Abstract
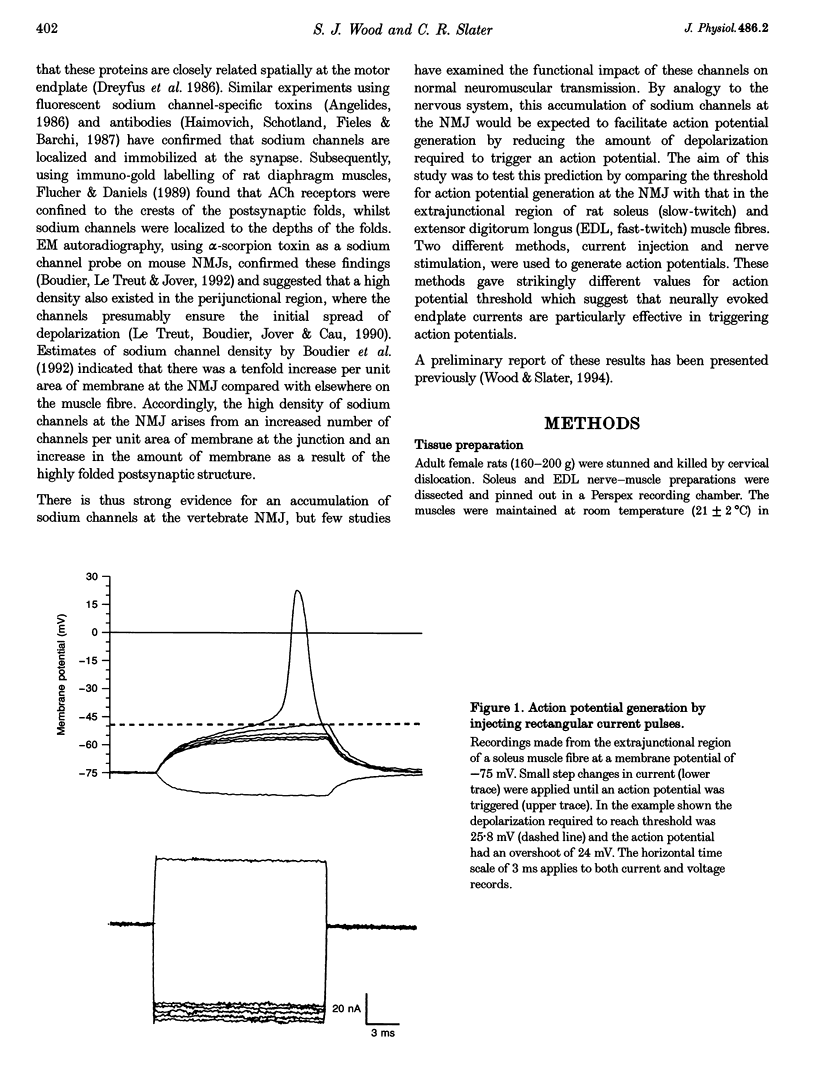
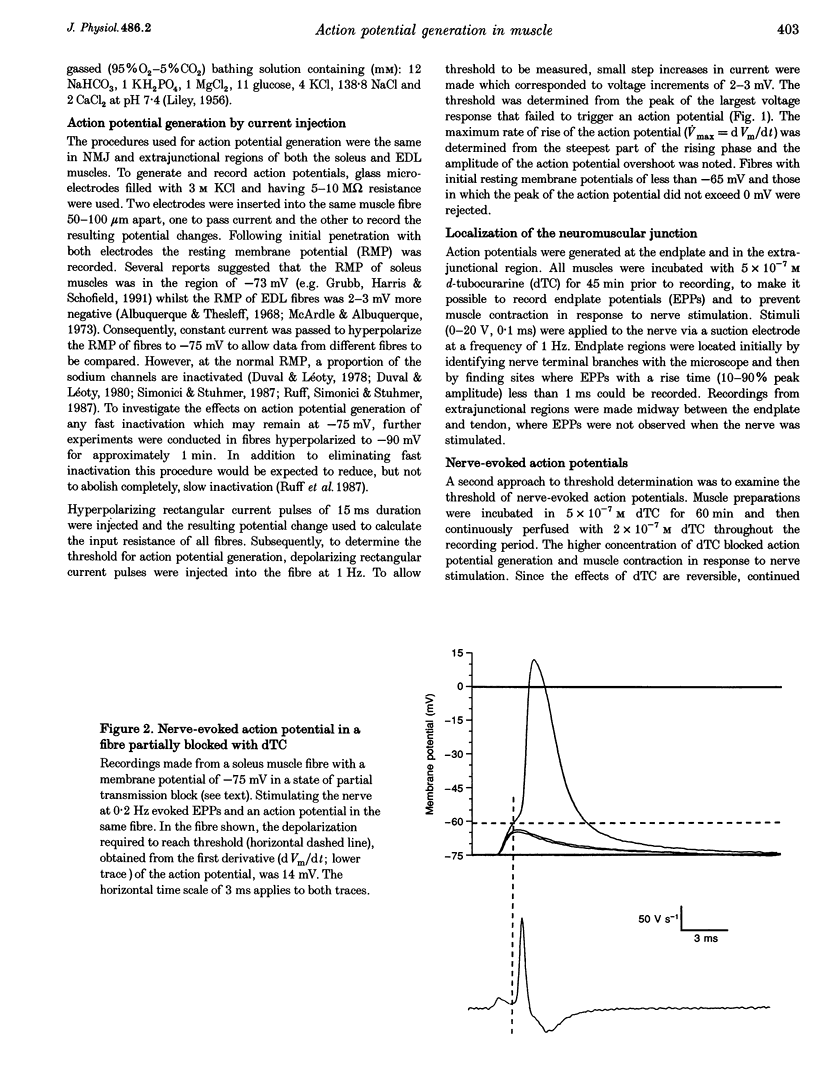
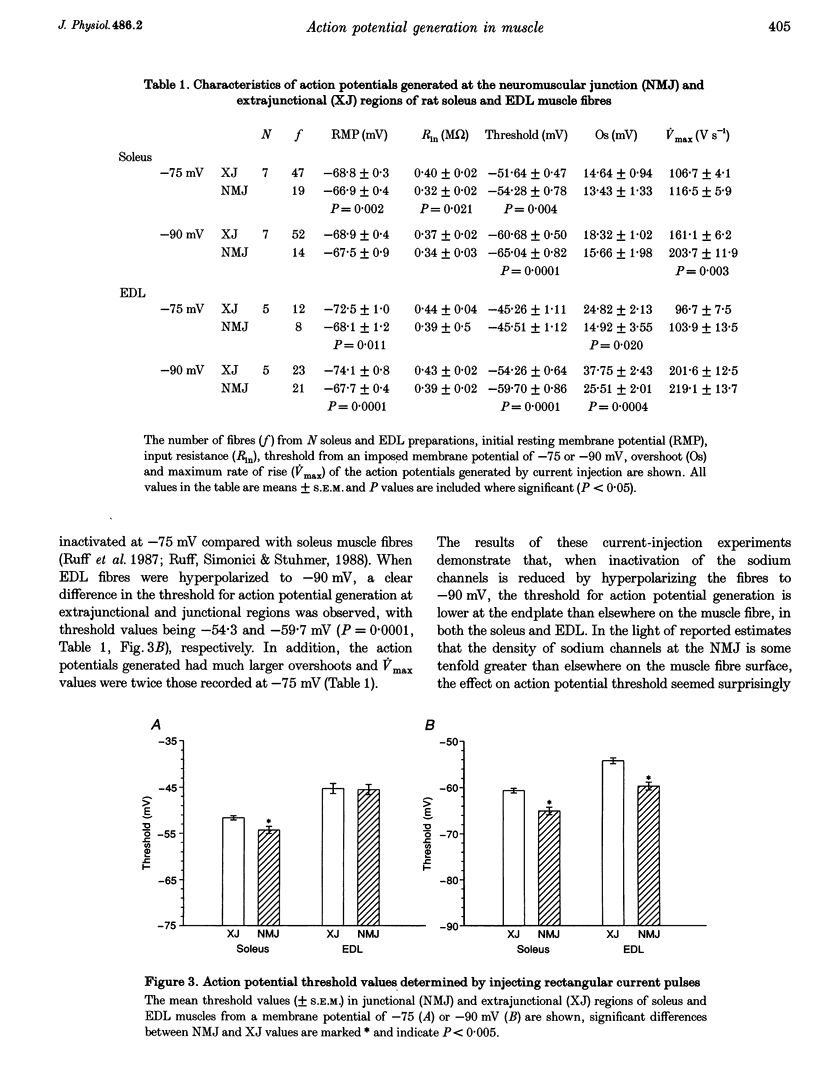
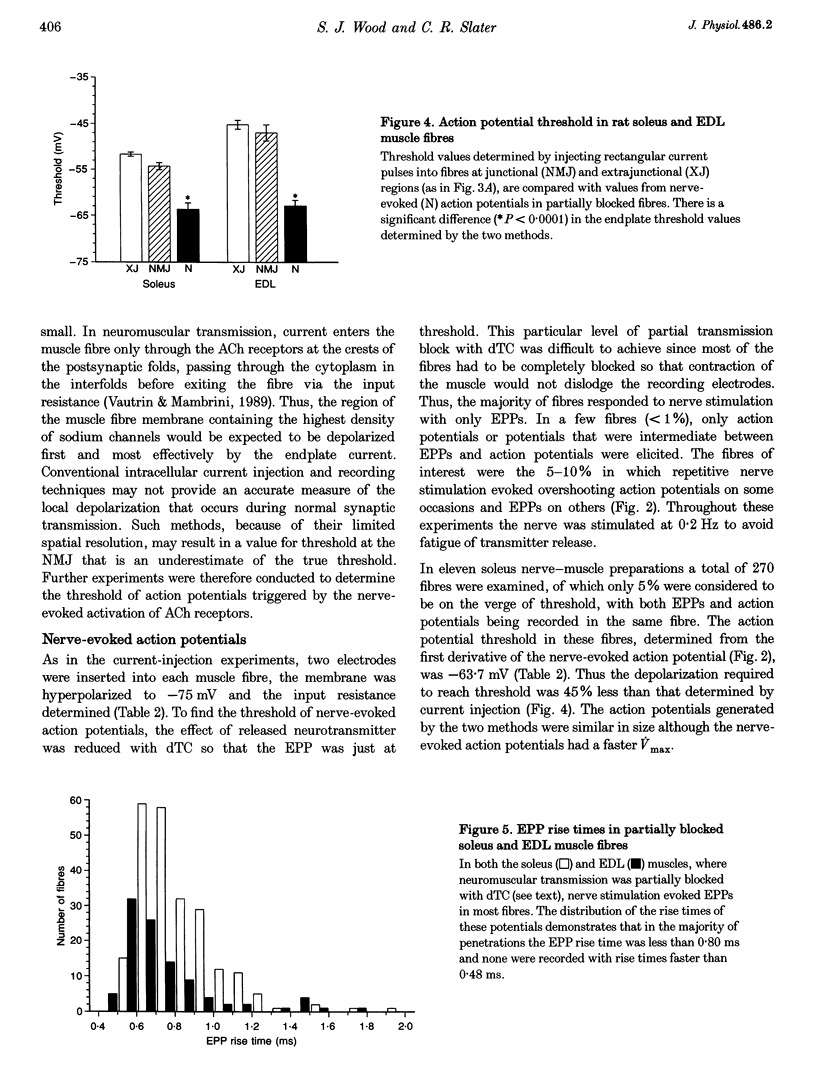
1. In skeletal muscle fibres, voltage-gated sodium channels are concentrated at the neuromuscular junction. The effect of this accumulation of sodium channels on action potential generation was investigated in rat slow- and fast-twitch muscle fibres. 2. Intracellular microelectrodes were used to generate and record action potentials, from an imposed membrane potential of -75 and -90 mV, in junctional and extrajunctional regions of the muscle fibre. To identify junctional regions, preparations were incubated with 5 x 10(-7) M d-tubocurarine (dTC) to block muscle contraction in response to nerve stimulation whilst allowing endplate potentials (EPPs) to be recorded. Injection of rectangular depolarizing current pulses initiated action potentials at the endplate with threshold values several millivolts lower than those generated elsewhere in the fibre. In addition, the maximum rate of rise of the action potential was greater at the endplate than in extrajunctional regions. 3. In other muscles, neuromuscular transmission was partially blocked with dTC (2 x 10(-7) M), such that repetitive nerve stimulation evoked action potentials and EPPs in the same fibre. The threshold of these nerve-evoked action potentials was approximately 50% lower than values derived from action potentials generated by current injection. 4. It is concluded that the threshold for action potential generation is significantly lower at the neuromuscular junction than in extrajunctional regions of skeletal muscle fibres. Furthermore, nerve-evoked current is more effective at generating an action potential than is injected current.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides K. J. Fluorescently labelled Na+ channels are localized and immobilized to synapses of innervated muscle fibres. Nature. 1986 May 1;321(6065):63–66. doi: 10.1038/321063a0. [DOI] [PubMed] [Google Scholar]
- Banker B. Q., Kelly S. S., Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983 Jun;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Caldwell J. H., Campbell D. T. Na channels in skeletal muscle concentrated near the neuromuscular junction. Nature. 1985 Feb 14;313(6003):588–590. doi: 10.1038/313588a0. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Kinnamon S. C. Increased sodium conductance in the synaptic region of rat skeletal muscle fibres. J Physiol. 1984 Jul;352:189–202. doi: 10.1113/jphysiol.1984.sp015286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudier J. L., Le Treut T., Jover E. Autoradiographic localization of voltage-dependent sodium channels on the mouse neuromuscular junction using 125I-alpha scorpion toxin. II. Sodium distribution on postsynaptic membranes. J Neurosci. 1992 Feb;12(2):454–466. doi: 10.1523/JNEUROSCI.12-02-00454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Campbell D. T., Beam K. G. Na channel distribution in vertebrate skeletal muscle. J Gen Physiol. 1986 Jun;87(6):907–932. doi: 10.1085/jgp.87.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus P., Rieger F., Murawsky M., Garcia L., Lombet A., Fosset M., Pauron D., Barhanin J., Lazdunski M. The voltage-dependent sodium channel is co-localized with the acetylcholine receptor at the vertebrate neuromuscular junction. Biochem Biophys Res Commun. 1986 Aug 29;139(1):196–201. doi: 10.1016/s0006-291x(86)80098-5. [DOI] [PubMed] [Google Scholar]
- Duval A., Léoty C. Ionic currents in mammalian fast skeletal muscle. J Physiol. 1978 May;278:403–423. doi: 10.1113/jphysiol.1978.sp012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A., Léoty C. Ionic currents in slow twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:23–41. doi: 10.1113/jphysiol.1980.sp013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B. E., Daniels M. P. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989 Aug;3(2):163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Gertler R. A., Robbins N. Differences in neuromuscular transmission in red and white muscles. Brain Res. 1978 Feb 17;142(1):160–164. doi: 10.1016/0006-8993(78)90186-5. [DOI] [PubMed] [Google Scholar]
- Grubb B. D., Harris J. B., Schofield I. S. Neuromuscular transmission at newly formed neuromuscular junctions in the regenerating soleus muscle of the rat. J Physiol. 1991 Sep;441:405–421. doi: 10.1113/jphysiol.1991.sp018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Schotland D. L., Fieles W. E., Barchi R. L. Localization of sodium channel subtypes in adult rat skeletal muscle using channel-specific monoclonal antibodies. J Neurosci. 1987 Sep;7(9):2957–2966. doi: 10.1523/JNEUROSCI.07-09-02957.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Treut T., Boudier J. L., Jover E., Cau P. Localization of voltage-sensitive sodium channels on the extrasynaptic membrane surface of mouse skeletal muscle by autoradiography of scorpion toxin binding sites. J Neurocytol. 1990 Jun;19(3):408–420. doi: 10.1007/BF01188407. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton R. L., Lupa M. T., Caldwell J. H. Fast and slow twitch skeletal muscle fibres differ in their distribution of Na channels near the endplate. Neurosci Lett. 1992 Jan 20;135(1):41–44. doi: 10.1016/0304-3940(92)90131-p. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A. 1977 Jan;74(1):211–215. doi: 10.1073/pnas.74.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. Na current density at and away from end plates on rat fast- and slow-twitch skeletal muscle fibers. Am J Physiol. 1992 Jan;262(1 Pt 1):C229–C234. doi: 10.1152/ajpcell.1992.262.1.C229. [DOI] [PubMed] [Google Scholar]
- Ruff R. L., Simoncini L., Stühmer W. Comparison between slow sodium channel inactivation in rat slow- and fast-twitch muscle. J Physiol. 1987 Feb;383:339–348. doi: 10.1113/jphysiol.1987.sp016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L., Simoncini L., Stühmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 1988 May;11(5):502–510. doi: 10.1002/mus.880110514. [DOI] [PubMed] [Google Scholar]
- Ruff R. L., Whittlesey D. Na+ current densities and voltage dependence in human intercostal muscle fibres. J Physiol. 1992 Dec;458:85–97. doi: 10.1113/jphysiol.1992.sp019407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L., Whittlesey D. Na+ currents near and away from endplates on human fast and slow twitch muscle fibers. Muscle Nerve. 1993 Sep;16(9):922–929. doi: 10.1002/mus.880160906. [DOI] [PubMed] [Google Scholar]
- Simoncini L., Stühmer W. Slow sodium channel inactivation in rat fast-twitch muscle. J Physiol. 1987 Feb;383:327–337. doi: 10.1113/jphysiol.1987.sp016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C. R., Lyons P. R., Walls T. J., Fawcett P. R., Young C. Structure and function of neuromuscular junctions in the vastus lateralis of man. A motor point biopsy study of two groups of patients. Brain. 1992 Apr;115(Pt 2):451–478. [PubMed] [Google Scholar]
- Thesleff S., Vyskocil F., Ward M. R. The action potential in end-plate and extrajunctional regions of rat skeletal muscle. Acta Physiol Scand. 1974 Jun;91(2):196–202. doi: 10.1111/j.1748-1716.1974.tb05676.x. [DOI] [PubMed] [Google Scholar]
- Vautrin J., Mambrini J. Synaptic current between neuromuscular junction folds. J Theor Biol. 1989 Oct 23;140(4):479–498. doi: 10.1016/s0022-5193(89)80110-9. [DOI] [PubMed] [Google Scholar]
- Wollner D. A., Catterall W. A. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]



