Abstract
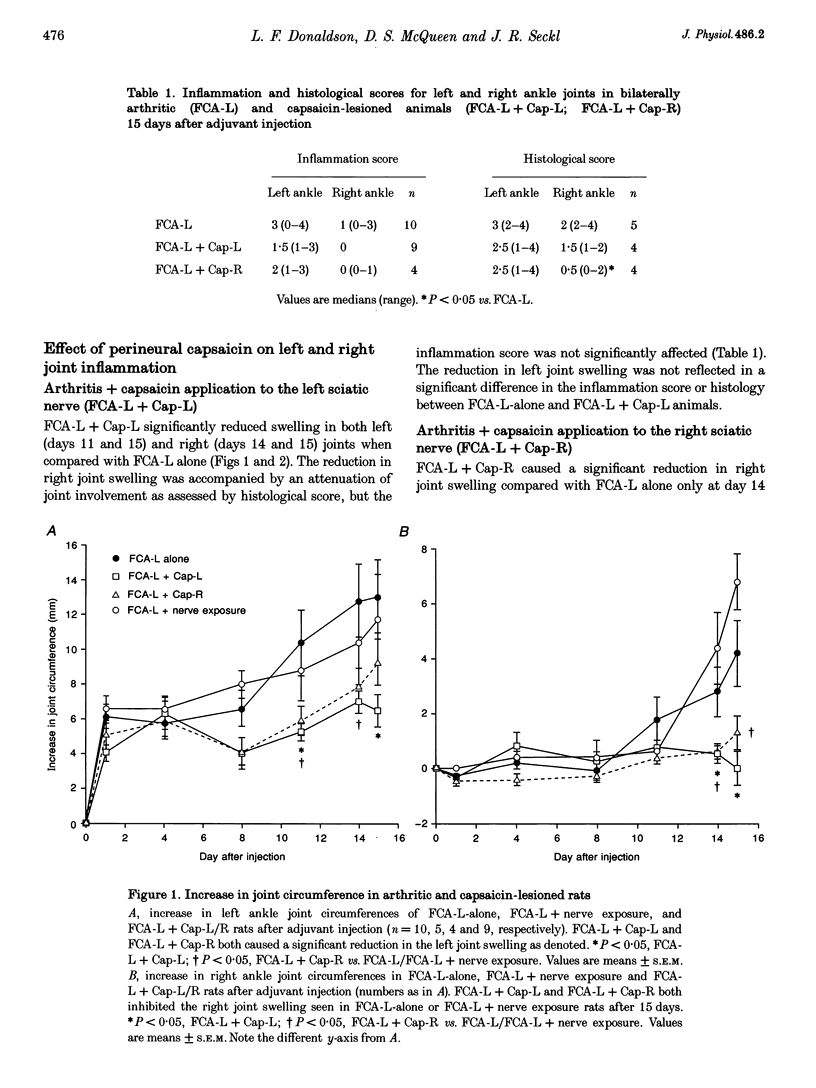
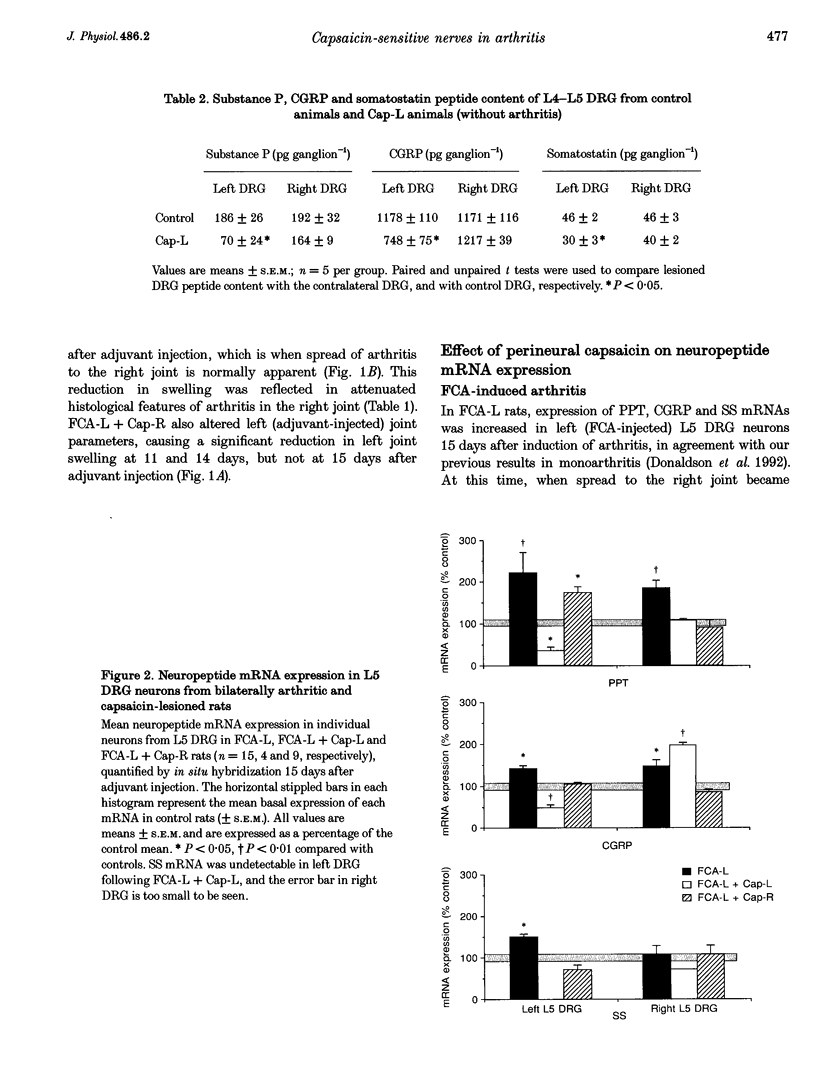
1. Many experimental and clinical arthritides are characterized by their bilateral nature. There is strong evidence to suggest that this bilateral spread may be mediated by a neuronal mechanism. We have previously shown early and sustained induction of mRNAs encoding preprotachykinin (PPT) and calcitonin gene-related peptide (CGRP) in dorsal root ganglion (DRG) neurons innervating an inflamed, arthritic joint. We have now investigated the involvement of capsaicin-sensitive primary afferents and the expression of neuropeptide mRNAs in the maintenance and bilateral spread of mild adjuvant-induced arthritis in the rat. 2. Capsaicin was applied perineurally to either the left (Cap-L) or right (Cap-R) sciatic nerve of halothane-anaesthetized male Han Wistar rats. Two weeks after capsaicin lesioning, arthritis was induced by injection of Freund's complete adjuvant (FCA) around the left ankle at a dose that caused inflammation of the left ankle joint, and a delayed (14 days) contralateral (right) ankle arthritis. Arthritis was monitored for 15 days after injection, when animals were killed and the lumbar DRG dissected. PPT, CGRP, somatostatin (SS), and vasoactive intestinal polypeptide (VIP) mRNA expression was determined in L5 DRG using in situ hybridization. 3. Spread of inflammation/arthritis to the right limb was associated with bilateral rises in PPT and CGRP mRNA expression in L5 DRG. SS mRNA expression in right DRG was unaffected by spread of inflammation. FCA-L+Cap-L reduced left joint swelling and prevented spread of arthritis to the right joint when assessed by joint swelling. This inhibition of spread of arthritis was associated with significant reductions in all left L5 DRG neuropeptide mRNAs compared with controls, and the rise in right L5 DRG PPT mRNA expression seen in FCA-L-alone animals was blocked. FCA-L+Cap-R also reduced left joint swelling and prevented the spread of inflammation to the right ankle. This lesion prevented the rise in PPT and CGRP mRNA expression seen in right DRG with FCA-L alone. 4. These findings suggest a role for capsaicin-sensitive primary afferents and the primary afferent neuropeptides encoded by PPT and CGRP mRNA in the maintenance and spread of arthritis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainsworth A., Hall P., Wall P. D., Allt G., MacKenzie M. L., Gibson S., Polak J. M. Effects of capsaicin applied locally to adult peripheral nerve. II. Anatomy and enzyme and peptide chemistry of peripheral nerve and spinal cord. Pain. 1981 Dec;11(3):379–388. doi: 10.1016/0304-3959(81)90637-0. [DOI] [PubMed] [Google Scholar]
- Allnatt J. P., Dickson K. E., Lisney S. J. Saphenous nerve injury and regeneration on one side of a rat suppresses the ability of the contralateral nerve to evoke plasma extravasation. Neurosci Lett. 1990 Oct 16;118(2):219–222. doi: 10.1016/0304-3940(90)90631-i. [DOI] [PubMed] [Google Scholar]
- Atkinson M. E., Shehab S. A. Peripheral axotomy of the rat mandibular trigeminal nerve leads to an increase in VIP and decrease of other primary afferent neuropeptides in the spinal trigeminal nucleus. Regul Pept. 1986 Dec 1;16(1):69–81. doi: 10.1016/0167-0115(86)90195-3. [DOI] [PubMed] [Google Scholar]
- Bileviciute I., Lundeberg T., Ekblom A., Theodorsson E. Bilateral changes of substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity in rat knee joint synovial fluid during acute monoarthritis. Neurosci Lett. 1993 Apr 16;153(1):37–40. doi: 10.1016/0304-3940(93)90071-r. [DOI] [PubMed] [Google Scholar]
- Bileviciute I., Lundeberg T., Ekblom A., Theodorsson E. Substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity (-LI) in rat knee joint synovial fluid during acute monoarthritis is not correlated with concentrations of neuropeptide-LI in cerebrospinal fluid and plasma. Neurosci Lett. 1994 Feb 14;167(1-2):145–148. doi: 10.1016/0304-3940(94)91048-0. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985 Dec;86(4):855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Courtright L. J., Kuzell W. C. Sparing effect of neurological deficit and trauma on the course of adjuvant arthritis in the rat. Ann Rheum Dis. 1965 Jul;24(4):360–368. doi: 10.1136/ard.24.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson L. F., Harmar A. J., McQueen D. S., Seckl J. R. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992 Nov;16(1-2):143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- Donaldson L. F., McQueen D. S., Seckl J. R. Local anaesthesia prevents acute inflammatory changes in neuropeptide messenger RNA expression in rat dorsal root ganglia neurons. Neurosci Lett. 1994 Jul 4;175(1-2):111–113. doi: 10.1016/0304-3940(94)91091-x. [DOI] [PubMed] [Google Scholar]
- Donaldson L. F., Seckl J. R., McQueen D. S. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Methods. 1993 Aug;49(1-2):5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- Donnerer J., Schuligoi R., Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992 Aug;49(3):693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Alterations in the ipsi- and contralateral afferent inputs of dorsal horn cells produced by capsaicin treatment of one sciatic nerve in the rat. Brain Res. 1982 Sep 23;248(1):97–107. doi: 10.1016/0006-8993(82)91151-9. [DOI] [PubMed] [Google Scholar]
- Grubb B. D., Stiller R. U., Schaible H. G. Dynamic changes in the receptive field properties of spinal cord neurons with ankle input in rats with chronic unilateral inflammation in the ankle region. Exp Brain Res. 1993;92(3):441–452. doi: 10.1007/BF00229032. [DOI] [PubMed] [Google Scholar]
- Hanesch U., Pfrommer U., Grubb B. D., Schaible H. G. Acute and chronic phases of unilateral inflammation in rat's ankle are associated with an increase in the proportion of calcitonin gene-related peptide-immunoreactive dorsal root ganglion cells. Eur J Neurosci. 1993 Feb 1;5(2):154–161. doi: 10.1111/j.1460-9568.1993.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Tölle T. R., Bravo R., Zieglgänsberger W., Zimmermann M. Sequential expression of JUN B, JUN D and FOS B proteins in rat spinal neurons: cascade of transcriptional operations during nociception. Neurosci Lett. 1991 Aug 19;129(2):221–224. doi: 10.1016/0304-3940(91)90466-7. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Walker T., Leah J. D., Bravo R., Zimmermann M. The KROX-24 protein, a new transcription regulating factor: expression in the rat central nervous system following afferent somatosensory stimulation. Neurosci Lett. 1990 Nov 27;120(1):21–24. doi: 10.1016/0304-3940(90)90158-6. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Hylden J. L., Nahin R. L., Traub R. J., Dubner R. Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain. 1991 Feb;44(2):187–193. doi: 10.1016/0304-3959(91)90136-L. [DOI] [PubMed] [Google Scholar]
- Inman R. D., Chiu B., Rabinovich S., Marshall W. Neuromodulation of synovitis: capsaicin effect on severity of experimental arthritis. J Neuroimmunol. 1989 Sep;24(1-2):17–22. doi: 10.1016/0165-5728(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Kayser V., Guilbaud G. Local and remote modifications of nociceptive sensitivity during carrageenin-induced inflammation in the rat. Pain. 1987 Jan;28(1):99–107. doi: 10.1016/0304-3959(87)91064-5. [DOI] [PubMed] [Google Scholar]
- Kidd B. L., Mapp P. I., Gibson S. J., Polak J. M., O'Higgins F., Buckland-Wright J. C., Blake D. R. A neurogenic mechanism for symmetrical arthritis. Lancet. 1989 Nov 11;2(8672):1128–1130. doi: 10.1016/s0140-6736(89)91491-8. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Chung J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992 Sep;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kolston J., Lisney S. J., Mulholland M. N., Passant C. D. Transneuronal effects triggered by saphenous nerve injury on one side of a rat are restricted to neurones of the contralateral, homologous nerve. Neurosci Lett. 1991 Sep 16;130(2):187–189. doi: 10.1016/0304-3940(91)90393-8. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y., Nanayama T., Ohno H., Fujii N., Otaka A., Yajima H., Satoh M. Calcitonin gene-related peptide increases in the dorsal root ganglia of adjuvant arthritic rat. Peptides. 1989 Mar-Apr;10(2):447–452. doi: 10.1016/0196-9781(89)90057-0. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Basbaum A. I., Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985 May;5(5):1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Mayer D. J., Hayes R. L., Price D. D. Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in 14C-2-deoxyglucose metabolic activity in rats with painful peripheral mononeuropathy. J Neurophysiol. 1993 Aug;70(2):470–481. doi: 10.1152/jn.1993.70.2.470. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Terenghi G., Walsh D. A., Chen S. T., Cruwys S. C., Garrett N., Kidd B. L., Polak J. M., Blake D. R. Monoarthritis in the rat knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neuroscience. 1993 Dec;57(4):1091–1096. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- Millan M. J., Colpaert F. C. Opioid systems in the response to inflammatory pain: sustained blockade suggests role of kappa- but not mu-opioid receptors in the modulation of nociception, behaviour and pathology. Neuroscience. 1991;42(2):541–553. doi: 10.1016/0306-4522(91)90396-6. [DOI] [PubMed] [Google Scholar]
- Minami M., Kuraishi Y., Kawamura M., Yamaguchi T., Masu Y., Nakanishi S., Satoh M. Enhancement of preprotachykinin A gene expression by adjuvant-induced inflammation in the rat spinal cord: possible involvement of substance P-containing spinal neurons in nociception. Neurosci Lett. 1989 Mar 13;98(1):105–110. doi: 10.1016/0304-3940(89)90382-0. [DOI] [PubMed] [Google Scholar]
- Morton C. R., Hutchison W. D., Hendry I. A., Duggan A. W. Somatostatin: evidence for a role in thermal nociception. Brain Res. 1989 May 29;488(1-2):89–96. doi: 10.1016/0006-8993(89)90696-3. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Morita Y., Kiyama H., Ono K., Tohyama M. A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry. Brain Res. 1988 Aug;464(1):31–35. doi: 10.1016/0169-328x(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Ohno H., Kuraishi Y., Nanayama T., Minami M., Kawamura M., Satoh M. Somatostatin is increased in the dorsal root ganglia of adjuvant-inflamed rat. Neurosci Res. 1990 Jul;8(3):179–188. doi: 10.1016/0168-0102(90)90019-b. [DOI] [PubMed] [Google Scholar]
- Pini Adrian, Baranowski Richard, Lynn Bruce. Long-Term Reduction in the Number of C-Fibre Nociceptors Following Capsaicin Treatment of a Cutaneous Nerve in Adult Rats. Eur J Neurosci. 1990 Jan;2(1):89–97. doi: 10.1111/j.1460-9568.1990.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Pini Adrian, Lynn Bruce. C-fibre Function During the 6 Weeks Following Brief Application of Capsaicin to a Cutaneous Nerve in the Rat. Eur J Neurosci. 1991;3(3):274–284. doi: 10.1111/j.1460-9568.1991.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Tal M. The transneuronal induction of sprouting and synapse formation in intact mouse muscles. J Physiol. 1985 Mar;360:387–396. doi: 10.1113/jphysiol.1985.sp015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H. G., Jarrott B., Hope P. J., Duggan A. W. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Res. 1990 Oct 8;529(1-2):214–223. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- Sluka K. A., Westlund K. N. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993 Dec;55(3):367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Harmar A. J., McQueen D. S., Seckl J. R. Increase in substance P and CGRP, but not somatostatin content of innervating dorsal root ganglia in adjuvant monoarthritis in the rat. Neurosci Lett. 1992 Mar 30;137(2):257–260. doi: 10.1016/0304-3940(92)90417-6. [DOI] [PubMed] [Google Scholar]
- Stevens C. W., Kajander K. C., Bennett G. J., Seybold V. S. Bilateral and differential changes in spinal mu, delta and kappa opioid binding in rats with a painful, unilateral neuropathy. Pain. 1991 Sep;46(3):315–326. doi: 10.1016/0304-3959(91)90114-D. [DOI] [PubMed] [Google Scholar]
- Stiller R. U., Grubb B. D., Schaible H. G. Neurophysiological evidence for increased kappa opioidergic control of spinal cord neurons in rats with unilateral inflammation at the ankle. Eur J Neurosci. 1993 Nov 1;5(11):1520–1527. doi: 10.1111/j.1460-9568.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Traub R. J., Solodkin A., Gebhart G. F. NADPH-diaphorase histochemistry provides evidence for a bilateral, somatotopically inappropriate response to unilateral hindpaw inflammation in the rat. Brain Res. 1994 May 30;647(1):113–123. doi: 10.1016/0006-8993(94)91405-2. [DOI] [PubMed] [Google Scholar]
- Verge V. M., Wiesenfeld-Hallin Z., Hökfelt T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey. Eur J Neurosci. 1993 Mar 1;5(3):240–250. doi: 10.1111/j.1460-9568.1993.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Wooley P. H. Animal models of rheumatoid arthritis. Curr Opin Rheumatol. 1991 Jun;3(3):407–420. doi: 10.1097/00002281-199106000-00013. [DOI] [PubMed] [Google Scholar]