Abstract
Purpose
Extranodal NK/T-cell lymphoma (ENKTCL) is rare in the Western Hemisphere and is commonly treated with combined modality therapy (CMT).
Methods and Materials
We retrospectively reviewed 35 patients treated with Ann Arbor stage I/II ENKTCL between 1994 and 2015 at a large academic cancer center in the United States.
Results
With 11.6 years median follow-up, median overall survival and progression-free survival were 13.5 and 7.5 years, respectively. Eighteen (51%) patients experienced disease relapse, with 5 regional nodal relapses, of which 2 experienced combined regional and distant relapses. All 5 regional nodal relapses occurred exclusively among patients not treated with elective nodal irradiation (ENI). ENI was associated with improved progression-free survival (hazard ratio [HR], 0.21; 95% CI, 0.09-0.52; P = .018) without significant association with OS (HR, 0.33; 95% CI, 0.11-0.94; P = .11). There was a trend toward improved local control with radiation dose to the primary tumor ≥50 Gy (HR, 0.29; 95% CI, 0.08-1.08; P = .098).
Conclusions
In this Western Hemisphere cohort of early-stage ENKTCL patients treated with CMT, ENI may have a potential clinical benefit, particularly in patients who are treated with non–asparaginase-containing CMT, such as in patients treated with radiation alone, patients treated with less intensive chemotherapy concurrently, or patients who are unable to tolerate intensive chemotherapy.
Introduction
Extranodal NK/T-cell lymphoma (ENKTCL), historically specified as nasal type, is a rare form of non-Hodgkin lymphoma observed primarily in Asian populations. ENKTCL is by definition associated with Epstein-Barr virus infection and is generally found in the upper aerodigestive tract, usually within the nasal cavity.1,2 Outcomes and optimal treatment strategies for ENKTCL patients remain opaque, with limited literature available3,4 for both patients from Asia and the Western Hemisphere. While anthracycline-based chemotherapy (ChT) regimens such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) are the mainstay of therapy for other aggressive lymphomas, outcomes with this approach in ENKTCL patients are poor, partially because of elevated tumor cell expression of the multidrug resistant O-glycoprotein, which mediates anthracycline resistance.5, 6, 7 Therefore, non–anthracycline-based ChT regimens have been used for limited and advanced stage ENKTCL with improved outcomes.8, 9, 10, 11 For patients with localized disease, radiation therapy (RT) is integral for cure and is often coupled with systemic therapy either concurrently or sequentially to maximize local and distant control.6, 7, 8, 9, 10, 11, 12, 13, 14 Combined modality therapy (CMT), including both RT and ChT, often platinum or L-asparaginase-based, is commonly used in the treatment of ENKTCL patients, although diverse regimens and treatment sequences have been described in the literature.15
Given the rarity of ENKTCL, randomized trials are challenging and treatment strategies are guided by retrospective studies. Current guidelines recommend involved site radiation therapy (ISRT) approaches to RT field design, where the RT field is primarily limited to sites of grossly identifiable disease and adjacent areas at risk for local microscopic disease spread from the primary tumor.16 Prophylactic nodal irradiation for nasal ENKTCL, in particular for patients without nodal involvement, is not currently recommended based on retrospective analyses of limited stage ENKTCL patients in Asian populations showing no local control (LC) or survival benefit but potential for increased toxicity secondary to large RT volumes.16, 17, 18 However, data regarding the potential benefit of elective nodal irradiation (ENI) among Western Hemisphere patients are lacking.
Here, we report a series of Western Hemisphere patients with early-stage (Ann Arbor stage I and II) ENKTCL treated mostly with non–asparaginase-containing ChT, specifically examining patterns of failure and predictors of oncologic outcomes. We examined rates of both local and regional nodal disease relapse. Given that current guidelines generally do not recommend ENI,16,18,19 we sought to determine whether ENI impacted outcomes in this Western Hemisphere ENKTCL cohort.
Methods and Materials
Patient selection
With institutional review board approval, a retrospective study was performed to identify patients with Ann Arbor stage I or II ENKTCL seen at our institution between January 1, 1994 and December 31, 2015. Beginning in 2015, early-stage ENKTCL patients were treated in a clinical trial evaluating RT and concurrent ChT (clinicaltrials.gov NCT02106988). Criteria for inclusion included the availability of pathologic material for confirmation of diagnosis according to standard criteria at the time, and all cases met the current World Health Organization diagnostic criteria.
Disease staging and evaluation
Patients were staged according to the Ann Arbor system. Radiographic staging evaluations usually included fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT), gallium-67 nuclear scan and/or computed tomography (CT) of the neck, chest, abdomen, and pelvis (generally with iodinated intravenous contrast), as well as magnetic resonance imaging (MRI).
Treatment strategies
Details of RT were extracted from the electronic medical records. Radiation was delivered to involved sites with the goal of targeting gross disease when RT was used as initial therapy or pre-ChT to sites of disease when RT was administered sequentially, with appropriate set-up margin used, according to guidelines from the International Lymphoma Radiation Oncology Group.19 Photons were generally used for therapy, either with traditional 3-dimensional conformal or contemporary RT with intensity modulated radiation therapy or volumetric modulated arc therapy. When ENI was used, coverage was based on expected patterns of drainage of the primary tumor and generally targeted the bilateral submandibular and cervical basins, including levels IB, II, III, IV, and V.
Systemic therapy was administered with CHOP, cyclophosphamide, vincristine, doxorubicin and dexamethasone (CVAD), hyper-CVAD (HCVAD), or dexamethasone, etoposide, ifosfamide, and carboplatin (DeVIC)-based regimens. For patients that received anthracyline-based regimens (CHOP, CVAD, hyper-CVAD), treatment was generally given sequentially with RT administered after systemic therapy. DeVIC-based regimens were administered concurrently with RT.
Response assessment
Treatment response was assessed on completion of all therapy with CT, MRI, or PET-CT according to response criteria for non-Hodgkin lymphoma.20 Following completion of therapy, patients were seen in follow-up approximately every 3 to 6 months for 2 years and at physician discretion thereafter.
Statistical analyses
Disease-related outcomes including overall survival (OS), progression-free survival (PFS), LC, and regional control were ascertained. All disease endpoints were calculated from the date of pathologic diagnosis. OS was defined as death from any cause. PFS was defined as disease progression, relapse, or death from any cause. LC was defined as the absence of disease relapse or progression within an initial site of disease involvement (either primary tumor or nodal site if involved at diagnosis). Regional control was defined as the absence of lymphoma relapse in a nodal basin that was not involved at diagnosis but a site of lymph node drainage of the primary tumor. Disease-related outcomes were estimated using Kaplan-Meier method.21 Median follow-up was calculated using reverse Kaplan-Meier method. Log-rank tests were used for univariate analyses of disease-related outcomes by subgroups. Statistical analyses were performed using the SPSS statistical package (version 24.0; IBM) and GraphPad Prism (version 10.0; GraphPad).
Results
Cohort characteristics
Thirty-five patients who fulfilled World Health Organization criteria for ENKTCL were Ann Arbor stage I and II and completed radiation treatment were identified. The demographic, disease, and treatment-related characteristics of this group are described in Table 1. The median age of the cohort was 45 years (range, 18-80 years) and 11 (31%) were female. Most patients had stage I disease (n = 27, 77%) and 83% received CMT (n = 29). At baseline prior to treatment, 22 patients had PET scans, 10 had baseline gallium-67 citrate nuclear scans, 1 patient had baseline CT and MRI, and 2 patients’ baseline imaging scan type(s) are unknown.
Table 1.
Patient and treatment characteristics
| Patient and treatment characteristics | Value or No. (%) |
|---|---|
| Age, y, median (range) | 45 (18-80) |
| Female | 11 (31%) |
| Primary tumor location | |
| Nasal | 32 (91%) |
| Other upper aerodigestive tract | 3 (9%) |
| Stage | |
| I | 27 (77%) |
| II | 8 (23%) |
| Baseline staging imaging modality | |
| PET scan | 22 (63%) |
| Gallium-67 scan | 10 (29%) |
| MRI scan | 16 (46%) |
| CT scan | 30 (86%) |
| Initial treatment strategy | |
| CMT | 29 (83%) |
| RT alone | 6 (17%) |
| If CMT, sequence of treatment | |
| ChT followed by RT | 16 (55%) |
| RT followed by ChT | 10 (34%) |
| Concurrent ChT and RT | 3 (10%) |
| RT total dose to primary | |
| Median, Gy (range) | 50 (39.6-60) |
| ENI delivered | 8 (23%) |
| Chemotherapy regimen | |
| Doxorubicin-based | 26 (74%) |
| Non-doxorubicin-based | 3 (9%) |
| No ChT | 6 (27%) |
| Consolidative SCT | 3 (8%) |
Abbreviations: ChT = chemotherapy; CMT = combined modality therapy; CT = computed tomography; ENI = elective nodal irradiation; MRI = magnetic resonance imaging; PET = positron emission tomography; RT = radiation therapy; SCT = stem cell transplantation.
Among patients receiving CMT, RT was administered either sequentially after ChT (n = 15, 43%), before ChT (n = 11, 31%), concurrent with systemic treatment (n = 3, 9%,) or alone (n = 6, 17%). The median radiation dose to the primary tumor was 50 Gy (range, 39.6-60 Gy). Most patients who received ChT received a doxorubicin-based regimen (n = 26, 74%). One patient was treated with 5 cycles of cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and L-asparaginase. Three (9%) patients received a consolidative stem cell transplant (SCT) according to physician preference.
Elective nodal irradiation
Eight (22%) patients treated with RT received ENI, including 5 patients with stage I ENKTCL and 3 patients with stage II disease. ENI targeted the nodal drainage regions of the primary tumor, including the bilateral submandibular and cervical basins, including levels IB, II, III, IV and V. For all 3 stage II patients who received ENI, there was evidence of nodal disease involving unilateral levels IB and II at diagnosis. All 3 of these patients received RT to the initial nodal sites of gross disease in addition to prophylactic RT to ipsilateral levels III-V and contralateral levels IB-V. There were no differences noted for patients who did and did not receive ENI in age, sex, stage, initial treatment strategy, RT dose to primary, and ChT regimen (all P > .05). Most (6/8) patients treated with ENI were treated with CMT.
Treatment strategies and outcomes
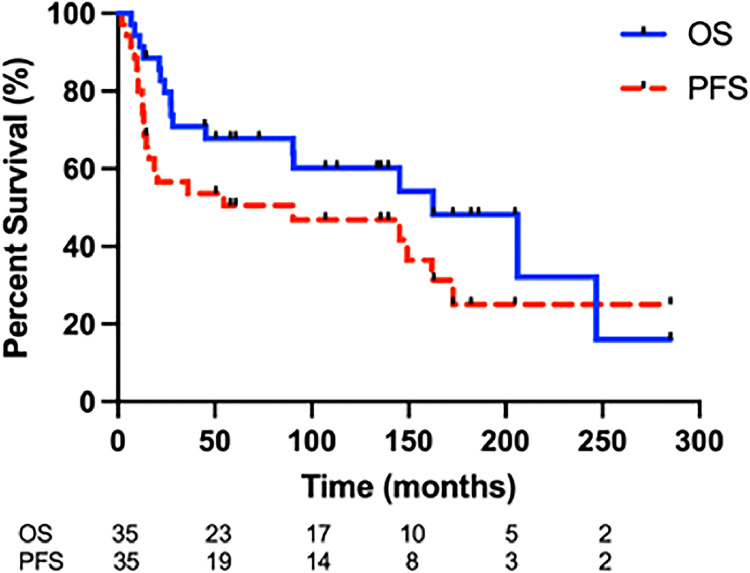
The median follow-up for all patients was 11.6 years (range, 6.7-285 months). Median OS was 13.5 years (Fig. 1). Median PFS was 7.5 years (Fig. 1). Eighteen (51%) patients had disease relapse: 7 with isolated local relapse at the site of the primary tumor; 3 with isolated regional lymph node relapse; 5 with isolated distant relapse; 1 with concurrent local and distant relapse; and 2 with concurrent regional and distant relapse. All 5 regional nodal relapses occurred in patients who did not receive ENI. Therefore, a 18% absolute regional nodal failure rate was observed for non-ENI patients. At baseline, 4 of these patients had PET staging, and 1 had gallium-67 nuclear scan. ENI was associated with improved PFS (hazard ratio [HR], 0.21; 95% CI, 0.09-0.52; P = .018) (Fig. 2). One nodal relapse occurred in a patient with stage II disease at diagnosis (Fig. 3). The remaining 4 of 5 nodal relapses occurred among patients with stage I disease at diagnosis. Four of 5 patients with nodal relapse had nasal ENKTCL with disease located in the nasal cavity and/or paranasal sinuses. One patient had disease originating in the left buccal mucosa upper aerodigestive tract. In addition, all of the nodal relapses occurred in draining lymph nodes relative to the primary tumor, including levels IB-V. Three of 5 patients with nodal relapse were treated with CMT with HCVAD (n = 1), DeVIC (n = 1), and CHOP-based (n = 1) ChT. ENI was not significantly associated with OS (HR, 0.33; 95% CI, 0.11-0.94; P = .11).
Figure 1.
Progression-free survival (PFS) and overall survival (OS) for all patients with early-stage extranodal natural killer/T-cell lymphoma.
Figure 2.
Progression-free survival (A) and overall survival (B) for all patients with early-stage extranodal natural killer/T-cell lymphoma who did and did not receive elective nodal irradiation (ENI).
Figure 3.
Relapsed nodal disease in a patient with stage IIAE ENKTCL that was not treated with elective nodal irradiation. PET-CT images and the corresponding RT plan of a 65-year-old patient with ENKTCL involving the entire left nasal cavity (anterior and posterior involvement; a) and a 2.5 cm left level 1B submandibular lymph node without other nodal involvement identified (b, c). No other nodal sites of involvement were detected by PET-CT, MRI, or contrasted CT. The patient was planned to receive sequential therapy with upfront RT alone followed by systemic therapy. The patient was treated to 53.2 Gy in 28 fractions with volumetric modulated arc therapy to the sites of gross disease (contoured in red; d, e) and 47.6 Gy in 28 fractions to areas at risk for microscopic extension (contoured in blue; d, e). The patient did not receive elective nodal radiation to draining nodal basins. At the 3-month follow-up, the original sites of gross disease in the nasal cavity (g) and left submandibular region resolved (h); however, recurrent disease in other draining nodal basins including the right 1B submandibular region (h), the left facial nodal region (i), and the right retropharyngeal region (not shown) were identified despite not being present at diagnosis (b, c). This patient developed a second malignancy known as chondroblastic osteosarcoma within the left nasal cavity 8.3 years after completion of radiation.
Abbreviations: CT = computed tomography; ENKTCL = extranodal natural killer/T-cell lymphoma; MRI = magnetic resonance imaging; PET = positron emission tomography; RT = radiation therapy.
Predictors of LC
Univariate analyses were performed to identify factors associated with LC. Treatment with RT dose to the primary tumor ≥50 Gy (n = 19) trended toward improved LC (HR, 0.29; 95% CI, 0.08-1.08; P = .098). Multivariable analysis was not performed owing to the limited number of local relapse events.
Salvage therapy for regional nodal relapse
There were 5 patients who developed a regional nodal relapse. Of these 5 patients, 4 patients had stage I disease without nodal involvement and were treated without ENI. One patient had stage II disease with involvement of a submandibular node and was treated with involved site radiation to the involved node without ENI, followed by ChT. Initial salvage treatment for 1 patient involved RT followed by Epstein-Barr virus-targeted cytotoxic T-lymphocyte therapy with consolidative allogeneic SCT. Another patient was initially salvaged with an etoposide, solumedrol, high-dose cytarabine, and cisplatin regimen followed by autologous SCT. The remaining 3 patients received asparagine-based SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) regimen of which 2 patients also received autologous SCT. Three patients with isolated regional relapse had successful salvage therapy and were alive without disease at last follow-up; 2 patients whose first relapse was both regional and distant had progression of disease despite salvage therapy and subsequently died because of disease progression.
Discussion
Early-stage ENKTCL is an aggressive disease with similar outcomes in patients from Asia and the Western Hemisphere.3,22 In this group of ENKTCL patients treated at a single US institution with RT and primarily non–asparaginase-based systemic therapy, the median PFS and OS were 7.5 and 13.5 years, respectively. Approximately one-quarter of relapses occurred in nodal basins that were primary drainage regions of the primary tumor site. As such, ENI was associated with a significant improvement in PFS. The 5 nodal relapses occurred exclusively among patients who were not treated with ENI as part of their RT, which conferred a 18% nodal failure rate for non-ENI patients. There was a trend toward improved LC with radiation dose to the primary tumor ≥50 Gy.
Few studies have examined outcomes of a Western Hemisphere population of limited stage ENKTCL patients treated with RT. An analysis from Memorial Sloan Kettering Cancer Center reported a 2-year OS of 87% and 2-year PFS of 56% in early-stage non-Asian patients treated with short-course ChT (either a CHOP-based regimen before 2009 or modified SMILE regimen after 2009) and ISRT of 45 Gy.3 Most of these early-stage patients were treated with an aggressive modified SMILE regimen without prophylactic nodal irradiation. The use of the modified SMILE regimen with ISRT without ENI in early-stage non-Asian patients with ENKTCL suggests that SMILE is effective, offering both local and distant control in the absence of prophylactic nodal irradiation. Furthermore, outcomes of these patients were comparable to outcomes in an Asian population with ENKTCL. Another study that examined 642 patients from the National Cancer Database with stage I/II ENKTCL identified and treated in the United States observed inferior outcomes with systemic therapy alone.23 Collectively, these data indicate that RT has an integral role in the outcomes of ENKTCL patients. Although the modified SMILE regimen with RT yields acceptable outcomes, the data we present raise the question of whether elective nodal RT may be beneficial in non-asparaginase-based regimens.
Existing data have established the role of RT dose in improving outcome for limited stage ENKTCL patients. Specifically, RT doses of ≥50 Gy are important for LC and in turn, PFS and OS.23,24 In a large series of 1332 patients with early-stage ENKTCL, Yang et al24 demonstrated that high-dose RT to doses ≥50 Gy were associated with significantly improved 5-year locoregional control (85% vs 73%, P < .001), PFS (61% vs 50%, P = .004), and OS (70% vs 58%, P = .04) compared to doses <50 Gy. In the current study we also observed a trend toward improved local control with RT doses ≥50 Gy, in support of the existing literature. The aforementioned study by Qi et al3 that incorporated RT after the aggressive L-aspariginase based SMILE ChT used lower doses of RT (45 Gy) with acceptable outcomes, likely indicating the ability of the intensive SMILE regimen to control local disease. LC can also be improved by incorporating RT in the initial portion of the CMT treatment strategy as demonstrated previously and suggested by our current data. A recent meta-analysis25 revealed a survival advantage for patients with limited stage disease treated with RT first, as opposed to after upfront ChT (hazard ratio, 0.70; P = .002). Furthermore, Qi et al26 demonstrated upfront radiation to be beneficial in the modern era of aspariginase/gemcitabine-based ChT in improving both PFS and locoregional control. Thus, the combination of upfront, optimal RT and effective systemic therapy may provide superior outcomes for patients with early-stage ENKTCL.
Several guidelines do not recommend ENI for limited stage ENKTCL patients, particularly those without nodal involvement.16,18,19 However, in the current study of patients treated largely with sequential therapy and anthracycline-based ChT regimens, ENI appears to show benefit for early-stage ENKTCL patients, likely by decreasing the risk of regional nodal recurrence. This notion is supported by the observed elevated rates of nodal failure among patients not treated with ENI, predominantly at first-echelon nodal stations. Precise targets for ENI remain unclear, but patterns of failure suggest a benefit to treating elective nodal basins relative to the local extent of the primary tumor, often with bilateral neck treatment. In our cohort, 4 of 5 patients with regional nodal relapse had stage I disease initially, arguing against the use of ENI exclusively for stage II patients.27
Given that anthracycline-based regimens have been shown to be inferior to current approaches with concurrent platinum-based chemoradiotherapy or with sequential L-asparaginase-based regimens, one major limitation of the current study is the use of anthracycline-based ChT in most patients and the potential limited applicability to modern treatment approaches. It is certainly plausible that in the context of RT administered sequentially after highly aggressive multiagent ChT regimens such as SMILE, ENI is not necessary and may only serve to increase RT field size with the potential for increased RT-related toxicity. On the other hand, systemic regimens administered concurrently with RT are not as intensive and may be less capable of controlling microscopic disease in draining regional nodal basins. Moreover, recent data from China suggests that some patients with early-stage ENKTCL may be best treated with RT alone. In this large retrospective study28 of 253 patients who received RT alone as frontline therapy, the 5-year PFS was 65% and OS was 70%, prompting the authors to recommend combined modality therapy only for those patients with risk factors such as age >60 at diagnosis, stage II disease, performance status >1, elevated lactate dehydrogenase, and primary tumor invasion. Given that relapsed disease can be challenging to salvage coupled with the increased utilization of advanced RT techniques such as intensity modulated radiation therapy/volumetric modulated arc therapy that may mitigate the toxicity of increased treatment volumes, our data suggests that ENI should be considered in the absence of intensive systemic therapy.
There are several limitations to this study including the inherent shortcomings of a retrospective analysis. The limited sample size and the heterogeneity in treatment regimens limits the generalizability of the data and thus, we are cautious in making definitive conclusions regarding treatment. In addition, it is difficult to draw firm conclusions because of the small number of events (3 patients who experienced isolated regional relapses and 2 patients with regional and distant relapses). We were also unable to obtain data on factors such as Epstein-Barr virus serum status and titers, which have been implicated in prognosis and treatment. Importantly, most patients in this study were treated with anthracycline-based regimens. Our data however are instructive regarding the potential for regional nodal failures when nonintensive systemic therapy regimens are used in a Western Hemisphere patient population. It will be important to evaluate the patterns of failure among Western Hemisphere populations treated with RT alone or with currently recommended concurrent platinum-based ChT regimens.
Conclusion
With disease-related outcomes comparable to previously reported data from Asia, this Western Hemisphere cohort of early-stage ENKTCL patients highlights a potential benefit for ENI in the setting of non–asparaginase-containing CMT, such as in patients treated with RT alone, patients treated with less intensive ChT concurrently, or patients who are unable to tolerate intensive ChT. This is supported by high rates of nodal failure in first-echelon nodal stations relative to the primary tumor.
Disclosures
Paolo Strati is a consultant/on the advisory board for Roche-Genentech, Genmab-AbbVie, Kite-Gilead, Sobi, ADC Therapeutics, and AstraZeneca Acerta and has research support from Kite-Gilead, Sobi, ADC Therapeutics, AstraZeneca Acerta, and ALX Oncology. Loretta J. Nastoupil has received honorariums from AbbVie, AstraZeneca, BMS, Genentech, Genmab, Gilead/Kite, Incyte, Ipsen, Janssen, Merck, Novartis, Regeneron, and Takeda and has received research support from BMS, Caribou Biosciences, Genentech, Genmab, Gilead/Kite, IGM Biosciences, Ipsen, Janssen, Merck, Novartis, and Takeda.
Acknowledgments
Penny Fang and Sonal Noticewala were responsible for statistical analysis.
Footnotes
Sources of support: This work was supported in part by the National Institutes of Health National Cancer Institute, Cancer Center Support (Core) (grant CA 016672) to the University of Texas MD Anderson Cancer Center. Paolo Strati's salary is supported by the Leukemia and Lymphoma Society Career Development Program, Kite-Gilead Scholar in Clinical Research Award, and Sabin Family Fellowship Award. No other funding was received for design, completion, or analysis of this study.
Research data are not available at this time.
References
- 1.Wang ZY, Liu QF, Wang H, et al. Clinical implications of plasma Epstein-Barr virus DNA in early-stage extranodal nasal-type NK/T-cell lymphoma patients receiving primary radiotherapy. Blood. 2012;120:2003–2010. doi: 10.1182/blood-2012-06-435024. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018;131:2528–2540. doi: 10.1182/blood-2017-12-791418. [DOI] [PubMed] [Google Scholar]
- 3.Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016;57:2575–2583. doi: 10.1080/10428194.2016.1180689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haverkos BM, Pan Z, Gru AA, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11:514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351–2356. doi: 10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12:349–352. doi: 10.1023/a:1011144911781. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Ahn YC, Kim WS, Ko YH, Kim K, Park K. The effect of pre-irradiation dose intense CHOP on anthracyline resistance in localized nasal NK/T-cell lymphoma. Haematologica. 2006;91:427–428. [PubMed] [Google Scholar]
- 8.Yamaguchi M, Tobinai K, Oguchi M, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2012;30:4044–4046. doi: 10.1200/JCO.2012.45.6541. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 10.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 11.Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 12.You JY, Chi KH, Yang MH, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol. 2004;15:618–625. doi: 10.1093/annonc/mdh143. [DOI] [PubMed] [Google Scholar]
- 13.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. Published correction appears in J Clin Oncol. 2006;24:2973. [DOI] [PubMed] [Google Scholar]
- 14.Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–174. doi: 10.1016/j.ijrobp.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Yoon SE, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review. J Hematol Oncol. 2018;11:140. doi: 10.1186/s13045-018-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi SN, Li YX, Specht L, et al. Modern radiation therapy for extranodal nasal-type NK/T-cell lymphoma: risk-adapted therapy, target volume, and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2021;110:1064–1081. doi: 10.1016/j.ijrobp.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Li YX, Wang H, Jin J, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1809–1815. doi: 10.1016/j.ijrobp.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Xia ZJ, Lu Y, Zhang YJ. Prophylactic cervical lymph node irradiation provides no benefit for patients of stage IE extranodal natural killer/T cell lymphoma, nasal type. Med Oncol. 2015;32:320. doi: 10.1007/s12032-014-0320-1. [DOI] [PubMed] [Google Scholar]
- 19.Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:11–31. doi: 10.1016/j.ijrobp.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group study JCOG0211. J Clin Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 23.Vargo JA, Patel A, Glaser SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer. 2017;123:3176–3185. doi: 10.1002/cncr.30697. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Cao JZ, Lan SM, et al. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type natural killer/T-cell lymphoma. JAMA Oncol. 2017;3:83–91. doi: 10.1001/jamaoncol.2016.5094. [DOI] [PubMed] [Google Scholar]
- 25.Hu S, Zhou D, Zhang W. The optimal timing of radiotherapy in the combined modality therapy for limited-stage extranodal NK/T cell lymphoma (ENKTL): a systematic review and meta-analysis. Ann Hematol. 2018;97:2279–2287. doi: 10.1007/s00277-018-3479-2. [DOI] [PubMed] [Google Scholar]
- 26.Qi F, Chen B, Wang J, et al. Upfront radiation is essential for high-risk early-stage extranodal NK/T-cell lymphoma, nasal type: comparison of two sequential treatment modalities combining radiotherapy and GDP (gemcitabine, dexamethasone, and cisplatin) in the modern era. Leuk Lymphoma. 2019;60:2679–2688. doi: 10.1080/10428194.2019.1599111. [DOI] [PubMed] [Google Scholar]
- 27.Qi F, Wang WH, He XH, et al. Phase 2 study of first-line intensity modulated radiation therapy followed by gemcitabine, dexamethasone, and cisplatin for high-risk, early stage extranodal nasal-type NK/T-cell lymphoma: the GREEN study. Int J Radiat Oncol Biol Phys. 2018;102:61–70. doi: 10.1016/j.ijrobp.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126:1424–1517. doi: 10.1182/blood-2015-04-639336. [DOI] [PubMed] [Google Scholar]