Abstract
Background
Changes in stent graft (SG) orientation after frozen elephant trunk (FET) treatment for aortic arch aneurysms are not well understood. This study investigated changes in SG orientation and their effects on aortic events after FET for aortic arch aneurysms.
Methods
This study included 57 patients with true aneurysms who underwent elective total arch replacement using FET between 2016 and 2023. Postoperative SG angle, descending aortic (DA) diameter at the end of the SG, and the DA angle were measured retrospectively by using enhanced computed tomography (CT).
Results
Median patient age was 74 years. The median stent size was 33 mm, and the stent length was 12 cm (range, 23–37 mm and 6–15 cm). Enhanced CT showed that all the patients had completely thrombosed aneurysms after the procedure. Aortic events (dissection or type Ib leaks) were observed in four patients during the follow-up period. The diameter of the DA at the end of the SG and the DA angle increased in recent periods (P=0.04 and P=0.01, respectively). The SG angle changed to an obtuse angle in the recent periods (P<0.0001). The probability of postoperative aortic events did not correlate with changes in SG orientation but with the ratio of recent postoperative descending diameter to preoperative descending diameter (P=0.04).
Conclusions
The descending aorta into which the SG was inserted showed flattening and widening movements owing to the spiring back force. One of the factors related to aortic events was dilation of the postoperative DA due to oversizing of the SG.
Keywords: Frozen elephant trunk (FET), aortic aneurysm, recoil, aortic events
Highlight box.
Key findings
• Change in stent graft (SG) orientation was observed during follow up periods in patients with total arch replacement (TAR) with frozen elephant trunk (FET).
What is known and what is new?
• SG orientation and its effects on distal stent graft-induced new entry have been reported in patients with dissection after TAR with FET.
• It is unclear whether aortic events occur in patients with aneurysms due to the changes in SG orientation.
What is the implication, and what should change now?
• The use of longer stents does not necessarily reduce changes in SG orientation.
Introduction
Background
The frozen elephant trunk (FET) procedure combines surgery and stent grafting to treat aortic arch aneurysms (1). The regulatory approval of this device has led to its increased use in Japan as well as worldwide. It is one of the available treatment options for acute type A dissection requiring total arch repair and can be employed for all aortic arch surgeries, including arch aneurysms and type B aortic dissection (2-4). It has the advantage of allowing FET implantation into the descending aorta (DA), which is difficult to anastomose via a median sternotomy. Pulmonary complications may occur if median sternotomy with additional left thoracotomy is performed in patients with aneurysms extending from the arch to the DA (5). In FET, self-expandable stent grafts (SGs) consist of a nitinol skeleton and a bloodtight polyester cover. Nitinol allows the SG to be mounted on a smaller introducer system for aortic delivery.
Rationale and knowledge gap
The nitinol memory attribute ensures the expansion of the SG to its original shape within the aorta (6,7). In addition, the spring-back force (elastic recoil) of nitinol acts along the outer curvature of the DA and may result in SG reorientation (8,9), which may complicate the distal stent graft-induced new entry (d-SINE) in patients with dissection (10,11). However, it is unclear whether aortic events occur in patients with aneurysms because of the spring-back force of the SG.
Objective
We aimed to investigate the changes in SG orientation and the incidence of aortic events in aortic arch aneurysms. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1169/rc).
Methods
From October 2016 to August 2023, 70 adult patients with true arch aneurysm and chronic dissecting aneurysm underwent total arch replacement (TAR) using Frozen J-graft (Japan Lifeline Inc., Tokyo, Japan) at Osaka Metropolitan University Hospital. Of these, 11 with chronic dissecting aneurysm and 2 with aneurysm rupture were excluded from this study. Therefore, 57 patients were enrolled in our study. Patients with acute aortic dissection were excluded from this study because compared with aneurysms, acute aortic dissection has a different pathology and requires different surgical strategies.
The patients’ clinical characteristics and pre-and postoperative enhanced computed tomography (CT) findings were retrospectively analyzed. We planned to perform secondary interventions after TAR with the Frozenix J-graft in patients who required it’s deployment distal to T8 for aortic aneurysms extending from the aortic arch to the distal DA. The Institutional Review Board of Osaka Metropolitan University Hospital approved this retrospective data analysis and waived the need for patient consent (Approval No. 2024-023). This study was conducted in accordance with the Declaration of Helsinki (revised in 2013).
Surgical technique
The approach used was a median sternotomy. Cardiopulmonary bypass was established with an arterial cannula to the ascending aorta and venous drainage with a two-stage cannula or bicaval venous cannula, as described previously (12). Body temperature was cooled to below 27 ℃ and measured in the pharynx. After cross clamping, we chose an arch translocation anastomosis technique for TAR. The aorta was dissected between the left common carotid artery and the left subclavian artery. The brachiocephalic artery, left common carotid artery, and left subclavian artery were dissected, and antegrade cerebral perfusion was started at the dissected end of each artery. Frozenix J-graft was inserted into the aorta with the non-stent part as short as possible and anastomosed with the 4-branch graft. After anastomosis, circulation was started from the lateral branch of the graft. The 4-branch graft was anastomosed with the left subclavian artery, the left common carotid artery, and the left brachiocephalic artery, respectively. Finally, a proximal anastomosis of the graft was completed with the aorta trimmed with sinotubular junction.
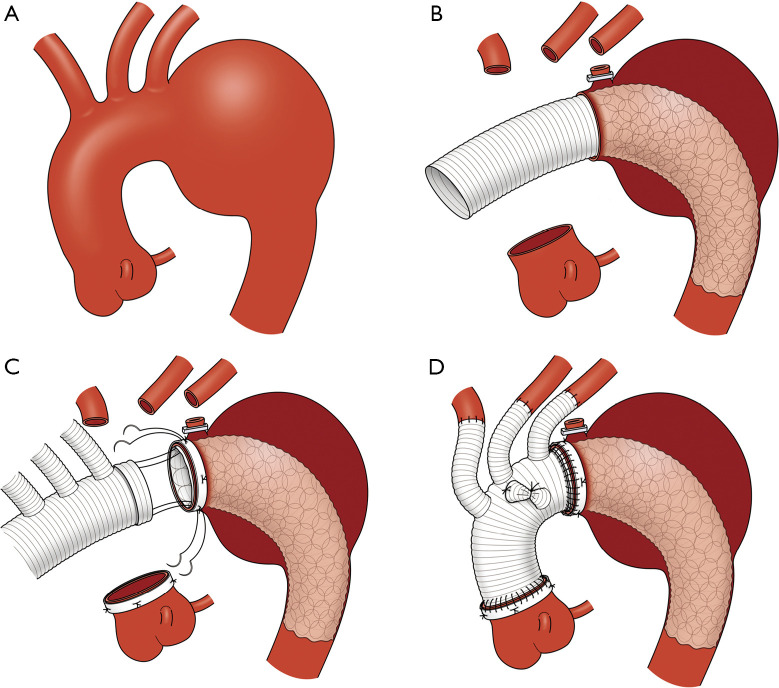
Figure 1A-1D show our total exclusion of the non-stent part of the Frozenix using the everting anastomosis (TENSE) technique, as described previously (12). The Frozenix J-graft was selected by 10–20% oversizing of the landing aorta. The length of the stent part of the Frozenix J-graft was >3 cm from the distal anastomosis site of the distal landing in the DA to prevent endoleaks.
Figure 1.
Schema of TENSE technique. (A) Schema of distal aortic arch aneurysm. (B) Deployment of the Frozenix J-graft with matching the proximal stump of the stent part to the distal anastomosis end of the aortic arch. (C) Anastomosis of the branched vascular graft with an everted distal end, the Frozenix J-graft with exclusion of the non-stent part, and the aortic arch. (D) Final schema of TENSE technique. Figure 1A-1D are reused with permission from AME Publishing Company (12). TENSE, total exclusion of the non-stent part of Frozenix using an everting anastomosis.
Follow-up examination and management
Fifty-seven patients were followed up every 6 to 12 months using CT data on an outpatient basis. Follow-up patients were censored on the last known date that they visited the hospital or based on patient referral documents from another hospital. The follow-up duration was 100%. The median follow-up duration was 512 days [interquartile range (IQR), 287–1,457 days].
CT measurement
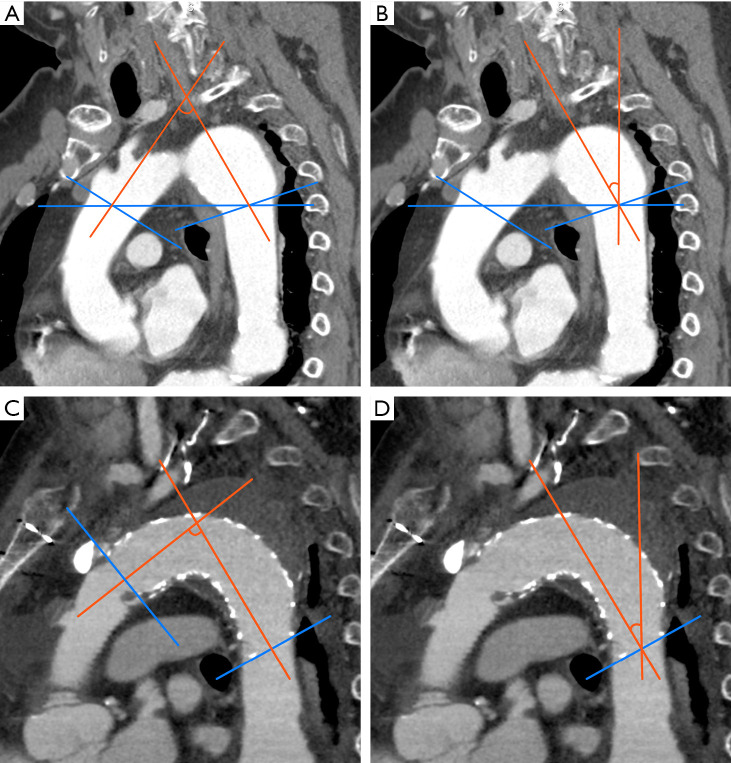
The aortic arch angle was defined as the angle between the coaxial lines of the ascending artery and DA at the level of the brachiocephalic artery (Figure 2A,2B). The stent-graft angle was defined as the angle between the coaxial lines of the proximal and distal stent-graft edges (Figure 2C). The DA angle of the SG was defined as the angle between the coaxial line of the distal edge of the SG and the vertical line (Figure 2D), as described previously (10). On the axial image, the diameter of the DA was measured at the site of the distal SG. Changes in SG orientation were evaluated using the ratio of the postoperative SG angle divided by the SG angle in recent periods, and the ratio of the preoperative DA angle divided by the DA angle in recent periods.
Figure 2.
CT parameters for SG orientation. (A) Aortic arch angle: the angle between the coaxial lines of the ascending aorta at the level of the brachiocephalic artery. (B) Distal DA angle: the angle between the coaxial lines of the DA at the level of the brachiocephalic artery. (C) SG angle: the angle between the coaxial lines of the proximal and distal SG edge. (D) Distal DA angle of SG: the angle between the coaxial line of the distal edge of the SG and vertical line. CT, computed tomography; DA, descending aorta; SG, stent graft.
Data collection and statistical analysis
Descriptive statistics for categorical variables are reported as absolute values and percentages, whereas continuous variables are shown as means and standard deviations or medians and interquartile ranges. When comparing the three groups, the Bonferroni correction was used after the nonparametric Kruskal-Wallis test. We analyzed the independent determinants of aortic events using Cox proportional hazard models. In Cox models, the hazard ratios (HRs), 95% confidence intervals (CIs), and P values were calculated. Statistical analyses were performed using R version 3.3.2 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P<0.05.
Results
Preoperative patient characteristics
The patient profiles are summarized in Table 1. There were no patients with connective tissue disorders including Marfan syndrome. The median patient age was 74 years. Seventy-seven percent of patients were men and 95% of patients were hypertensive. Forty percent of the patients exhibited chronic renal failure. Eleven of the 57 patients had preoperative recurrent nerve palsy. Thirteen patients (23%) had a history of cerebral infarction. The median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 4.2.
Table 1. Preoperative patient characteristics and echocardiographic data.
| Variables | All (n=57), median [IQR] or (%) |
|---|---|
| Age (years) | 77 [70–81] |
| Sex (male/female) | 44/13 (77%/23%) |
| BSA (m2) | 1.6 [1.5–1.8] |
| HT | 54 (95%) |
| Dyslipidemia | 30 (53%) |
| CRF | 23 (40%) |
| Hemodialysis | 1 (1.9%) |
| Cerebral disease | 13 (23%) |
| NIDDM | 18 (32%) |
| COPD | 10 (18%) |
| Extrathoracic vascular disease | 22 (39%) |
| Atrial fibrillation | 3 (5.3%) |
| RLNP | 6 (11%) |
| Redo | 2 (3.6%) |
| EuroSCORE II (%) | 4.2 [2.4–8.3] |
IQR, interquartile range; BSA, body surface area; HT, hypertension; CRF, chronic renal failure; NIDDM, non-insulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease; RLNP, recurrent laryngeal nerve palsy; EuroSCORE II, European System for Cardiac Operative Risk Evaluation II.
Surgical procedures and perioperative data
Surgical procedures and perioperative data are summarized in Table 2. The median operation and circulatory arrest times were 380 and 46 min, respectively. The size of the SG used in the operation ranged from 23 to 37 mm. Most of the SG sizes were 31, 33, and 35 mm. Most SG lengths were 9 and 12 cm. The mean SG size divided by the preoperative DA diameter was 1.2 (IQR, 1.1–1.3).
Table 2. Surgical procedures and perioperative data.
| Variables | All (n=57), median [IQR] or (%) |
|---|---|
| Operative data | |
| Operation time (min) | 380 [311–449] |
| Cardiopulmonary bypass time (min) | 203 [170–230] |
| Cross clamp time (min) | 130 [108–163] |
| Antegrade cerebral perfusion time (min) | 131 [99–155] |
| Circulatory arrest time (min) | 46 [39–52] |
| Fronzenix J graft t diameter (mm) | |
| 23 | 1 (1.8) |
| 27 | 1 (1.8) |
| 29 | 5 (8.8) |
| 31 | 16 (28.1) |
| 33 | 8 (14.0) |
| 35 | 20 (35.1) |
| 37 | 6 (10.5) |
| Stent-pert length (mm) | |
| 60 | 1 (1.8) |
| 90 | 15 (26.3) |
| 120 | 29 (50.9) |
| 150 | 12 (21.1) |
| Simultaneous procedures | |
| Aortic valve repair/replacement | 7 (12.3) |
| Bentall/remodeling | 2 (3.5) |
| Mitral valve repair | 6 (10.5) |
| Tricuspid valve repair | 3 (5.3) |
| CABG | 21 (36.8) |
| Stent size (mm)/pre distal DA diameter (mm) | 1.2 [1.1–1.3] |
| 1.0–1.10 | 7 |
| 1.11–1.20 | 20 |
| 1.21–1.30 | 28 |
| ≥1.31 | 2 |
IQR, interquartile range; CABG, coronary artery bypass grafting; DA, descending aorta.
Postoperative complications
Postoperative complications are shown in Table 3. Operative mortality was 0%. Severe morbidity during hospitalization was as follows: three patients required re-exploration for bleeding, one patient had acute myocardial infarction, two patients required continuous hemodialysis, two patients had a deep sternal wound infection, three patients had cerebral infarction, one patient developed paraplegia, and two patients developed paraparesis. All three cases were with shaggy DA. In patients with aortic arch aneurysms, postoperative CT angiography showed no kinking of the Frozenix J-graft or type Ia endoleaks. All aortic arch aneurysms were completely thrombosed using the Frozenix J-graft.
Table 3. Postoperative complications.
| Variables | All (N=57) (%) |
|---|---|
| Mortality | 0 (0) |
| Morbidity | |
| Re-exploration for bleeding | 3 (5.3) |
| Cardiac tamponade | 4 (7.0) |
| Myocardial infarction | 1 (1.8) |
| Need for continuous hemodialysis | 2 (3.5) |
| Cerebral infarction | 3 (5.2) |
| Paraplegia | 1 (1.8) |
| Paraparesis | 2 (3.5) |
| New RLNP | 2 (3.5) |
| Tracheotomy | 2 (3.5) |
| Pneumonia | 4 (7.0) |
| Deep sternal wound infection | 2 (3.5) |
| Aortic events in the follow up periods | |
| Type Ib endoleak | 2 (3.5) |
| Dissection | 2 (3.5) |
RLNP, recurrent laryngeal nerve palsy.
Aortic events
During the follow-up period, four patients had aortic events (Table 3). There were two patients with type Ib endoleaks: one was complicated 950 days after the initial operation and the other (Figure 3A) (12) 646 days after the initial operation. Two patients underwent thoracic endovascular aortic repair (TEVAR) for type Ib endoleaks. Two patients underwent dissection: one was complicated 77 days after the operation and the other 1,064 days after the operation (Figure 3B). One patient underwent TEVAR to repair the dissection, and the other received conservative treatment to decrease systemic blood pressure.
Figure 3.
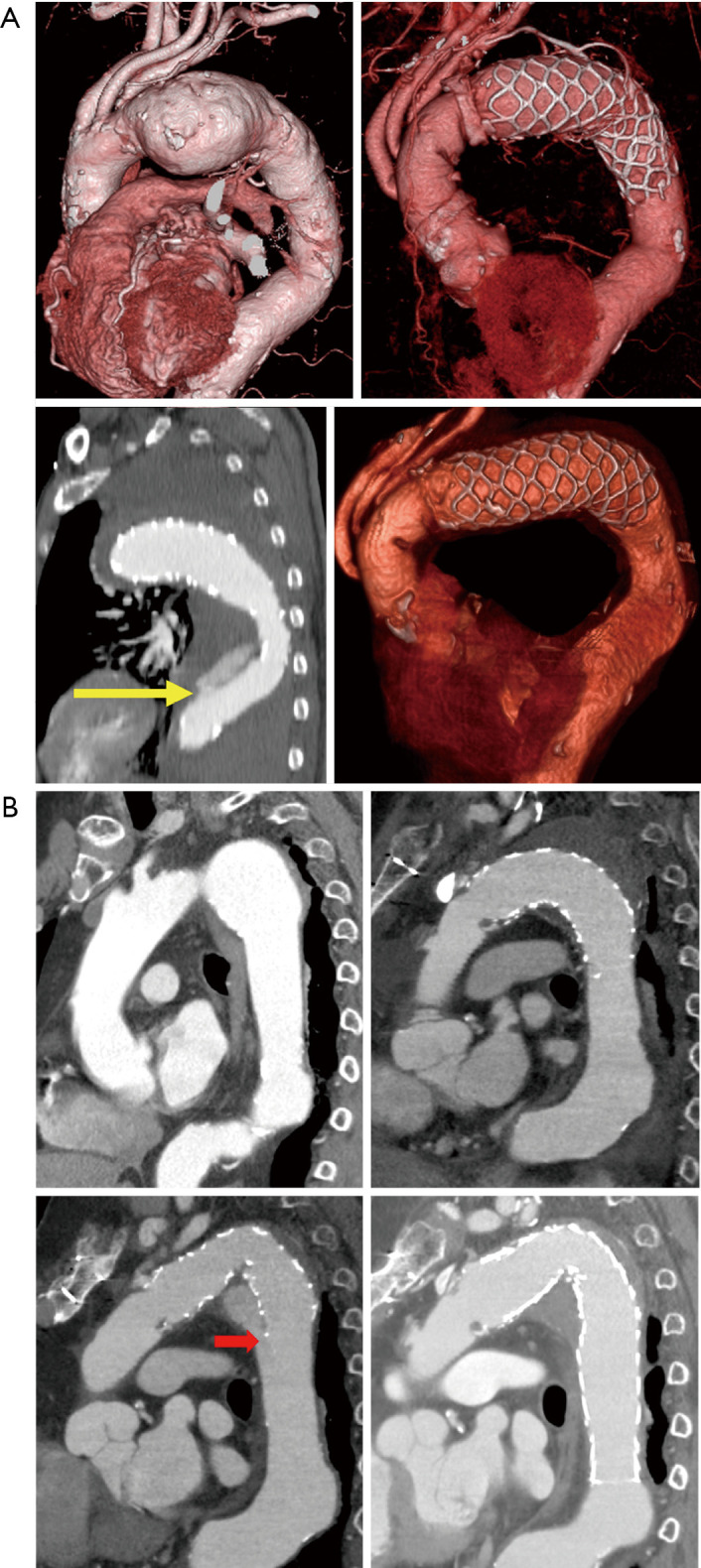
CT image of patients with aortic events. (A) CT angiography image of a patient with dissection. (B) CT angiography image of a patient with type Ib endoleak. The yellow arrow shows the distal end of the Frozenix J-graft, and the red arrow shows a type Ib endoleak. Figure 3A are reused with permission from AME Publishing Company (12). CT, computed tomography.
Evaluation of the changes in SG orientation
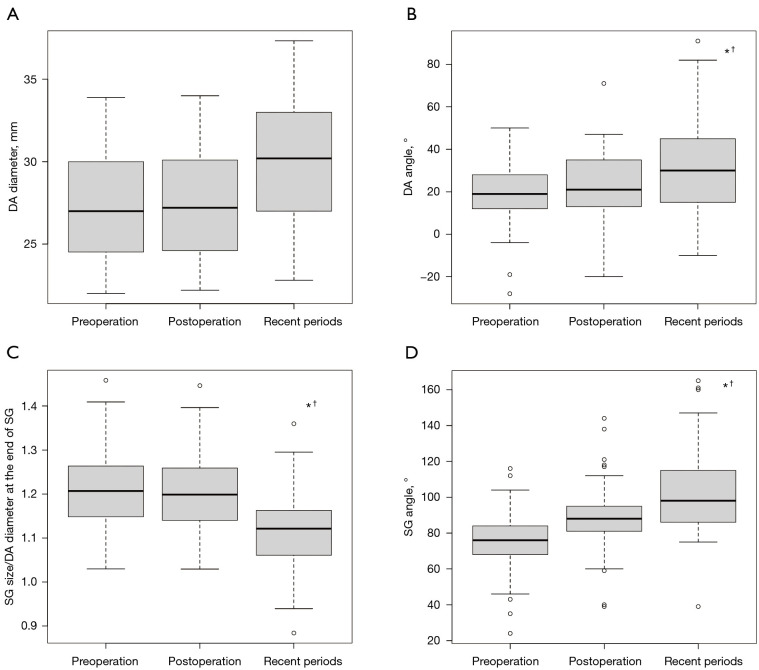
Changes in DA diameter, DA angle, SG size (diameter)/distal DA diameter at the end of the SG, and SG angle are shown in Figure 4A-4D, respectively.
Figure 4.
SG size and orientation in each period. (A) Change in the DA diameter, (B) DA angle, (C) SG size (diameter)/distal DA diameter at the end of the SG, and (D) SG angle. *, means P<0.05 between preoperative vs. recent periods; †, means P<0.05 between postoperative vs. recent periods. DA, descending aorta; SG, stent graft.
The median preoperative, postoperative DA diameter and recent periods DA diameters were 27 mm (25–30 mm), 27 mm (25–30 mm), and 31 mm (27–33 mm), respectively (preoperative DA diameter vs. recent periods DA diameter, P=0.04; postoperative DA diameter vs. recent periods DA diameter, P=0.08). The median preoperative, postoperative, and recent DA angles were 21° (13–35°), 21° (13–35°), and 30° (15–45°), respectively (preoperative vs. recent periods DA angle, P=0.013; postoperative vs. recent periods DA angle, P=0.06).
Median preoperative, postoperative, and recent periods SG size (diameter)/distal DA diameter at the end of the SG were 1.21 (1.15–1.26), 1.20 (1.14–1.26), and 1.11 (1.06–1.16), respectively [postoperative (SG size/distal DA diameter at the end of SG) vs. recent periods (SG size/distal DA diameter at the end of the SG), P<0.0001].
The median preoperative arch angle, postoperative SG angle, and recent periods SG angle were 76° (68–84°), 88° (81–95°), and 98° (86–115°), respectively (preoperative arch angle vs. postoperative SG angle, P<0.0001; preoperative arch angle vs. recent periods SG angle, P<0.0001; postoperative SG angle vs. recent periods SG angle, P=0.02).
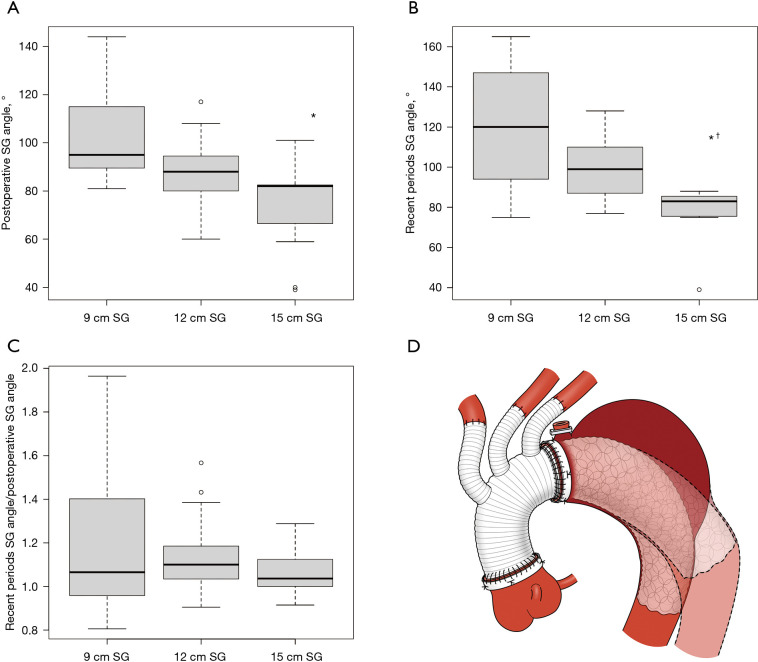
Relation between the SG length and the SG angle
The relationship between SG length and SG angle is shown in Figure 5A,5B, respectively. The relationship between SG length and change in SG angle is shown in Figure 5C. Patients with a 15 cm SG length had a lower SG angle in the postoperative periods and in recent periods [9 cm (95°) vs. 15 cm (82°) in the postoperative periods, P=0.007; 9 cm (120°) vs. 15 cm (83°) in recent periods, P=0.006]. However, when looking at the change in SG angle due SG length, there was no relationship between SG length and changes in SG angle during the follow-up period.
Figure 5.
The SG angle and the change in SG angle during follow up periods in each SG size. (A) Change in postoperative SG angle; (B) change in recent period SG angle; and (C) change in SG angle postoperatively to recent periods; (D) changes in SG orientation after TAR with FET. *, means P<0.05 between preoperative vs. recent periods; †, means P<0.05 between postoperative vs. recent periods. SG, stent graft, TAR, total arch replacement; FET, frozen elephant trunk.
Predictors of postoperative aortic events
Univariate analyses were performed to determine risk factors for aortic events (Table 4). Among patient preoperative parameters and perioperative and postoperative data, the ratio of distal DA diameter in recent periods divided by preoperative distal DA diameter was a predictor of postoperative aortic events (P=0.02).
Table 4. Predictors of postoperative aortic events.
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (years) | 0.955 | 0.8558–1.067 | 0.42 |
| SG size (mm) | 1.297 | 0.8081–2.082 | 0.28 |
| SG length (mm) | 1.332 | 0.7481–2.373 | 0.33 |
| Pre distal DA diameter (mm) | 1.162 | 1.162–0.8317 | 0.38 |
| Pre proximal aortic diameter (mm) | 1.095 | 0.8731–1.372 | 0.43 |
| Distal DA diameter in the recent periods (mm) | 1.76 | 0.6557–4.725 | 0.26 |
| Distal DA diameter in the recent periods (mm)/preoperative distal DA diameter (mm) | 10,420 | 3.94–27,580,000 | 0.02 |
| SG size (diameter) (mm)/pre distal DA diameter (mm) | 3 | 0.0001108–81,280 | 0.83 |
| Preoperative arch angle (°) | 0.998 | 0.911–1.092 | 0.96 |
| Postoperative SG angle (°) | 0.998 | 0.911–1.092 | 0.96 |
| SG angle in the recent periods (°) | 0.991 | 0.9406–1.044 | 0.73 |
| Preoperative DA angle (°) | 1.001 | 0.9008–1.113 | 0.98 |
| Postoperative DA angle (°) | 0.955 | 0.8603–1.06 | 0.38 |
| DA angle in the recent periods (°) | 0.993 | 0.9419–1.048 | 0.80 |
| Arch angle in the recent periods (°)/postoperative SG angle (°) | 0.792 | 0.01504–41.65 | 0.91 |
| Distal DA angle in the recent periods (°)/postoperative distal DA angle (°) | 1.266 | 0.8424–1.903 | 0.26 |
CI, confidence interval; SG, stent graft; DA, descending aorta.
Discussion
Our data revealed the following: (I) a change in SG orientation was found during the follow-up period; (II) no correlation between changes in SG orientation and SG length was observed; and (III) aortic events were associated with post-operative enlargement of the DA at the stent insertion site compared to the preoperative DA diameter and not with changes in SG orientation.
Aortic enlargement at stent insertion site was reported in TEVAR for patients with dissection, where the stent inserted into the true lumen expanded over time, reaching the original stent size in 22% of proximal sites and 17% of the distal sites (13). Aortic dilation is a common benign finding after TEVAR and is most pronounced at the distal end of the SG (14). Our findings are similar to those of previous studies. We performed TAR with FET using TENSE to treat the proximal anastomosis with a four-branch prosthesis because the TENSE technique leaves as few non-stent parts as possible (12). Hence, we speculate that FET stent movement reflects distal spring-back because the proximal portion of the SG is fixed at the anastomotic site (Figure 5D). If the long stent is inserted, the SG angle may be sharply angled because the end of the stent is in the straight portion of the DA. In our study, there was no statistical difference in the rate of change in SG angle for each stent length. On the other hand, the rate of change in SG angle varied for the 9 cm stent, suggesting that insertion of a longer stent may be less likely to result in a change in positions orientation.
FET-based recoil has been reported in patients with dissection in whom aortic events, including d-SINE, are a major problem. d-SINE incidence of 13% was reported after FET procedure (15). The cumulative incidences of d-SINE was reported as 7.1%, 12.4% and 21.4% after 12, 36 and 60 months, respectively (10). The recoil phenomenon is a risk factor for d-SINE in patients with aortic dissection. No studies have been conducted on aortic events induced by the recoil phenomenon in patients with aortic aneurysms. Our data revealed that the DA diameter covered by the FET tended to approach the original stent size and that the FET spring-back force yielded the SG angle. In all four cases of aortic events, an oversized FET was inserted. Two cases of fusiform aneurysm were complicated by type Ib leaks because the DA initially provided sufficient landings by the stent, but recoil caused the stent tip to move toward the distal portion near the aneurysm, resulting in an inadequate landing length. Considering the spring-back force of the SG, type Ib leaks are expected to be less likely to occur if a longer SG is selected to cover an aneurysm. However, the choice of SG length must be made with caution because of the risk of the spinal cord injury.
With regard to dissection, the cases in this study were not d-SINEs. Considering that the postoperative DA diameter was enlarged compared to the preoperative one and the SG angle changed significantly in recent periods in both cases, it might be concluded that the spring-back force or expansion force of the SG caused the dissection. Stent length is another relevant parameter as shorter grafts develop d-SINEs because the landing zone is not in a straight portion of the aorta (16). Longer thoracic DA coverage, although more prone to develop spinal cord injury, may provide lower parietal shear stress at the distal end of the stent (17). In our study, aortic events were associated with post-operative enlargement of the DA at the stent insertion site compared to the preoperative DA diameter but not with changes in SG orientation and length. Further studies are necessary to elucidate the relationship between dissection and SG orientation.
Limitations
Our study has several limitations. First, it was a retrospective study with a small number of patients. Nevertheless, our data originated from a single-reference surgical team committed to attempting TAR with the FET using the TENSE technique. Second, the CT data may have measurement bias. Third, each CT parameter was measured in 2D coordinates (axial and sagittal) and not evaluated in 3D coordinates. Fourth, other factors pertaining to the aortic disease itself or risk factors, such as undiagnosed connective tissue disorders, especially of a non-syndromic type, or uncontrolled hypertension, may have been the reason for the aortic diameter growth instead of the chosen endograft size. Finally, the patients included in the study were not a homogeneous group as they underwent a variety of surgeries other than TAR with FET.
Conclusions
Changes in SG orientation were observed in patients who underwent TAR with FET. The aortic events occurring after TAR-FET in patients with aortic aneurysms were partly due to DA dilatation at the end of SG insertion during follow-up.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Editage (www.editage.jp) for the English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Boards of Osaka Metropolitan University Hospital and Osaka City General Hospital (Approval No. 2024-023), and the need for patient consent was waived owing to the retrospective nature of the study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1169/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1169/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1169/dss
References
- 1.Song K, Kim YS, Jang WS, et al. Total arch replacement versus hybrid operation for aortic arch aneurysm in elderly patients: a retrospective cohort analysis. J Thorac Dis 2023;15:4357-66. 10.21037/jtd-23-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakob H, Moughal S, Bashir M. Frozen elephant trunk with straight vascular prosthesis: single-center experience with a review of current trends. J Cardiovasc Surg (Torino) 2020;61:301-7. 10.23736/S0021-9509.20.11401-0 [DOI] [PubMed] [Google Scholar]
- 3.Roselli EE, Idrees JJ, Bakaeen FG, et al. Evolution of Simplified Frozen Elephant Trunk Repair for Acute DeBakey Type I Dissection: Midterm Outcomes. Ann Thorac Surg 2018;105:749-55. 10.1016/j.athoracsur.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 4.Ho JYK, Kim CH, Chow SCY, et al. Initial Asian experience of the branched E-vita open NEO in complex aortic pathologies. J Thorac Dis 2023;15:484-93. 10.21037/jtd-22-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida N, Shibamura H, Katayama A, et al. Long-term results of the frozen elephant trunk technique for the extensive arteriosclerotic aneurysm. J Thorac Cardiovasc Surg 2010;139:913-7. 10.1016/j.jtcvs.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 6.Cao P, Verzini F, De Rango P, et al. Different types of thoracic endografts. J Cardiovasc Surg (Torino) 2009;50:483-92. [PubMed] [Google Scholar]
- 7.De Bock S, Iannaccone F, De Beule M, et al. Filling the void: a coalescent numerical and experimental technique to determine aortic stent graft mechanics. J Biomech 2013;46:2477-82. 10.1016/j.jbiomech.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Osswald A, Weymann A, Tsagakis K, et al. First insights into the role of wall shear stress in the development of a distal stent graft induced new entry through computational fluid dynamics simulations. J Thorac Dis 2023;15:281-90. 10.21037/jtd-22-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jánosi RA, Tsagakis K, Bettin M, et al. Thoracic aortic aneurysm expansion due to late distal stent graft-induced new entry. Catheter Cardiovasc Interv 2015;85:E43-53. 10.1002/ccd.25614 [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka A, Iida Y, Furukawa T, et al. Predictive factors of distal stent graft-induced new entry after frozen elephant trunk procedure for aortic dissection. Eur J Cardiothorac Surg 2022;62:ezac325. 10.1093/ejcts/ezac325 [DOI] [PubMed] [Google Scholar]
- 11.Osswald A, Schucht R, Schlosser T, et al. Changes of stent-graft orientation after frozen elephant trunk treatment in aortic dissection. Eur J Cardiothorac Surg 2021;61:142-9. 10.1093/ejcts/ezab297 [DOI] [PubMed] [Google Scholar]
- 12.Morisaki A, Takahashi Y, Fujii H, et al. Early outcomes of the Frozenix J-graft with exclusion of the non-stent part at a single center. J Thorac Dis 2022;14:1031-41. 10.21037/jtd-21-1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkarda Z, Kondov S, Kreibich M, et al. Landing Zone Remodelling after Endovascular Repair of Dissected Descending Aorta. Eur J Vasc Endovasc Surg 2020;59:939-45. 10.1016/j.ejvs.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Tran K, Li M, Stern JR, et al. Thoracic Aortic Dilation after Endovascular Repair of Blunt Traumatic Aortic Injury. Ann Vasc Surg 2021;70:101-8. 10.1016/j.avsg.2020.06.049 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Zhang S, Lu Q, et al. Impact of Oversizing on the Risk of Retrograde Dissection After TEVAR for Acute and Chronic Type B Dissection. J Endovasc Ther 2016;23:620-5. 10.1177/1526602816647939 [DOI] [PubMed] [Google Scholar]
- 16.Furutachi A, Takamatsu M, Nogami E, et al. Early and mid-term outcomes of total arch replacement with the frozen elephant trunk technique for type A acute aortic dissection. Interact Cardiovasc Thorac Surg 2019;29:753-60. 10.1093/icvts/ivz154 [DOI] [PubMed] [Google Scholar]
- 17.Berger T, Weiss G, Voetsch A, et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur J Cardiothorac Surg 2019;56:572-8. 10.1093/ejcts/ezz037 [DOI] [PubMed] [Google Scholar]