasibility Assessment
Following the identification of relevant evidence, a feasibility assessment was carried out to determine whether a connected network of direct and indirect evidence for a given outcome of interest could be established, and whether the comparability and transitivity assumption of the NMA was violated or not. Studies deemed eligible for evidence synthesis were assessed for presence and extent of between-study heterogeneity by reviewing key features of study designs, patient populations (eTable 5 in Supplement 1), and study outcomes (eTable 6 in Supplement 1).
NMA
NMA is an established evidence synthesis method that extends pairwise meta-analysis to larger networks, connecting multiple treatments from several different studies. NMA considers both direct evidence (ie, from head-to-head comparisons) and indirect evidence (via ≥1 common treatment groups) in a network of evidence, where different studies are linked by means of common comparators. Bayesian NMA was conducted using Stan11 through R version 4.2.1 or higher (R Project for Statistical Computing)12 within the RStudio interface,13 using the multinma package.14 The method followed guidance from the NICE Decision Support Unit Technical Support Document 2.15,16 Vague (flat or uninformative) priors were used for all models (eTable 7 in Supplement 1). Both fixed and random effects models were fitted. The deviance information criterion17 and the total residual deviance were calculated for the outcomes assessed.
Statistical Analysis
For continuous outcomes, the posterior mean difference (MD) was used to reflect the relative treatment effects between interventions, while for binary outcomes, posterior odds ratios (ORs) were used, each of which were reported with associated 95% credible intervals (CrIs). The unrelated means method was used to address potential inconsistency between direct and indirect comparisons. To determine the robustness of the treatment recommendations following the NMA, threshold analysis was conducted to explore how hypothetical changes to the data impact recommendations produced from the original analyses.18 In this analysis, thresholds up to 60% were explored for both point estimates and SEs. P values were 2-sided, and statistical significance was set at P ≤ .05.
Results
Study Selection
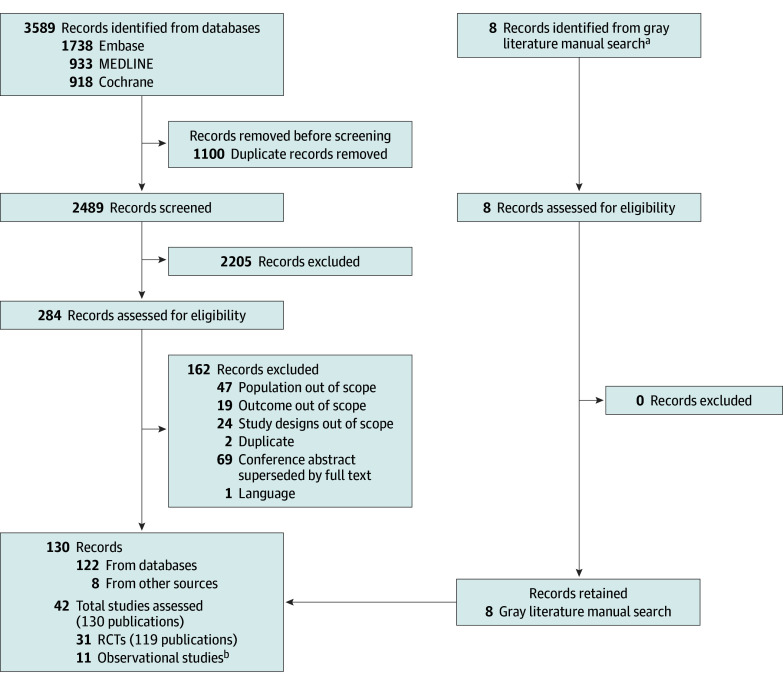
Database searches identified a total of 2489 unique records. After the initial screening of titles and abstracts, 284 references were considered as potentially relevant. Following a detailed examination of the full articles by 2 independent reviewers, 130 publications met the eligibility criteria, including 11 postrandomization observational studies that could not be considered because their uncontrolled nature did not allow incorporating them in the networks of evidence. Therefore, 31 RCTs19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 from 119 publications were moved forward for data extraction and feasibility assessment. The study selection flowchart is provided in Figure 1.
Figure 1. Study Selection Flowchart.
aOf the 8 references, 6 were ClinicalTrials.gov entries for randomized clinical trials (RCTs) linked to the identified studies from the database searches, which were checked manually for additional information. The remaining 2 references were peer-reviewed articles obtained from bibliographies of published systematic literature reviews.
bObservational studies with safety data were not extracted.
An assessment of the risk of bias across included studies is provided in eFigure 1 in Supplement 1. The assessment indicated that 26 of 31 studies had high risk of bias. However, common biases identified in these studies could be attributed to inherent challenges of sham (placebo) implementation in studies of this type (ie, patients can often perceive whether a device is on or off),50 impossibility of physician outcome assessment due to the personal nature of pain, and adequate blinding for device placement requiring surgical implantation.
A summary of patient demographic and disease characteristics in the included studies is provided in eTable 5 in Supplement 1. We found low to moderate levels of heterogeneity with regard to age, gender or sex, pain etiology, and duration of pain.
Networks of Evidence
The feasibility assessment found that connected networks of evidence could be created, subject to specific analytical and clinical assumptions. SCS therapies, either as monotherapy or in combination with CMM, were pooled into 1 node. Similarly, the sham or CMM node included either sham alone or standard care, with different studies referring to CMM or conventional medical practice or best medical treatment or management. Most importantly, analyses pooled different novel waveforms, including high frequency, burst, differential target multiplexed, and closed-loop SCS together in a novel SCS treatment node, while tonic SCS waveforms were pooled in a conventional SCS node. This analytic decision was made based on clinical advancements in the technology over the past decade from original waveforms to newer technologies commonly in use today.
Comparisons were feasible for 6 outcomes of interest: pain intensity in the back, pain intensity in the leg, proportion of patients achieving at least 50% pain reduction in back, proportion of patients achieving at least 50% pain reduction in leg, EQ-5D index score, and Oswestry Disability Index score. Analyses for safety outcomes were not feasible in an NMA setting, given they were reported as procedure-related adverse events, which were not reported for the control groups across all included studies.
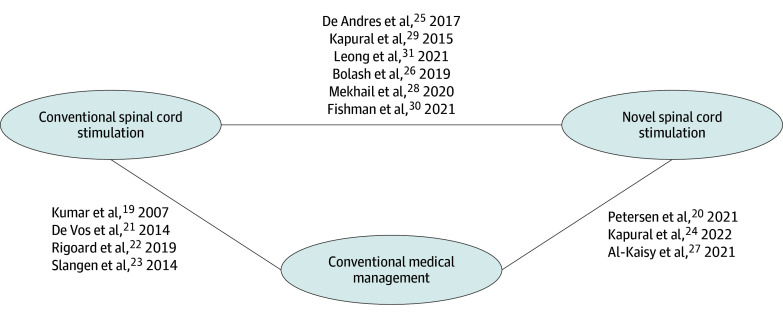
An overall connected network of evidence was established for 13 RCTs19,20,21,22,23,24,25,26,27,28,29,30,31 of 31 RCTs identified in the SLR (Figure 2). Eighteen trials were excluded due to data not available for the follow-up assessment time points of interest, no measure of uncertainty reported, or no separate data provided for back vs leg pain.32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 All RCTs with a sham group32,33,36 were excluded from the subsequent analysis at this step. Therefore the remaining feasible comparisons were SCS (conventional and novel) vs CMM. An overview of the study design characteristics of the included studies is presented in Table 1.
Figure 2. Overall Network of Evidence.
Overall network of evidence used in the network meta-analysis.
Table 1. Overview of Study Design Across Included Studies.
| Source | Study phase | Blinding | Intervention | Comparator | Study population | Sample size | Duration of follow-up |
|---|---|---|---|---|---|---|---|
| Kumar et al,19 2007 | NA | Open label | SCS + CMM | CMM alone | Patients with neuropathic pain (FBSS) | 100 | 24 mo |
| Petersen et al,20 2021 | NA | Open label | 10 kHz SCS + CMM | CMM | Patients with neuropathic pain (PDN) | 216 | 52 mo |
| de Vos et al,21 2014 | NA | Open label | SCS + CMP | CMP | Patients with neuropathic pain (PDN) | 60 | 6 mo |
| Rigoard et al,22 2019 | 4 | Open label | SCS + OMM | OMM alone | Patients with FBSS diagnosis, mixed population | 218 | 24 mo |
| Slangen et al,23 2014 | NA | Open label | SCS + BMT | BMT | Patients with neuropathic pain (moderate to severe PDPN) | 36 | 6 mo |
| Kapural et al,24 2022 | NA | Open label | 10 kHz SCS + CMM | CMM | Patients with neuropathic pain (NSRBP) | 159 | 12 mo |
| De Andres et al,25 2017 | NA | Double-blind | High-frequency (10 kHz) SCS | Conventional SCS | Patients with neuropathic pain (FBSS) | 55 | 12 mo |
| Bolash et al,26 2019 | NA | Open label | High-frequency (10 kHz) SCS | Low-frequency SCS | Patients with neuropathic pain (FBSS) | 99 | 6 mo |
| Al-Kaisy et al,27 2021 | NA | Open label | SCS 10 kHz + CMM | CMM alone | Patients with possible neuropathic pain (NSRBP) | 58 | 12 mo |
| Mekhail et al,28 2020 | NA | Double-blind | Closed-loop SCS (ECAP-controlled) | Open-loop SCS (manual stimulation) | Patients with chronic, intractable pain of the back and legs | 134 | 3 y |
| Kapural et al,29 2015 | NA | Open label | High-frequency (10 kHz) SCS | Low-frequency therapy SCS | Chronic, intractable pain of the trunk or extremities | 198 | 24 mo |
| Fishman et al,30 2021 | NA | Open label | Differential target multiplexed SCS | Traditional SCS | Chronic LBP and leg pain | 128 | 12 mo |
| Leong et al,31 2021 | NA | Open label | Burst stimulation SCS | Tonic stimulation SCS | Chronic intractable pain in the extremities and trunk | 100 | 24 wk |
Abbreviations: BMT, best medical treatment; CMM, conventional medical management; CMP, conventional medical practice; FBSS, failed back surgery syndrome; kHz, kilohertz; LBP, low back pain; NA, not available; NSRBP, non-surgical refractory back pain; OMM, optimal medical management; PDN, painful diabetic neuropathy; PDPN, painful diabetic peripheral neuropathy; SCS, spinal cord stimulation.
Analyses were conducted using data from 3-, 6-, 12-, and 24-month follow-ups; however, only the results from analyses using the longest mutually reported time point for all 6 end points of interest (6 months) are reported in Table 2. Results for all outcomes at all available follow-up time points are provided in eTable 9 in Supplement 1.
Table 2. Matrix of Bayesian NMA Resultsa.
| Measure | Estimate vs CMM |
|---|---|
| Proportion of patients achieving ≥50% pain reduction in back b | |
| Conventional SCS, OR (95% CrI) | 3.00 (1.49 to 6.72)c |
| Novel SCS, OR (95% CrI) | 8.76 (3.84 to 22.31)c |
| DIC | 14.07 |
| Studies in the network, No. | 5 |
| Total sample size, No. | 683 |
| Proportion of patients achieving ≥50% pain reduction in leg d | |
| Conventional SCS, OR (95% CrI) | 6.93 (0.67 to 49.35)e |
| Novel SCS, OR (95% CrI) | 10.13 (0.45 to 129.31)e |
| DIC | 26.15 |
| Studies in the network, No. | 7 |
| Total sample size, No. | 831 |
| Pain intensity in back b | |
| Conventional SCS, MD (95% CrI) | −1.17 (−1.64 to −0.70)c |
| Novel SCS, MD (95% CrI) | −2.34 (−2.96 to −1.73)c |
| DIC | 23.87 |
| Studies in the network, No. | 6 |
| Total sample size, No. | 738 |
| Pain intensity in leg d | |
| Conventional SCS, MD (95% CrI) | −2.89 (−4.03 to −1.81)c |
| Novel SCS, MD (95% CrI) | −4.01 (−5.31 to −2.75)c |
| DIC | 37.98 |
| Studies in the network, No. | 10 |
| Total sample size, No. | 1014 |
| EQ-5D Index score b | |
| Conventional SCS, MD (95% CrI) | 0.15 (0.09 to 0.21)c |
| Novel SCS, MD (95% CrI) | 0.17 (0.13 to 0.21)c |
| DIC | 19.49 |
| Studies in the network, No. | 6 |
| Total sample size, No. | 700 |
| ODI b | |
| Conventional SCS, MD (95% CrI) | −7.10 (−10.91 to −3.36)c |
| Novel SCS, MD (95% CrI) | −4.98 (−10.78 to 0.62)e |
| DIC | 11.13 |
| Studies in the network, No. | 3 |
| Total sample size, No. | 367 |
Abbreviations: CMM, conventional medical management; CrI, Credible interval; DIC, deviance information criterion; EQ-5D, EuroQol-5 Dimensions; MD, mean difference; ODI, Oswestry Disability Index; OR, odds ratio; SCS, spinal cord stimulation.
Additional follow-up times are provided in eTable 9 in Supplement 1.
Fixed-effects model.
Favorability of SCS vs CMM and achieved superiority (statistical significance).
Random-effects model.
Did not achieve superiority (statistical significance).
Efficacy Outcomes
Responder Rate
Five studies19,22,26,29,30 reported responder rates for back pain. OR values larger than 1 imply favorability of SCS therapies compared with CMM. Analysis found that patients treated with either conventional or novel SCS were more likely to achieve at least 50% pain reduction in the back than patients treated with CMM, with novel SCS demonstrating superiority over CMM (OR, 8.76; 95% CrI, 3.84 to 22.31). Both novel and tonic SCS therapies were found to be statistically superior to CMM (eFigure 2 in Supplement 1).
For pain in leg, 7 studies were analyzed.19,20,21,22,23,29,30 Analysis did not find statistically significant differences for SCS therapies compared with CMM at 6 months (eFigure 3 in Supplement 1). For longer follow-ups (12 and 24 months), novel SCS continued to achieve superiority to CMM.
Pain Intensity Score
Six studies involving 3 treatments reported pain intensity score in the back.19,22,25,26,29,30 Patients treated with either conventional SCS or novel SCS achieved a statistically significantly greater reduction in pain intensity in the back compared with patients treated with CMM, with novel SCS showing the greatest MD (−2.34; 95% CrI −2.96 to −1.73) (eFigure 4 in Supplement 1). Furthermore, both SCS therapy types demonstrated the minimal clinically important change of at least 1.5 points on the visual analog scale.51
For pain in lower leg, 10 studies were included in the network of evidence.19,20,21,22,23,25,26,27,29,30 Analysis results showed statistical superiority of SCS therapies compared with CMM, with novel SCS having the greatest MD (−4.01, 95% CrI −5.31 to −2.75) (eFigure 5 in Supplement 1).
EQ-5D Index Score
Six studies reported EQ-5D index score and were pooled for analysis.19,20,21,22,23,24 Patients treated with either conventional or novel SCS reported higher utility scores than patients treated with sham or CMM, with novel SCS showing the highest MD (0.17; 95% CrI, 0.13 to 0.21) (eFigure 6 in Supplement 1). Furthermore, both SCS therapies demonstrated the minimal clinically important difference of at least 0.074.52
Functional Disability
Three studies reported functional disability as measured by the Oswestry Disability Index.19,22,25 Results showed that patients treated with either conventional or novel SCS had better functionality than patients treated with CMM, with conventional SCS showing the lowest MD (−7.10; 95% CrI −10.91 to −3.36) (eFigure 7 in Supplement 1). Statistical significance was achieved for conventional SCS, while the difference for novel SCS was not statistically significant.
Discussion
In this systematic review and network meta-analysis, we estimated the relative efficacy associated with SCS therapies (novel and conventional) vs sham (placebo) or CMM for patients with chronic pain in the back or leg who had not previously used SCS to provide a more complete view than reported in previous meta-analyses of SCS. We included a different set of selection criteria applied in the SLR by extending the analytical framework from pairwise meta-analysis to network meta-analysis. NMA allowed for both direct and indirect evidence to be synthesized and thus to incorporate additional evidence for the comparison of SCS vs CMM.
Prior to 2015, SCS clinical trials were primarily designed to compare tonic stimulation (conventional SCS) with CMM. With the advent of novel SCS paradigms, newer RCTs were designed to assess the incremental benefit of novel SCS waveforms vs conventional SCS. The recently published meta-analyses by O’Connell et al4 and Traeger et al5 drew criticism from several practicing SCS clinicians (eg, Russo et al7 and Durbhakula et al6), the most notable critique being the exclusion of RCTs comparing novel SCS to conventional SCS. Moreover, these reviews excluded postcrossover data from studies comparing SCS vs sham or CMM, mentioning randomization issues, while it is known that there are serious ethical concerns with long-term maintenance of a sham group arm and not implementing a crossover phase in a chronic pain population.7 Given the differences in study selection and method used in our study vs previous studies, a formal comparison with the results of the Cochrane review analyses is challenging. Ultimately, our analysis did not include a higher number of studies than the previous meta-analyses but achieved a more homogenous and contemporaneous evidence base, thereby increasing representativeness and reducing risk of bias. Generally, researchers need to carefully balance, justify, and transparently report the inclusion criteria for evidence synthesis to minimize selection bias.
Analysis results suggest statistical superiority of conventional and novel SCS therapies over CMM in 5 of the 6 outcomes evaluated at 6-month follow-up, with the remaining outcome (responder rate in leg) yielding no statistically significant results. Specifically, patients treated with SCS therapies were more likely to achieve at least 50% pain reduction in either back or leg when measured at 6 months of follow-up. To assess the uncertainty around these results, a threshold analysis was carried out for the pain-related outcomes, results of which indicated that superiority was maintained for both SCS therapies up to a 60% decrease in the treatment effect estimate and corresponding SEs. The results for leg pain could be attributed to the fact that there was only one study meeting the inclusion criteria, thus entailing a small sample size which resulted in higher uncertainty.
Limitations
This study has some limitations. Although our study attempted to include studies with long-term data, a lack of RCTs with long-term follow-up precluded their inclusion in the NMA. This limitation is inherent to pain-related randomized study designs, given the ethical obligation to offer the CMM groups the option to cross over to the active treatment group. Nearly all trials offered crossover at 6 months, and nearly all patients (>90%) in the CMM groups opted to cross over. As such, researchers must balance the need for rigorous, controlled study designs with the ethical imperative to offer patients with chronic intractable pain the option to cross over to the intervention group. High risk of bias was detected in most included studies; however, their study design and the nature of the intervention (implantable device with surgery) inherently does not allow for standard blinding in randomized trials. Relative safety estimates could not be assessed since safety end points were reported as procedure-related adverse events, which are not applicable for CMM. Additionally, given that we could not include sham-controlled studies within the structure of our NMA approach, comparison of SCS with sham was not feasible.
Inherent limitations also apply to the network meta-analytical approach, which pools evidence from studies with different patient eligibility criteria, therefore entailing the possibility of introducing bias to the investigated comparisons as a result of between-study heterogeneity. Chronic pain is highly individualized and subject to variability in the underlying cause of pain, duration of symptoms, psychological factors, and prior treatments. Our study sought to strike a balance between capturing key chronic pain populations (ie, chronic back and/or leg pain, leg pain due to complex regional pain syndrome and painful diabetic polyneuropathy) while retaining homogeneity in terms of focusing on patients who had never previously used SCS, whereas the Cochrane review by O’Connell et al4 included a mix of SCS-naive and SCS-experienced populations, as well as trials with broader conditions assessed (ie, myelopathy, myelomalacia, multiple sclerosis, and irritable bowel syndrome).4 Network meta-regression controlling for study-level covariates to account for heterogeneity was not conducted in our analysis due to the relatively small number of studies, decreasing the likelihood of network meta-regression detecting meaningful estimates with reasonable precision. Wide CrIs were observed in most analyses, indicating moderate to high levels of uncertainty around point estimates. Therefore, meta-analysis results should be interpreted with caution. The observed uncertainty can be predominantly attributed to the low number of events in the comparator groups, the low number of studies included in most networks, low sample sizes in some included studies, the network geometry, and undetected heterogeneity across included studies.
Conclusions
In this systematic review and network meta-analysis of SCS vs CMM for the treatment of patients with chronic pain in back and/or lower extremities who had not previously used SCS, we found that SCS was associated with improved pain and QOL and reduced disability compared with CMM after 6 months of follow-up. Our study provides important insights for clinical decision-making as well as assessment by health technology agencies based on a more representative evidence base and a more inclusive analytical framework than that reported by prior reviews comparing SCS technologies.
eTable 1. Population, Intervention, Comparator, Outcomes, Study Design (PICOS) Search Strategy
eTable 2. Embase Search Strategy (1974 to September 2, 2022)
eTable 3. MEDLINE Search Strategy (1946 to September 2, 2022)
eTable 4. Cochrane Search Strategy (2005 to August 31, 2022)
eTable 5. Summary of Available Patient Characteristics Across Included Studies
eTable 6. Study Results for Evidence Synthesis of the Included Studies
eTable 7. Prior Distributions Used in the Bayesian NMAs for All Outcomes
eTable 9. Matrix of Bayesian NMA Results for All Follow-Up Assessment Timepoints
eFigure 1. Overall Risk of Bias Across the Identified Studies
eFigure 2. Forest Plot for Proportion of Patients Achieving at Least 50% Pain Reduction in Back, FE Model (Assessment Timepoint: 6 Months)
eFigure 3. Forest Plot for Proportion of Patients Achieving at Least 50% Pain Reduction in Lower Limbs/Leg, RE Model (Assessment Timepoint: 6 Months)
eFigure 4. Forest Plot for Pain Intensity Score in Back, FE Model (Assessment Timepoint: 6 Months)
eFigure 5. Forest Plot for Pain Intensity Score in Lower Limbs/Leg, RE Model (Assessment Timepoint: 6 Months)
eFigure 6. Forest Plot for EQ-5D Index Score, FE Model (Assessment Timepoint: 6 Months)
eFigure 7. Forest Plot for Functional Disability: ODI Score, FE Model (Assessment Timepoint: 6 Months)
Data Sharing Statement
References
- 1.Breivik H, Eisenberg E, O’Brien T; OPENMinds . The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi: 10.1186/1471-2458-13-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmer Z, Fraser K, Grol-Prokopczyk H, Zajacova A. A global study of pain prevalence across 52 countries: examining the role of country-level contextual factors. Pain. 2022;163(9):1740-1750. doi: 10.1097/j.pain.0000000000002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12(12):CD013756. doi: 10.1002/14651858.CD013756.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traeger AC, Gilbert SE, Harris IA, Maher CG. Spinal cord stimulation for low back pain. Cochrane Database Syst Rev. 2023;3(3):CD014789. doi: 10.1002/14651858.CD014789.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durbhakula S, Broachwala MY, Schuster NM, McCormick ZL. Striking errors in the methodology, execution, and conclusions of the Cochrane Library review of spinal cord stimulation for low back pain by Traeger et al. Pain Med. 2023;24(8):923-925. doi: 10.1093/pm/pnad047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo MA, Bhatia A, Hayek S, et al. Problems with O’Connell et al, “Implanted Spinal Neuromodulation Interventions for Chronic Pain in Adults” (Cochrane Review). Neuromodulation. 2023;26(5):897-904. doi: 10.1016/j.neurom.2023.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutton B, Catalá-López F, Moher D. The PRISMA Statement Extension for Systematic Reviews Incorporating Network Meta-Analysis: PRISMA-NMA. Article in Spanish. Med Clin (Barc). 2016;147(6):262-266. doi: 10.1016/j.medcli.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 10.Cochrane. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Accessed October 10, 2024. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 11.Carpenter B, Gelman A, Hoffman MD, et al. Stan: a probabilistic programming language. J Stat Softw. 2017;76(1):1. doi: 10.18637/jss.v076.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Project for Statistical Computing . R: A language and environment for statistical computing. http://www.R-project.org/2016
- 13.Racine JS. RStudio: a platform-independent IDE for R and Sweave. J Appl Econ. 2012;27:167-172. doi: 10.1002/jae.1278 [DOI] [Google Scholar]
- 14.Phillippo DM. multinma: an R package for Bayesian network meta-analysis of individual and aggregate data. Paper presented at Evidence Synthesis and Meta-Analysis in R Conference 2021; January 21, 2021; virtual. Accessed October 10, 2024. https://research-information.bris.ac.uk/en/publications/multinma-an-r-package-for-bayesian-network-meta-analysis-of-indiv [Google Scholar]
- 15.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 16.Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. 2015;34(6):984-998. doi: 10.1002/sim.6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64(4):583-639. doi: 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 18.Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Ann Intern Med. 2019;170(8):538-546. doi: 10.7326/M18-3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi: 10.1016/j.pain.2007.07.028 [DOI] [PubMed] [Google Scholar]
- 20.Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687-698. doi: 10.1001/jamaneurol.2021.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426-2431. doi: 10.1016/j.pain.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 22.Rigoard P, Basu S, Desai M, et al. ; PROMISE Study Group . Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160(6):1410-1420. doi: 10.1097/j.pain.0000000000001510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016-3024. doi: 10.2337/dc14-0684 [DOI] [PubMed] [Google Scholar]
- 24.Kapural L, Jameson J, Johnson C, et al. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10 kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. J Neurosurg Spine. 2022;37(2):188-199. doi: 10.3171/2021.12.SPINE211301 [DOI] [PubMed] [Google Scholar]
- 25.De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401-2421. doi: 10.1093/pm/pnx241 [DOI] [PubMed] [Google Scholar]
- 26.Bolash R, Creamer M, Rauck R, et al. Wireless high-frequency spinal cord stimulation (10 kHz) compared with multiwaveform low-frequency spinal cord stimulation in the management of chronic pain in failed back surgery syndrome subjects: preliminary results of a multicenter, prospective randomized controlled study. Pain Med. 2019;20(10):1971-1979. doi: 10.1093/pm/pnz019 [DOI] [PubMed] [Google Scholar]
- 27.Al-Kaisy A, Baranidharan G, Gempt J, et al. Randomized, controlled trial comparing high-frequency SCS (10 kHz) to conventional medical management for treatment of non-surgical-refractory-back-pain. Paper presented at: North American Neuromodulation Society’s 2021 Virtual Meeting; January 15-16, 2021; virtual. doi: 10.1111/ner.13385 [DOI] [Google Scholar]
- 28.Mekhail N, Levy RM, Deer TR, et al. ; Evoke Study Group . Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123-134. doi: 10.1016/S1474-4422(19)30414-4 [DOI] [PubMed] [Google Scholar]
- 29.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. doi: 10.1097/ALN.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 30.Fishman M, Cordner H, Justiz R, et al. Twelve-month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021;21(8):912-923. doi: 10.1111/papr.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong SL, De Ridder D, Deer T, Vanneste S. Potential therapeutic effect of low amplitude burst spinal cord stimulation on pain. Neuromodulation. 2021;24(3):574-580. doi: 10.1111/ner.13090 [DOI] [PubMed] [Google Scholar]
- 32.Sokal P, Malukiewicz A, Kierońska S, et al. Sub-perception and supra-perception spinal cord stimulation in chronic pain syndrome: a randomized, semi-double-blind, crossover, placebo-controlled trial. J Clin Med. 2020;9(9):2810. doi: 10.3390/jcm9092810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation. 2018;21(5):457-465. doi: 10.1111/ner.12771 [DOI] [PubMed] [Google Scholar]
- 34.Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618-624. doi: 10.1056/NEJM200008313430904 [DOI] [PubMed] [Google Scholar]
- 35.Rigoard P, Billot M, Ingrand P, et al. How should we use multicolumn spinal cord stimulation to optimize back pain spatial neural targeting: a prospective, multicenter, randomized, double-blind, controlled trial (ESTIMET study). Neuromodulation. 2021;24(1):86-101. doi: 10.1111/ner.13251 [DOI] [PubMed] [Google Scholar]
- 36.Hara S, Andresen H, Solheim O, et al. Effect of spinal cord burst stimulation vs placebo stimulation on disability in patients with chronic radicular pain after lumbar spine surgery: a randomized clinical trial. JAMA. 2022;328(15):1506-1514. doi: 10.1001/jama.2022.18231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: part II-clinical outcomes. Neurosurgery. 2005;57(5):990-996. doi: 10.1227/01.NEU.0000180030.00167.b9 [DOI] [PubMed] [Google Scholar]
- 38.Breel J, Wille F, Wensing AGCL, et al. A comparison of 1000 Hz to 30 Hz spinal cord stimulation strategies in patients with unilateral neuropathic leg pain due to failed back surgery syndrome: a multicenter, randomized, double-blinded, crossover clinical study (HALO). Pain Ther. 2021;10(2):1189-1202. doi: 10.1007/s40122-021-00268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Kaisy A, Baranidharan G, Sharon H, et al. Comparison of paresthesia mapping with anatomic placement in burst spinal cord stimulation: long-term results of the prospective, multicenter, randomized, double-blind, crossover CRISP study. Neuromodulation. 2022;25(1):85-93. doi: 10.1111/ner.13467 [DOI] [PubMed] [Google Scholar]
- 40.Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669-681. doi: 10.1097/j.pain.0000000000000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Heteren EPZ, van Roosendaal BWP, van Gorp EJAA, et al. Spinal cord stimulation with additional peripheral nerve/field stimulation versus spinal cord stimulation alone on back pain and quality of life in patients with persistent spinal pain syndrome. Neuromodulation. 2023;26(3):658-665. doi: 10.1016/j.neurom.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 42.Wallace MS, North J, Phillips G, et al. Two-year outcomes using an SCS system capable of combination therapy: a randomized controlled trial (COMBO). Neuromodulation. 2022;25(8):S3. doi: 10.1016/j.neurom.2022.10.009 [DOI] [Google Scholar]
- 43.ClinicalTrials.gov . Safety and effectiveness study of the precision SCS systems adapted for high-rate spinal cord stimulation (ACCELERATE). Accessed October 10, 2024. https://clinicaltrials.gov/study/NCT02093793
- 44.ClinicalTrials.gov . Evaluation of conventional and long pulse widths during a temporary spinal cord stimulation trial Accessed October 10, 2024. https://clinicaltrials.gov/study/NCT03526055
- 45.ClinicalTrials.gov . Spinal cord stimulation in patients with post-laminectomy syndrome in testing phase. Accessed October 10, 2024. https://clinicaltrials.gov/study/NCT03702010
- 46.Pope JE, Schu S, Sayed D, et al. Anatomic lead placement without paresthesia mapping provides effective and predictable therapy during the trial evaluation period: results from the prospective, multicenter, randomized, DELIVERY study. Neuromodulation. 2020;23(1):109-117. doi: 10.1111/ner.13019 [DOI] [PubMed] [Google Scholar]
- 47.Eldabe S, Duarte RV, Gulve A, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM): a randomised controlled trial. Pain. 2020;161(12):2820-2829. doi: 10.1097/j.pain.0000000000001977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67-76. doi: 10.1111/ner.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician. 2012;15(1):1-12. [PubMed] [Google Scholar]
- 50.Takroni R, Sharma S, Reddy K, et al. Randomized controlled trials in neurosurgery. Surg Neurol Int. 2022;13:379. doi: 10.25259/SNI_1032_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94. doi: 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 52.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523-1532. doi: 10.1007/s11136-004-7713-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Population, Intervention, Comparator, Outcomes, Study Design (PICOS) Search Strategy
eTable 2. Embase Search Strategy (1974 to September 2, 2022)
eTable 3. MEDLINE Search Strategy (1946 to September 2, 2022)
eTable 4. Cochrane Search Strategy (2005 to August 31, 2022)
eTable 5. Summary of Available Patient Characteristics Across Included Studies
eTable 6. Study Results for Evidence Synthesis of the Included Studies
eTable 7. Prior Distributions Used in the Bayesian NMAs for All Outcomes
eTable 9. Matrix of Bayesian NMA Results for All Follow-Up Assessment Timepoints
eFigure 1. Overall Risk of Bias Across the Identified Studies
eFigure 2. Forest Plot for Proportion of Patients Achieving at Least 50% Pain Reduction in Back, FE Model (Assessment Timepoint: 6 Months)
eFigure 3. Forest Plot for Proportion of Patients Achieving at Least 50% Pain Reduction in Lower Limbs/Leg, RE Model (Assessment Timepoint: 6 Months)
eFigure 4. Forest Plot for Pain Intensity Score in Back, FE Model (Assessment Timepoint: 6 Months)
eFigure 5. Forest Plot for Pain Intensity Score in Lower Limbs/Leg, RE Model (Assessment Timepoint: 6 Months)
eFigure 6. Forest Plot for EQ-5D Index Score, FE Model (Assessment Timepoint: 6 Months)
eFigure 7. Forest Plot for Functional Disability: ODI Score, FE Model (Assessment Timepoint: 6 Months)
Data Sharing Statement