Abstract
Background
Stereotactic body radiotherapy (SBRT) is a precise and effective treatment for pulmonary oligometastases, offering high local control (LC) rates. However, the optimal SBRT dose when combined with immunotherapy remains unclear, and there is a lack of comprehensive studies focusing on dose optimization in this setting. This study addresses this knowledge gap by exploring different SBRT dose regimens and their impact on progression-free survival (PFS), overall survival (OS), and LC in patients receiving concurrent immunotherapy, offering novel insights into the synergistic effects of these treatments.
Methods
A retrospective cohort study was conducted of 101 patients with 141 pulmonary oligometastases treated from April 2018 to April 2022. Inclusion criteria included patients with a maximum of five lung metastases and an Eastern Cooperative Oncology Group performance status of ≤2. Patients received SBRT with doses ranging from 50–70 Gy in 5–10 fractions. Follow-up was performed quarterly, and the best dose was determined by comparing survival outcomes across different dose groups. The patients received SBRT with doses ranging from 50–70 Gy in 5–10 fractions. Patient demographics, tumor characteristics, treatment details, and outcomes were collected. The Kaplan-Meier method was used for the survival analysis, and Cox regression models were used to identify prognostic factors for LC, PFS, and OS.
Results
The median follow-up for the 101 patients was 22.4 months (range, 1–58 months). The cohort comprised 82.2% male patients with a median age of 64 years (range, 36–81 years). The majority of the patients (64.4%) had primary tumors originating from non-lung sites, with adenocarcinoma being the predominant histological subtype (47.5%). The median tumor size was 13.5 mm. Across the entire cohort, the median OS was 39 months, and the median PFS was 11 months. Pre-treatment with immunotherapy significantly improved outcomes: the PFS increased to 13 months compared to 7 months for those who did not receive immunotherapy [P=0.02, hazard ratio (HR) = 0.523, 95% confidence interval (CI): 0.302–0.906], and the OS was also significantly improved (P=0.008, HR =0.411, 95% CI: 0.214–0.792). The SBRT regimen of 60 Gy in 10 fractions provided the best outcomes, with a median OS of 39 months, a median PFS of 10 months, and a LC rate of 92.4%, with relatively low toxicity compared to other regimens.
Conclusions
SBRT is a potent, minimally invasive option for managing pulmonary oligometastases, especially when preceded by immunotherapy. The 60 Gy in 10 fractions regimen demonstrated significant efficacy in terms of OS and LC, while maintaining manageable toxicity. Although the retrospective nature of the study introduces some selection bias, this dose regimen appears to offer a promising therapeutic option for pulmonary oligometastases. Further validation through well-designed prospective studies would help confirm the optimal SBRT dose and clarify the role of immunotherapy in this setting.
Keywords: Pulmonary oligometastases, stereotactic body radiotherapy (SBRT), immunotherapy, dosage regimen
Highlight box.
Key findings
• This study showed that stereotactic body radiotherapy (SBRT) at a dose of 60 Gy in 10 fractions is effective for managing pulmonary oligometastases, particularly when combined with immunotherapy. Compared to other regimens (50 Gy in 5 fractions, 50 Gy in 10 fractions, and 70 Gy in 10 fractions), the 60 Gy in 10 fractions regimen provided significant improvements in local control (LC), progression-free survival (PFS), and overall survival (OS), with relatively low toxicity.
What is known, and what is new?
• It is well established that SBRT offers precise radiation delivery, minimizes injury to surrounding healthy tissues, and achieves high LC in various cancers. However, the optimal SBRT dosage regimen for pulmonary oligometastases and the effect of concurrent immunotherapy require further exploration.
• This study provided evidence that a regimen of 60 Gy in 10 fractions was the most effective for maximizing therapeutic outcomes while maintaining a favorable toxicity profile, especially when integrated with immunotherapy.
What is the implication, and what should change now?
• The findings suggest that an SBRT regimen of 60 Gy in 10 fractions shows promise for the treatment of pulmonary oligometastases, particularly for patients who are eligible for concurrent immunotherapy. This approach could lead to improved patient outcomes, including increased survival rates and reduced treatment-related adverse effects. While these results are encouraging, further prospective trials are needed to confirm the efficacy of this regimen and to explore the synergistic effects of SBRT and immunotherapy.
Introduction
Approximately 30% of patients diagnosed with cancer develop pulmonary metastases (1). Metastases predominantly originate from primary lung cancers or other malignancies, such as gastrointestinal cancers and soft tissue sarcomas (2). Introduced by Hellman and Weichselbaum in 1995, the concept of oligometastases represents a transitional state between localized and widely disseminated disease (3). This concept has significantly influenced cancer management strategies, as it suggests that localized treatment could potentially be curative in a metastatic setting (4,5). Pulmonary oligometastases represent a distinct clinical scenario in the metastatic cascade, and they are characterized by limited metastatic lesions that might benefit from localized treatment strategies. Advances in imaging and targeted therapies have enabled the identification and treatment of such metastatic sites, which could improve patient outcomes.
Stereotactic body radiotherapy (SBRT) has emerged as a highly precise and effective treatment modality for pulmonary oligometastases, providing superior local control (LC) rates with minimal toxicity (6,7). SBRT is characterized by the delivery of high doses of radiation to small, well-defined targets over a limited number of fractions (f). However, despite its growing use, the optimal SBRT dose for patients receiving concurrent immunotherapy remains unclear. Current clinical practice in determining SBRT doses largely depends on biological equivalent dose (BED), which takes into account both the total dose and the fractionation scheme. A study has suggested that BED values greater than 150 Gy may yield better LC outcomes, but at the expense of increased toxicity, particularly in larger or centrally located tumors (8).
In clinical practice, the choice of SBRT dose is influenced by multiple factors, including tumor size, location, and proximity to critical organs at risk (OARs), as well as the patient’s overall treatment plan. For peripheral lung tumors, higher doses are often achievable with reduced risk of severe toxicity, whereas central tumors close to major bronchi or vessels may require more conservative dose limits (9). Additionally, the integration of immunotherapy into SBRT treatment regimens has raised new considerations for dose optimization. Evidence from early studies suggests that SBRT may enhance the effects of immunotherapy, potentially through mechanisms such as the abscopal effect, in which localized radiation induces systemic anti-tumor responses (10). However, the ideal SBRT dose in this combined treatment context has not been well-defined in the literature.
Several studies have investigated the histologic and clinical effects of SBRT when combined with other treatment modalities, including surgery and immunotherapy. For instance, Begum et al. highlighted the potential for neoadjuvant SBRT followed by metastasectomy to improve outcomes (9). Palma et al. have indicated that combining SBRT after effective systemic therapies may enhance survival outcomes, further validating the role of SBRT in multimodal therapeutic strategies (11). Meanwhile, Piao et al. demonstrated the efficacy of SBRT combined with immunotherapy for pulmonary oligometastases, showing improved progression-free survival (PFS) and overall survival (OS) with the use of SBRT doses around 60 Gy in 10 f (12). But there remains a significant gap in understanding the optimal SBRT dose with immunotherapy.
This retrospective cohort study aimed to evaluate the efficacy of different SBRT dose-fractionation regimens on LC, PFS, and OS in patients with pulmonary oligometastases. Additionally, the study sought to assess whether pre-treatment with immunotherapy could enhance survival outcomes. By identifying the optimal SBRT regimen and gaining an understanding of the synergistic effects of immunotherapy, this study sought to provide evidence-based recommendations for the management of pulmonary oligometastases, focusing on survival and prognostic factors in a retrospective analysis from April 2018 to April 2022. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1624/rc).
Methods
Study design and patient cohort
This retrospective cohort study was conducted at the Department of Radiation Oncology of The Affiliated Lihuili Hospital, Ningbo University from April 2018 to April 2022. The study aimed to evaluate the effectiveness of different SBRT dose regimens and the role of immunotherapy in enhancing survival outcomes in patients with pulmonary oligometastases.
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) with a score of ≤2; (II) have inoperable disease due to severe comorbidities or unresectable tumors; (III) have well-managed primary and extrapulmonary metastatic sites; (IV) have oligometastatic disease characterized by up to five metastatic lesions in the lung and the involvement of two metastatic organs; (V) have pulmonary oligometastases measuring ≤7 cm; and (VI) have confirmation of pulmonary oligometastases by histopathologic analysis or imaging.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Medical Ethics Committee of The Affiliated Lihuili Hospital, Ningbo University approved the study (reference number 2022-330), and the requirement for informed consent was waived due to the retrospective nature of the study. This study involved the use of previously collected treatment information, without disclosing any personal patient information or collecting biological samples, thereby ensuring patient confidentiality. A flowchart detailing the study procedure is shown in Figure S1.
Treatment regimen
SBRT was administered using a tailored approach based on lesion size and anatomical location, with radiation doses ranging from 50–70 Gy delivered in 5–10 f. The protocol aimed to optimize the radiation dose to the metastatic sites while protecting the surrounding healthy tissues. Dose fractionation was determined based on a comprehensive assessment of the tumor’s radiobiological characteristics and the patient’s overall treatment plan. Multidetector planning computed tomography (CT) scans with 3-mm slices of the entire chest were performed, incorporating respiratory management to minimize treatment-related toxicity. The gross tumor volume was delineated from four-dimensional CT data sets, resulting in the internal target volume and planning target volume with a 5-mm safety margin. BED10 was used to evaluate the biological effect of varying dose fractionation schedules. The dose constraints for OARs are referenced in Table S1.
Data collection and follow-up
Detailed clinical and treatment data were systematically collected, including patient demographics, primary and metastatic tumor characteristics, specific SBRT dosing regimens, and concurrent systemic therapies. Follow-up assessments included routine imaging and hematology testing 1-month post-treatment, followed by quarterly visits for the first 2 years. If recurrence was suspected, pathologic biopsies or radiologic studies were conducted to confirm recurrence. Local-regional failure was defined according to the revised (version 1.1) Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Treatment-related adverse events were classified according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE, version 4.0). The key outcomes of interest in this study were LC, PFS, and OS.
Loss to follow-up
Of the 108 patients included in the study, seven patients were lost to follow-up. These patients were excluded from the final survival analysis to avoid introducing bias. Sensitivity analyses were conducted to ensure that the exclusion of these patients did not significantly alter the study findings. The primary endpoints, including LC, PFS, and OS, were calculated based on the remaining 101 patients who completed the follow-up.
Statistical analysis
Survival functions were estimated using the Kaplan-Meier method. To control for confounding factors, univariate and multivariate Cox proportional hazards models were used to analyze the effects of prognostic factors on survival outcomes. Subgroup analyses were conducted to examine the effects of different variables, such as the primary tumor site, and pre-treatment with immunotherapy, on PFS and OS. Interactions between significant prognostic factors were also examined using interaction terms in the Cox models. To address the issue of missing data, multiple imputation methods were used to ensure that the analyses included all the available data and to minimize bias. To address the issue of patients lost to follow-up, sensitivity analyses were conducted to assess the potential impact of their exclusion on the study results. Specifically, these analyses compared the outcomes of patients who completed follow-up with those who were lost to follow-up to ensure the robustness of the findings. All the statistical analyses were performed using SPSS Statistics software (version 26.0), except for the bar charts in the supplementary materials, which were created using the ggplot package in R (version 4.3.3). All the statistical tests were bidirectional, and a P value of less than 0.05 was considered statistically significant. Hazard ratios (HRs) and 95% confidence intervals (CIs) were determined to facilitate the multivariate analysis of patient demographics, tumor attributes, and other critical determinants on the study endpoints.
Results
Patient and tumor profile
Between April 2018 and April 2022, 126 patients were initially evaluated for inclusion in this retrospective study. Of these patients, 18 were excluded for the following reasons: 10 did not meet the inclusion criteria (e.g., poor PS or extensive metastases), and 8 were lost to follow-up before treatment initiation. This left 108 patients, whose eligibility for the study was confirmed. Of these patients, 101 were included in the final analysis after seven additional patients were lost to follow-up during the study. A total of 141 pulmonary oligometastases from these 101 patients underwent SBRT treatment. The study population predominantly comprised male patients, who comprised 82.2% of the study cohort. The age of the patients ranged from 36 to 81 years (median age: 64 years). Adenocarcinoma was the main histological subtype, comprising 47.5% of cases. In terms of the primary tumor origins, the majority (64.4%) originated from non-lung cancers, including colorectal cancer (28 cases), liver cancer (17 cases), esophageal cancer (6 cases), oral cancer (4 cases), cholangiocarcinoma (3 cases), nasopharyngeal cancer (1 case), cervical cancer (1 case), gastric cancer (1 case), pancreatic cancer (1 case), breast cancer (1 case), hypopharyngeal cancer (1 case), and salivary gland cancer (1 case). Of the patients, 29.7% had metachronous tumors, and 52.5% had a history where more than two years passed between the primary tumor diagnosis and the development of lung metastases. From a therapeutic standpoint, the prevalent dose regimen was 60 Gy delivered over 10 f, which was administered to 65.2% of the patients. The median BED10 was 96 Gy (range, 75–119 Gy). The median tumor diameter measured 13.5 mm (range, 5.0–68.0 mm). Patients who did not receive immunotherapy were clinically distinct from those who did, due to eligibility factors and contraindications. Notably, the majority of patients (93.1%) received an adjuvant systemic therapy before SBRT, while 7 patients (6.9%) underwent surgery alone without additional systemic treatment. It should be noted that the surgeries reported in the cohort primarily involved the removal of the primary tumors rather than the lung oligometastatic lesions, which were treated exclusively with SBRT. In some cases, radiofrequency ablation (RFA) was employed to manage liver metastases, particularly in colorectal cancer patients, while the lung oligometastatic lesions remained untreated until SBRT. For further details of the patient demographics and tumor features, see Table 1.
Table 1. Patients and tumor characteristics.
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 83 (82.2) |
| Female | 18 (17.8) |
| Age (years) | 64 [36–81] |
| ECOG-PS score | |
| ≤1 | 92 (91.1) |
| >1 | 9 (8.9) |
| Primary tumor location | |
| Lung | 36 (35.6) |
| Non-lung | 65 (64.4) |
| Histologic subtype | |
| Adenocarcinoma | 48 (47.5) |
| Squamous cell carcinoma | 23 (22.8) |
| Other | 30 (29.7) |
| Number of metastases in the lung | |
| ≤2 | 95 (94.1) |
| >2 | 6 (5.9) |
| Distribution of metastasis | |
| Lung only | 71 (70.3) |
| Extrapulmonary and lung | 30 (29.7) |
| Time from diagnosis to lung metastasis (years) | |
| <2 | 48 (47.5) |
| ≥2 | 53 (52.5) |
| Dose (Gy/fraction) | |
| 50/5 | 12 (8.5) |
| 50/10 | 28 (19.9) |
| 60/10 | 92 (65.2) |
| 70/10 | 9 (6.4) |
| BED10 (Gy) | 96 [75–119] |
| Diameter of tumor (mm) | 13.5 [5.0–68.0] |
| Pulmonary lobe | |
| Superior lobe of left lung | 26 (18.4) |
| Inferior lobe of left lung | 38 (27.0) |
| Superior lobe of right lung | 34 (24.1) |
| Inferior lobe of right lung | 35 (24.8) |
| Middle lobe of right lung | 8 (5.7) |
| Adjuvant systemic therapy before SBRT | |
| Surgery | 7 (6.9) |
| Surgery + chemotherapy/targeted therapy/radiochemotherapy | 39 (38.6) |
| Surgery + chemotherapy + targeted therapy/immunotherapy | 19 (18.8) |
| Surgery + chemotherapy + immunotherapy + targeted therapy/radiation | 13 (12.9) |
| Radiofrequency ablation or chemotherapy ± targeted therapy | 23 (22.8) |
Data are presented as median [range] or n (%). ECOG-PS, Eastern Cooperative Oncology Group performance status; BED, biological equivalent dose; SBRT, stereotactic body radiotherapy.
Survival analysis
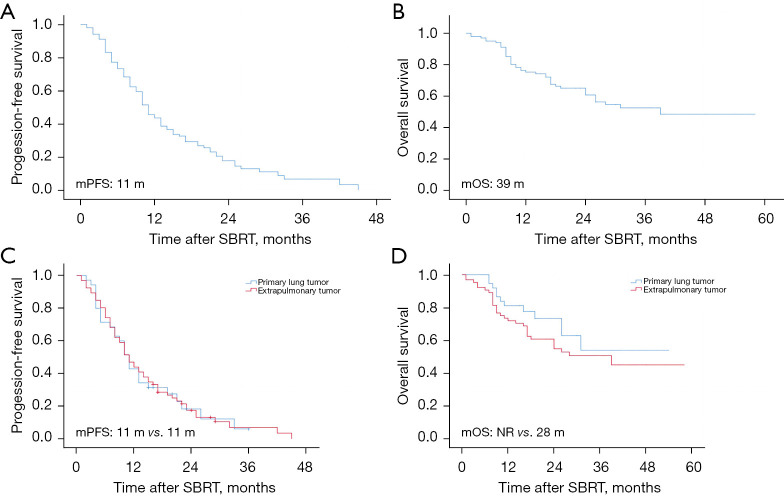
The average duration of patient follow-up was 22.4 months (range, 1–58 months). By the end of the follow-up period, 42 patients (41.6%) had died, and the overall 2-year LC rate for the entire population was 89.4%. A survival analysis was performed for all patients who were further stratified according to whether the primary tumor originated in the lung. For the entire cohort, the observed median PFS was 11 months, and the median OS was 39 months. The 2-year OS rates for patients with primary lung tumors and extrapulmonary tumors were 72.2% and 58.5%, respectively. While the 3-year OS rates for patients with primary lung tumors and extrapulmonary tumors were 5.6% and 10.8%, respectively. The subgroup analysis showed that median PFS remained consistent at 11 months regardless of whether the primary tumor was lung cancer. In relation to the median OS, the primary lung tumor group had not yet reached the median OS endpoint, while the extrapulmonary tumor group had a median OS of 28 months, as depicted in the Kaplan-Meier curves for both OS and PFS in Figure 1. All the analyses were conducted based on the number of patients, which totaled 101.
Figure 1.
Kaplan-Meier survival analysis. (A) PFS for all groups. (B) OS for all groups. (C) PFS comparing the primary lung tumor group and the extrapulmonary tumor group. (D) OS comparing the primary lung tumor group and the extrapulmonary tumor group. mPFS, median progression-free survival; mOS, median overall survival; m, months; NR, not reached; SBRT, stereotactic body radiotherapy.
Prognostic factors
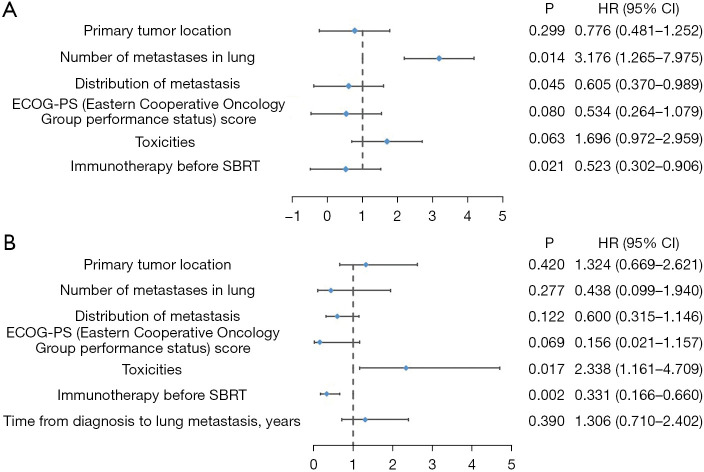
In relation to PFS, the univariate analyses indicated that the presence of lung metastases (P=0.02, HR =0.585) and the prior administration of immunotherapy before SBRT (P=0.02, HR =0.541) were significantly associated with the PFS outcomes. Conversely, a greater number of lung metastases (≥2) was significantly associated with decreased PFS (P=0.003, HR =3.749). In the multivariable analysis, only the presence of lung metastases (P=0.045, HR =0.605) and receiving immunotherapy before SBRT (P=0.02, HR =0.523) remained independent factors positively associated with better OS. In addition, patients with a higher number of lung metastases (≥2) had a poorer prognosis (P=0.01, HR =3.176) (Table 2).
Table 2. Prognostic factors in univariable and multivariable analyses for PFS (either patients or lesions).
| Prognostic factors affecting survival | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender (male/female) | 1.007 | 0.576–1.761 | 0.98 | – | – | – | |
| Age (</≥65 years) | 1.029 | 0.672–1.574 | 0.90 | – | – | – | |
| Primary tumor location (yes/no) | 0.991 | 0.633–1.551 | 0.97 | 0.776 | 0.481–1.252 | 0.3 | |
| Number of metastasis in the lung (≤/>2) | 3.749 | 1.576–8.916 | 0.003 | 3.176 | 1.265–7.975 | 0.01 | |
| Distribution of metastasis (yes/no) | 0.585 | 0.370–0.923 | 0.02 | 0.605 | 0.370–0.989 | 0.045 | |
| ECOG-PS score (>/≤1) | 0.568 | 0.283–1.140 | 0.11 | 0.534 | 0.264–1.079 | 0.08 | |
| BED10 (</≥100 Gy) | 0.888 | 0.620–1.271 | 0.52 | – | – | – | |
| Toxicities (no/yes) | 1.457 | 0.844–2.516 | 0.18 | 1.696 | 0.972–2.959 | 0.06 | |
| Immunotherapy before SBRT (no/yes) | 0.541 | 0.322–0.909 | 0.02 | 0.523 | 0.302–0.906 | 0.02 | |
| Time from diagnosis to lung metastasis (</≥2 years) | 1.471 | 0.947–2.284 | 0.09 | – | – | – | |
| Diameter of tumor (</≥15 mm) | 0.826 | 0.531–1.286 | 0.40 | – | – | – | |
PFS, progression-free survival; ECOG-PS, Eastern Cooperative Oncology Group performance status; BED, biological equivalent dose; SBRT, stereotactic body radiotherapy; HR, hazard ratio; CI, confidence interval.
In relation to OS, the univariate analyses showed that receiving immunotherapy before SBRT (P=0.008, HR =0.411) was significantly associated with better OS. Conversely, treatment-related toxicities were negatively and significantly associated with OS (P=0.03, HR =2.129). The multivariable analysis showed that the prior administration of immunotherapy before SBRT (P=0.002, HR =0.331) was an independent factor, positively correlated with improved OS, while the presence of toxicities (P=0.02, HR =2.338) indicated a poor prognosis (Table 3). Figure 2 shows the forest plots of the prognostic factors for PFS and OS.
Table 3. Prognostic factors in univariable and multivariable analyses for OS (either patients or lesions).
| Prognostic factors affecting survival | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender (male/female) | 1.008 | 0.466–2.177 | 0.99 | – | – | – | |
| Age (</≥65 years) | 1.347 | 0.740–2.451 | 0.33 | – | – | – | |
| Primary tumor location (yes/no) | 1.459 | 0.748–2.846 | 0.27 | 1.324 | 0.669–2.621 | 0.42 | |
| Number of metastases in the lung (≤/>2) | 0.713 | 0.172–2.952 | 0.64 | 0.438 | 0.099–1.940 | 0.28 | |
| Distribution of metastasis (yes/no) | 0.634 | 0.341–1.181 | 0.15 | 0.600 | 0.315–1.146 | 0.12 | |
| ECOG-PS score (>/≤1) | 0.166 | 0.023–1.209 | 0.08 | 0.156 | 0.021–1.157 | 0.07 | |
| BED10 (</≥100 Gy) | 0.615 | 0.303–1.247 | 0.18 | – | – | – | |
| Toxicities (no/yes) | 2.129 | 1.071–4.230 | 0.03 | 2.338 | 1.161–4.709 | 0.02 | |
| Immunotherapy before SBRT (no/yes) | 0.411 | 0.214–0.792 | 0.008 | 0.331 | 0.166–0.660 | 0.002 | |
| Time from diagnosis to lung metastasis (</≥2 years) | 1.202 | 0.660–2.190 | 0.55 | 1.306 | 0.710–2.402 | 0.39 | |
| Diameter of tumor (</≥15 mm) | 1.097 | 0.666–1.807 | 0.72 | – | – | – | |
OS, overall survival; ECOG-PS, Eastern Cooperative Oncology Group performance status; BED, biological equivalent dose; SBRT, stereotactic body radiotherapy; HR, hazard ratio; CI, confidence interval.
Figure 2.
Forest plots showing prognostic factors for PFS and OS. (A) Forest plot of prognostic factors associated with PFS. (B) Forest plot of prognostic factors associated with OS. SBRT, stereotactic body radiotherapy; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; OS, overall survival.
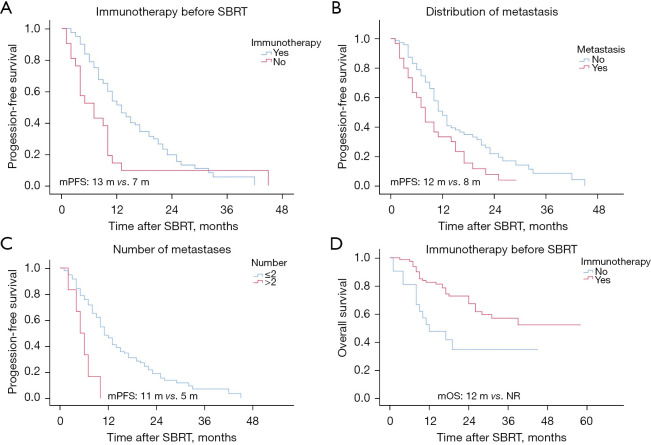
Finally, we conducted a subgroup survival analysis of the significant prognostic factors to understand their effect on PFS and OS. In the subgroup analyses, PFS differed significantly based on treatment and metastatic patterns. Patients treated with immunotherapy before SBRT had a median PFS of 13 months, while those who did not receive immunotherapy before SBRT had a median PFS of 7 months (Figure 3A). The analysis of the metastatic site showed that patients with lung metastases only had a median PFS of 12 months, while those with both lung and extrapulmonary metastases had a shorter median PFS of 8 months (Figure 3B). The analysis based on the number of lung metastases revealed that patients with ≤2 metastases (which includes 68 patients with 1 metastasis and 27 patients with 2 metastases) had a median PFS of 11 months, while those with >2 metastases (5 patients with 3 metastases and 1 patient with 4 metastases) had a median PFS of 5 months (Figure 3C). Additionally, the OS analysis showed that patients who received pre-SBRT immunotherapy did not reach the median OS endpoint, while those who did not receive immunotherapy before SBRT had a median OS of 12 months (Figure 3D).
Figure 3.
Subgroup analysis of prognostic factors using Kaplan-Meier curves for OS and PFS. (A) Effect of prior immunotherapy on PFS in all groups. (B) Effect of metastasis distribution on PFS in all groups. (C) Effect of metastasis number on PFS. (D) Effect of prior immunotherapy on OS in all groups. mPFS, median progression-free survival; mOS, median overall survival; m, months; NR, not reached; SBRT, stereotactic body radiotherapy.
Different dose-splitting modalities
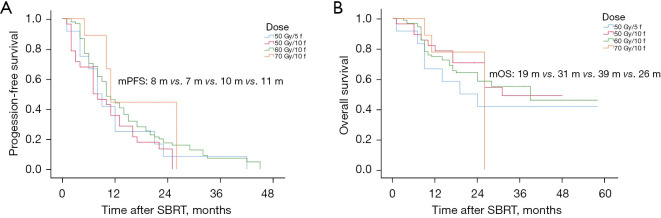
Various dose-fractionation schemes were used in treatment, including 50 Gy in 5–10 f and 60–70 Gy in 10 f. We determined the biological effects of each dose-fractionation regimen by converting the physical dose to the BED10. We then analyzed the effect of these modalities on OS and PFS. The 70 Gy/10 f modality yielded the longest median PFS at 11 months (Figure 4A). In relation to the median OS, the 60 Gy/10 f modality had the longest duration at 39 months (Figure 4B). We also examined the LC rates associated with each dose fractionation approach. The 60 Gy/10 f modality had the highest LC rate at 92.4% (Figure S2). As some patients had multiple pulmonary oligometastatic lesions, and different lesions received different radiation doses, all the analyses involving dose group data were based on the number of lung treatments, which totaled 141.
Figure 4.
Survival analysis based on different dose-splitting modes across all groups. (A) Effect of dose-splitting modes on PFS. (B) Effect of dose-splitting modes on OS. mPFS, median progression-free survival; mOS, median overall survival; m, months; f, fractions; SBRT, stereotactic body radiotherapy.
Toxicities
Pneumonitis was the most frequently observed toxicity, affecting 17 patients (16.8%). Among these, 11 patients (10.9%) had grade 2 pneumonitis, while six (5.9%) had grade 1 pneumonitis. Notably, there were no instances of acute grade 3 or 4 pneumonitis. Additionally, a single case of myelosuppression (0.9%) was recorded. There were no reports of chest wall pain, rib fractures, brachial plexopathy, or other significant adverse reactions, and no treatment-related fatalities occurred. A bar graph detailing the distribution of toxicities is shown in Figure S3A. In addition, we evaluated whether prior immunotherapy increased toxicity with SBRT. The results suggest that the immunotherapy group had slightly increased toxicities compared to the non-immunotherapy group (Figure S3B). An examination of the correlation between dose fractionation modality and toxicities revealed that the 50 Gy/5 f dose was associated with a peak toxicity of 41.7% (Figure S3C).
Discussion
In patients with oligometastatic disease, aggressive localized treatment modalities combined with systemic therapy may be appropriate. SBRT is emerging as the optimal non-surgical method for the treatment of oligometastases, as it offers precise dose delivery while sparing normal tissues from radiation (13,14). Notably, SBRT has shown significant antitumor effects, improving both local and distant control and providing survival benefits in various solid tumors (15,16).
In our analysis of patient and tumor characteristics, we observed a higher prevalence of pulmonary oligometastases in middle-aged and older men, which might be related to their greater exposure to smoking and other environmental risks. In addition, most of the primary tumors originated from non-pulmonary organs, which underscores the frequency of pulmonary oligometastases in individuals with cancers that originate outside the lung.
The principal aim of our research was to examine the survival benefits of SBRT for patients with pulmonary oligometastases. Our findings showed that in the primary lung tumor cohort, the LC rates for SBRT were as high as approximately 90% at both one and 2 years of follow-up. These results are consistent with previous studies that have reported similar success in LC rates and prolonged survival among patients with limited pulmonary metastases (17,18). Similarly, in the extrapulmonary tumor cohort, the 1- and 2-year LC rates were also remarkable, both exceeding 80%. This high LC rate might be attributed to our definition of LC, which was considered effective if there was no progression at the irradiated site before death.
In the analysis of the effects of different fractionation doses on 2-year LC rates, except for the 50 Gy/10 f plan, which had LC rates below 80%, the rest were above 80%, correlating with the BED. To achieve effective LC, a BED10 of at least 96 Gy was required. Both groups showed comparable median PFS. In terms of the median OS, the primary lung tumor group outperformed the extrapulmonary tumor group. This might be due to the differential efficacy of SBRT in different tumor origins, or the fact that OS determinants include both local and systemic treatments. In conclusion, while the short-term efficacy of SBRT appears to be consistent regardless of tumor type and systemic therapy (19), prolonged survival requires systemic therapy after SBRT, especially in patients with an increased risk of recurrence, or those with extrapulmonary metastases (20).
In both the univariate and multivariate analyses, we identified prognostic factors that significantly affected both PFS and OS. Notably, undergoing immunotherapy before SBRT was found to be an independent factor that significantly enhanced both PFS and OS. The interplay between SBRT and immunotherapy heralds a new era in cancer treatment, leveraging the immunomodulatory effects of radiation and the systemic efficacy of immune checkpoint inhibitors (ICIs). By inducing immunogenic cell death, SBRT not only enhances antigen release and dendritic cell activation, but also enhances Major histocompatibility complex (MHC) class I expression, which increases tumor-cell recognition by cluster of differentiation 8-positive (CD8+) T cells (21,22). Additionally, it can also remodel the tumor microenvironment (23,24). At the same time, ICIs neutralize the potential immunosuppressive effects of radiotherapy and sensitize the responsiveness of tumors to such treatments through the promotion of tumor-cell ferroptosis (25-27). Thus, an increasing number of researchers are investigating the combined efficacy of both treatments.
The synergy of integrating ICIs with SBRT to induce an abscopal response (a therapeutic effect outside the irradiated region) has attracted considerable interest. The efficacy of this combination has been observed (28-30). However, the optimal sequence and scheduling (whether concurrent or sequential) for this combination is unclear. Ideally, the chosen regimen should effectively strengthen immune responses against tumors. This recommendation is supported by the scant evidence currently available (31-33). However, the efficacy of the combination remains relatively consistent regardless of whether SBRT precedes or follows ICIs. Nevertheless, the different toxicity profiles warrant further investigation (15). In addition, the number of lung metastases and the occurrence of metastases outside the lungs have surfaced as crucial prognostic factors, underscoring the necessity of aggressive treatment strategies in patients bearing a substantial metastatic load.
Presently, there is no consensus as to the optimal SBRT dosage for the treatment of pulmonary oligometastases. Ricco et al. reported that lung metastasis patients receiving SBRT doses of BED10 ≥100 Gy showed enhanced LC, achieving a 3-year LC rate of 77.1%, while those treated with doses of BED10 <100 Gy achieved a rate of only 45% (34). Sharma et al. confirmed that achieving a BED10 of at least 100 Gy significantly enhanced LC and OS (35). Similarly, other research indicated that a BED10 of ≥115 Gy at the isocenter is closely linked to both higher LC rates and better outcomes in recurrence-free survival and OS (36). In our analysis comparing the effect of different dose fractionation schemes on PFS, OS and LC, we found that 60 Gy/10 f yielded the longest median OS and the highest LC rate. In addition, 70 Gy/10 f yielded the highest median PFS, likely due to its increased BED10.
Consistent with previous studies, SBRT was well-tolerated regardless of the treatment site (37,38). The predominant adverse events included acute toxicities (grade 1–2), among which pneumonitis was the predominant condition, occurring in 16.8% of the patient cohort. Notably, the absence of documented chronic toxicities over the average monitoring duration of 22.4 months shows the favorable toxicity profile of SBRT. However, in our analysis, toxicities were indicative of a worse prognosis when considering OS in multivariable assessments. In the subgroup analysis focused on prior immunotherapy before SBRT, we observed that the combined immunotherapy group did not experience a significant increase in toxicities. This finding may prove critical in guiding immunotherapy and SBRT combinations in clinical practice.
We also investigated the effect of different dose fractionation regimens on toxicities. The results showed that the 60 Gy/10 f regime, which was administered to the majority of patients, had relatively lower toxicities, while the 50 Gy/5 f regime, though received by a smaller proportion of patients (8.5%), was associated with higher toxicities. Due to the limited number of patients receiving the 50 Gy/5 f regimen, the comparison between these groups should be interpreted with caution. This suggests that a higher single dose may be correlated with increased toxicity, but further investigation with larger sample sizes is needed to confirm these findings. Therefore, the challenge remains to select the optimal SBRT dose to both maximize efficacy and minimize adverse effects (39). While our study indicates that 60 Gy/10 f may provide a reasonable balance between efficacy and tolerability, more comprehensive prospective trials are required to validate these observations.
This research showed that SBRT fractionation schemes are effective in managing pulmonary oligometastases and highlights the advantages of incorporating pre-SBRT immunotherapy. It showed that the 60 Gy in 10 f regimen was optimal for maximizing therapeutic efficacy while minimizing toxicity. Our comparative analysis with other research, as detailed in Table 4 (40-42), revealed that while our LC rates were relatively lower, mainly due to patient deaths from distant metastases, we reported higher overall PFS and OS. This indicates that effective management of systemic therapies, in conjunction with SBRT, can substantially prolong patient survival, especially in those with smaller tumor burdens. The integration of immunotherapy into the treatment protocol appears to further enhance survival outcomes across the patient population.
Table 4. Comparative table of SBRT outcomes for pulmonary oligometastases.
| Studies | Year of study | Study design | Patient cohort | PFS (months) | OS (months) | Fractionation regimen | LC rate | Toxicities | Key prognostic indicators |
|---|---|---|---|---|---|---|---|---|---|
| Sharma et al. (40) | 2019 | Retrospective | 206 patients | – | 33 | 51–60 Gy/3 f | 63% (2-year) |
– | Synchronous metastases, colorectal primary cancer |
| Niibe et al. (41) | 2015 | Retrospective | 34 patients | – | 20 | BED10 ≥75 Gy | 79.1% (2-year) |
No severe toxicities | – |
| Yamamoto et al. (42) | 2020 | Multicentre retrospective | 1,378 patients (1,547 lesions) | – | 60.3% (3-year) | BED10 ≥75 Gy | 86% (3-year) |
– | Maximum tumor diameter, dose calculation algorithm, overall treatment time, and colorectal primary origin |
| This study | 2022 | Retrospective | 101 patients (141 lesions) | 11 | 39 | 50–70 Gy/5–10 f | 89.4% (2-year) |
Grade 2 pneumonitis (10.9%) | Pre-treatment with immunotherapy |
SBRT, stereotactic body radiotherapy; PFS, progression-free survival; OS, overall survival; LC, local control; BED, biological equivalent dose; f, fractions.
A previous study explored the role of microRNA expression in elucidating the molecular basis of oligometastatic spread (43). MicroRNA expression may serve as guide to identify patients in the oligometastatic or polymetastatic phases, and to develop metastasis-targeted therapeutic strategies appropriate for the different stages of metastasis (44). In the foreseeable future, understanding tumor biology will be critical in identifying oligometastatic patients who may benefit from either SBRT alone or combined therapies (40). When juxtaposed with the broader literature, these findings underscore the complexity of fine-tuning SBRT protocols, and highlight the necessity for individualized treatment plans that consider tumor characteristics, patient status, and strategically incorporate combination therapies, paving the way for vital avenues for future research (45-48).
While our study demonstrates that the 60 Gy in 10 f regimen offers promising results in terms of OS and LC, several limitations must be acknowledged. First, this was a retrospective study, and the dose schedules were not randomized but rather tailored to individual patients based on factors such as lesion size, anatomical location, tumor radiobiology, and the overall treatment plan. This inherent selection bias may have influenced the observed outcomes, as patients with different tumor characteristics likely received different dose regimens. As a result, while the data suggest that the 60 Gy in 10 f regimen was associated with better outcomes, these results should not be interpreted as universally applicable to all patients with pulmonary oligometastases. Moreover, the dose-response relationship, particularly for LC, could be confounded by this bias. Future prospective randomized controlled trials are required to eliminate selection bias and rigorously evaluate the optimal dose-fractionation strategy. Additionally, the generalizability of these results to all patient populations remains limited, and further studies are necessary to validate these findings across different clinical settings.
Conclusions
Our research demonstrated that SBRT at a dose of 60 Gy in 10 f is an effective treatment for managing pulmonary oligometastases, especially when combined with immunotherapy. This approach not only led to significant improvements in OS and PFS but also exhibited a favorable toxicity profile compared to other dosing regimens. Specifically, the 60 Gy in 10 f regimen provided superior LC, with relatively lower toxicity levels than the 50 Gy in 5 f regimen, making it a promising option for clinical use. However, it is important to acknowledge that the individualized dose selection, based on tumor characteristics and patient-specific factors, introduces an inherent selection bias. As a result, the superior outcomes observed with the 60 Gy in 10 f regimen should be interpreted with caution. This study’s retrospective design and the heterogeneity in patient cohorts and dose-fractionation protocols further limit the generalizability of these findings. Future studies must be meticulously planned as prospective trials to determine the optimal sequencing and timing of immunotherapy and SBRT. Randomized controlled trials or matched-pair analyses should serve as a robust framework for these investigations. Combining relevant biomarkers and radiomics could further refine patient selection and personalize treatment strategies. Additionally, controlled clinical studies into the potential abscopal effect may open new therapeutic avenues, particularly for patients with refractory pulmonary metastases. These efforts will build upon the foundation of our results, validating and expanding the observed therapeutic synergy between SBRT and immunotherapy, ultimately laying the groundwork for developing more effective and tailored cancer treatment approaches. In conclusion, while our results indicate that 60 Gy in 10 f provides a reasonable balance between efficacy and safety, the potential impact of confounding variables necessitates further investigation. Prospective studies should also explore the synergistic effects of SBRT combined with immunotherapy to establish more robust guidelines for clinical practice.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The study received support from the Zhejiang Provincial Health Commission’s Medical Science and Technology Project (No. 2018KY728) and Ningbo Natural Science Foundation Project (No. 2019A610227).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Affiliated Lihuili Hospital, Ningbo University’s Institutional Medical Ethics Committee approved this retrospective study (reference number 2022-330), and the requirement for informed consent was waived due to the retrospective nature of the study
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1624/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1624/coif). S.K.J. serves as research consultant for Merck & Co, Inc., reviewer for IMX medical, adjudication committee of Syntactx, and consultant for Novocure, Radialogica, Astra Zeneca, and Beigene, participates on the advisory board of Advarra (unrelated to study), and receives grants from Merck& Co, Inc., Beigene, Guardant, outside the submitted study. The other authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1624/dss
References
- 1.Mangiameli G, Cioffi U, Alloisio M, et al. Lung Metastases: Current Surgical Indications and New Perspectives. Front Surg 2022;9:884915. 10.3389/fsurg.2022.884915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pretell-Mazzini J, Seldon CS, D'Amato G, et al. Musculoskeletal Metastasis From Soft-tissue Sarcomas: A Review of the Literature. J Am Acad Orthop Surg 2022;30:493-503. 10.5435/JAAOS-D-21-00944 [DOI] [PubMed] [Google Scholar]
- 3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 4.Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 2020;148:157-66. 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Beckham TH, Yang TJ, Gomez D, et al. Metastasis-directed therapy for oligometastasis and beyond. Br J Cancer 2021;124:136-41. 10.1038/s41416-020-01128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De la Pinta C. SBRT in non-spine bone metastases: a literature review. Med Oncol 2020;37:119. 10.1007/s12032-020-01442-1 [DOI] [PubMed] [Google Scholar]
- 7.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. 10.1016/j.ijrobp.2009.07.1751 [DOI] [PubMed] [Google Scholar]
- 8.Capone L, Antonia Allegretta S, Bianciardi F, et al. The impact of a mono-institutional experience in lung metastases treated with stereotactic body radiation therapy (SBRT): a retrospective analysis. Ther Radiol Oncol 2023;7:13. 10.21037/tro-21-45 [DOI] [Google Scholar]
- 9.Begum H, Swaminath A, Lee Y, et al. The histologic effects of neoadjuvant stereotactic body radiation therapy (SBRT) followed by pulmonary metastasectomy-rationale and protocol design for the Post SBRT Pulmonary Metastasectomy (PSPM) trial. Transl Cancer Res 2022;11:918-27. 10.21037/tcr-22-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashrafizadeh M, Farhood B, Eleojo Musa A, et al. Abscopal effect in radioimmunotherapy. Int Immunopharmacol 2020;85:106663. 10.1016/j.intimp.2020.106663 [DOI] [PubMed] [Google Scholar]
- 11.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 12.Piao MN, Xie J, Jin MM, et al. Efficacy and prognostic factors of stereotactic body radiotherapy combined with immunotherapy for pulmonary oligometastases: a preliminary retrospective cohort study. Transl Lung Cancer Res 2024;13:1950-63. 10.21037/tlcr-24-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada Y, Hashimoto M. Modern evidence and future prospects of external body radiation therapy for lung oligometastases of breast cancer. Transl Cancer Res 2020;9:5077-86. 10.21037/tcr.2020.02.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano MT, Biswas T, Simone CB, 2nd, et al. Oligometastases: history of a hypothesis. Ann Palliat Med 2021;10:5923-30. 10.21037/apm.2020.03.31 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Gao M, Huang Z, et al. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol 2020;13:105. 10.1186/s13045-020-00940-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayinger M, Kotecha R, Sahgal A, et al. Stereotactic Body Radiotherapy for Lung Oligo-metastases: Systematic Review and International Stereotactic Radiosurgery Society Practice Guidelines. Lung Cancer 2023;182:107284. 10.1016/j.lungcan.2023.107284 [DOI] [PubMed] [Google Scholar]
- 17.Pasalic D, Lu Y, Betancourt-Cuellar SL, et al. Stereotactic ablative radiation therapy for pulmonary metastases: Improving overall survival and identifying subgroups at high risk of local failure. Radiother Oncol 2020;145:178-85. 10.1016/j.radonc.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 18.Allen AJ, Labella DA, Kowalchuk RO, et al. Effect of histology on stereotactic body radiotherapy for non-small cell lung cancer oligometastatic pulmonary lesions. Transl Lung Cancer Res 2023;12:66-78. 10.21037/tlcr-22-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu MX, Carvalho C, Milan-Chhatrisha B, et al. Stereotactic Body Radiotherapy for Management of Pulmonary Oligometastases in Stage IV Colorectal Cancer: A Perspective. Clin Colorectal Cancer 2023;22:402-10. 10.1016/j.clcc.2023.09.001 [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Zhang S, Sun X, et al. Narrative review of stereotactic body radiation therapy combined with tyrosine kinase inhibitors for oligometastatic EGFR-mutated non-small cell lung cancer: present and future developments. Transl Lung Cancer Res 2024;13:1383-95. 10.21037/tlcr-24-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014;3:e28518. 10.4161/onci.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol 2016;1:EAAG1266. 10.1126/sciimmunol.aag1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Chi A. Combining stereotactic body radiotherapy with immunotherapy in stage IV non-small cell lung cancer. Front Oncol 2023;13:1211815. 10.3389/fonc.2023.1211815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. 10.1038/s41591-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang X, Green MD, Wang W, et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov 2019;9:1673-85. 10.1158/2159-8290.CD-19-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turchan WT, Chmura SJ. The role of immunotherapy in combination with oligometastasis-directed therapy: a narrative review. Ann Palliat Med 2021;10:6028-44. 10.21037/apm-20-1528 [DOI] [PubMed] [Google Scholar]
- 29.Cushman TR, Gomez D, Kumar R, et al. Combining radiation plus immunotherapy to improve systemic immune response. J Thorac Dis 2018;10:S468-79. 10.21037/jtd.2018.01.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez Plá M, Dualde Beltrán D, Ferrer Albiach E. Immune Checkpoints Inhibitors and SRS/SBRT Synergy in Metastatic Non-Small-Cell Lung Cancer and Melanoma: A Systematic Review. Int J Mol Sci 2021;22:11621. 10.3390/ijms222111621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434-41. 10.1093/annonc/mdv622 [DOI] [PubMed] [Google Scholar]
- 32.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 34.Ricco A, Davis J, Rate W, et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry's experience. Radiat Oncol 2017;12:35. 10.1186/s13014-017-0773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A, Baker S, Duijm M, et al. Prognostic factors for local control and survival for inoperable pulmonary colorectal oligometastases treated with stereotactic body radiotherapy. Radiother Oncol 2020;144:23-9. 10.1016/j.radonc.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Niibe Y, Matsumoto Y, et al. Analyses of local control and survival after stereotactic body radiotherapy for pulmonary oligometastases from colorectal adenocarcinoma. J Radiat Res 2020;61:935-44. 10.1093/jrr/rraa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes WA, Robin TP, Nath SK, et al. Stereotactic Body Radiation Therapy (SBRT) for Lung Metastases. In: Trifiletti DM, Chao ST, Sahgal A, et al. editors. Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy: A Comprehensive Guide. Cham: Springer International Publishing; 2019:247-64. [Google Scholar]
- 38.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. 10.1200/JCO.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 39.Kimura T, Fujiwara T, Kameoka T, et al. Stereotactic body radiation therapy for metastatic lung metastases. Jpn J Radiol 2022;40:995-1005. 10.1007/s11604-022-01323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma A, Duijm M, Oomen-de Hoop E, et al. Survival and prognostic factors of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol 2019;58:74-80. 10.1080/0284186X.2018.1521986 [DOI] [PubMed] [Google Scholar]
- 41.Niibe Y, Yamashita H, Sekiguchi K, et al. Stereotactic Body Radiotherapy Results for Pulmonary Oligometastases: A Two-Institution Collaborative Investigation. Anticancer Res 2015;35:4903-8. [PubMed] [Google Scholar]
- 42.Yamamoto T, Niibe Y, Aoki M, et al. Analyses of the local control of pulmonary Oligometastases after stereotactic body radiotherapy and the impact of local control on survival. BMC Cancer 2020;20:997. 10.1186/s12885-020-07514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One 2011;6:e28650. 10.1371/journal.pone.0028650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Gao S, Li T, et al. Exploring the microRNA profiles as potential diagnostic probes for oligo- and polymetastatic prognosis of lung metastasis(es) patients. Medicine (Baltimore) 2018;97:e10958. 10.1097/MD.0000000000010958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegner RE, Abel S, Hasan S, et al. Stereotactic Body Radiotherapy (SBRT) for Oligometastatic Lung Nodules: A Single Institution Series. Front Oncol 2019;9:334. 10.3389/fonc.2019.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee TH, Kim HJ, Kim JH, et al. Treatment outcomes of stereotactic body radiation therapy for pulmonary metastasis from sarcoma: a multicenter, retrospective study. Radiat Oncol 2023;18:68. 10.1186/s13014-023-02255-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berkovic P, Gulyban A, Defraene G, et al. Stereotactic robotic body radiotherapy for patients with oligorecurrent pulmonary metastases. BMC Cancer 2020;20:402. 10.1186/s12885-020-06906-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutkin PM, Gore E, Charlson J, et al. Stereotactic body radiotherapy for metastatic sarcoma to the lung: adding to the arsenal of local therapy. Radiat Oncol 2023;18:42. 10.1186/s13014-023-02226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]