Abstract
Purpose
To compare comfort outcomes between a novel daily disposable contact lens – designed to maximize comfort – and an established control. The hypothesis was that the test lens would be superior to the control for four key comfort questionnaire items: end-of-day comfort, all-day comfort, visual comfort while driving at night, and reduction of ocular fatigue from digital device use.
Methods
This randomized, controlled, subject-masked, parallel-arm study enrolled young (18–39 years), healthy, myopic, contact lens wearers with an up-to-date prescription at 19 investigational sites in the United States. Subjects wore either the test (ACUVUE® OASYS MAX 1-Day, senofilcon A) or control (Dailies Total1®, delefilcon A) lens for 2 weeks of bilateral, daily disposable wear before completing comfort questionnaire items, each of which had 5 or 6 response options. For each item, the odds ratio for positive (top-two-box) responses was estimated from a binomial generalized linear mixed model. A gatekeeping approach combined with the truncated Hochberg procedure was used for multiplicity adjustment.
Results
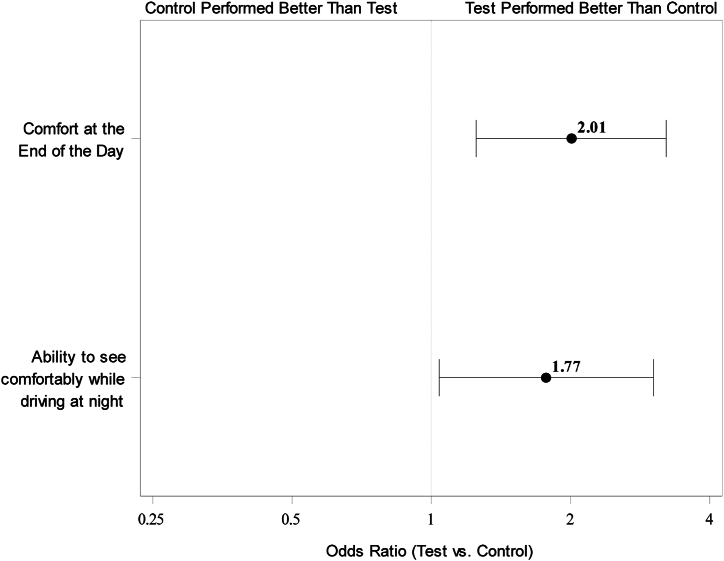
Of 344 enrolled subjects, 342 subjects were randomized and dispensed lenses, with 171 subjects per lens group. Among the 342 subjects, 68.4% were female, 83.6% were White, and the average age was 29.7 (±5.53) years. The test lens was statistically superior to the control for all four comfort questions: odds ratios (test vs. control) were 2.01 (95% CI: 1.25, 3.22) for end-of-day comfort, 2.17 (alpha-adjusted CI: 1.30, 3.64) for all-day comfort, 2.00 (alpha-adjusted CI: 1.18, 3.41) for reducing ocular fatigue from digital device use, and 1.77 (95% CI: 1.04, 3.02) for comfortable vision while driving at night.
Conclusion
The test lens demonstrated statistically superior physical and visual comfort, as measured by the four comfort endpoints, compared to the control. The test lens had significantly greater odds of favorable responses for all comfort items compared to the control.
Keywords: Daily disposable, Contact lens comfort, Tear-film stability, Blue-violet-light filtering, Digital device use, Night-time driving
Highlights
-
•
A large-scale, randomized, controlled, multi-site, subject-masked, parallel-arm study investigated the clinical performance of a novel test contact lens against an established control contact lens.
-
•
The test lens is statistically superior to a control lens for the physical comfort metrics of end-of-day comfort and all-day comfort.
-
•
The test lens is also statistically superior to a control for the visual comfort metrics of ocular fatigue from digital device use and visual comfort while driving at night.
1. Introduction
Despite significant advances in contemporary contact lens designs, symptoms of discomfort and dryness continue to be a common experience for wearers. A survey of 1092 soft contact lens wearers found that 31% have a reduced comfortable wear time (>2hr of uncomfortable wear), 28% experience dryness, and 17% experience discomfort [1]. Overall, symptoms of discomfort and dryness affect approximately 50% of contact lens wearers [1,2]. Moreover, symptoms of discomfort and dryness are more common and more severe among contact lens wearers than non-contact lens wearers [[2], [3], [4]]. Because of their high prevalence, these symptoms remain a common cause of permanent contact lens discontinuation [2,5,6].
Two key elements driving the overall comfort experience with contact lenses are physical (e.g., ocular surface comfort) and perceptual (e.g., visual comfort) [7]. Ocular surface comfort can be highly dependent on the tear film [8], which lubricates, moisturizes, and protects the ocular surfaces [9]. However, the tear film can be disrupted by factors such as contact lens wear and digital device use [[10], [11], [12]]. Recent studies have estimated that adults spend an average of over 13 h daily on digital devices, which is a 33% increase since 2019 [13], and 71% say screen time or the total demands of work and life have increased in the last two years [14]. Viewing digital screens results in 60% less blinking, compromising the tear film and creating discomfort [15,16]. The importance of the tear film in contact lens comfort is highlighted by the finding that those who discontinue due to discomfort are significantly more likely to have symptoms of dryness than successful contact lens wearers [17]. The tear film also provides the eye with an optically smooth surface, and therefore a decreased tear-film stability significantly reduces subjective visual performance [18].
Visual comfort is also an important aspect of the overall comfort experience, with a significant association between comfort scores and subjective vision quality reported among soft toric contact lens wearers [19]. Additionally, patients often cite poor vision as a reason for discontinuation from contact lens wear [6,20]. One possible contributor to visual discomfort is blue-violet light, which is especially uncomfortable to view [[21], [22], [23]]. For example, LED (light-emitting diode) headlamps with a greater short-wavelength content cause more intense discomfort glare [24].
Given the aforementioned concerns with current soft contact lenses, the possibility of identifying a new lens with a reduced symptomatology serves as the rationale to conduct this clinical trial. A novel contact lens, containing technologies designed to enhance physical and visual comfort by supporting a stable tear film and reducing the effect of blue-violet light, has recently entered the market. The purpose of this study was to compare the comfort of the new contact lens with that of an established control lens. The hypotheses were that the test lens would be superior to the control lens in four key physical and visual comfort questionnaire items: end-of-day comfort, all-day comfort, reduction of ocular fatigue from digital device use, and visual comfort while driving at night. To test these hypotheses, young, healthy, myopic, contact lens wearers with an up-to-date prescription were enrolled and randomized to 2 weeks of subject-masked, daily disposable wear of either the test or control lens before they were each given all four questionnaire items.
2. Materials and methods
2.1. Study design
This study randomized subjects to either a test contact lens with ultraviolet (UV) and blue-violet light filtering (ACUVUE® OASYS MAX 1-Day, senofilcon A) or a control contact lens (Dailies Total1®, delefilcon A) for 2 weeks (14 ± 1 days) of bilateral, daily disposable wear with the aim of comparing their clinical performance. Dailies Total1® was chosen as the control lens because it is a widely used daily disposable silicone hydrogel lens reported to reduce symptoms of discomfort among symptomatic contact lens wearers [25] and to have superior comfort compared with several other daily disposable lenses [[26], [27], [28]].
Subjects were enrolled by the investigators and randomly assigned to the two study lenses by randomly-permuted block randomization, stratified by site and type of habitual lens wear (daily disposable and daily wear reusable). All subjects were masked to the identity of the contact lenses (Appendix: Table S1) by over-labelling. Although lens identity was masked, the lenses differed in physical appearance (color). Subjects knowing the study treatments are different may presume a difference in performance. A parallel study design was therefore chosen to eliminate the risk of bias that may occur in a within-subjects study design (e.g., crossover design). Preservative-free rewetting drops (single-use EyeCept® Rewetting Drops) or saline (LacriPure Saline Solution or ScleralFil® Preservative Free Saline Solution) could be instilled by the subject as needed. The study occurred from March 2022 through May 2022.
2.2. Subjects
Nineteen investigational sites in the United States enrolled the subjects. Eligible subjects were myopic, aged 18–39 years, and habitual wearers of up-to-date, daily wear, spherical, silicone hydrogel contact lenses in both eyes. Habitual wear was defined as at least 6 h of wear per day for at least 5 days per week during the 30 days before screening. An up-to-date lens was one that had been prescribed or checked as being the correct prescription within the past 6 months and had been worn for at least 2 weeks. At baseline, each eye of eligible subjects had a best-corrected distance visual acuity of 20/25 or better, a vertex-corrected spherical equivalent refraction of −1.00 to −6.00 DS, and a vertex-corrected cylindrical refraction of 0.00–1.00 DC.
Individuals were ineligible for enrolment if they had ocular or systemic pathology, were using monovision, multifocal, or extended-wear contact lenses, had habitually worn rigid or hybrid lenses within the last 6 months, had undergone ocular surgery, planned to receive ocular surgery during the study period, had participated in a contact lens-related study within 14 days before screening, or were an employee or immediate family member of an employee at an investigational site.
2.3. Investigators
To ensure the accuracy and reliability of data, only qualified investigators [29] and appropriate investigational sites were selected for participation. Additionally, the protocol procedures were reviewed with the Principal Investigator, who, in turn, ensured that all sub-investigators and study staff were familiar with the protocol and all study-specific procedures and had appropriate knowledge of the study lenses.
2.4. Questionnaire items
At the 2-week follow-up visit, subjects were asked to rate the lens performance with the following questionnaire items: “Comfort at the end of the day”, “I could wear these contact lenses comfortably for as long as I wanted to”, “Reduction in the feeling of tired eyes from using a computer or other digital device”, and “Ability to see comfortably while driving at night”. Each questionnaire item had a 5-point Likert response scale, with a “Not Applicable” option for some items since not everyone uses digital devices or drives at night (Table 1). These items were chosen because they help address current performance gaps in contact lens wear. These performance gaps, created by increasingly demanding lifestyles and significant digital use, can be the reasons why a patient discontinues lens wear.
Table 1.
Research questionnaire items.
| Item | Response options |
|---|---|
| Comfort at the End of the Day | Very Satisfied, Satisfied, Neither Satisfied nor Dissatisfied, Dissatisfied, Very Dissatisfied, |
| I could wear these contact lenses comfortably for as long as I wanted to | Strongly Disagree, Disagree, Neither Agree Nor Disagree, Agree, Strongly Agree |
| Reduction in the feeling of tired eyes from using a computer or other digital device | Not Applicable, Excellent, Very Good, Good, Fair, Poor |
| Ability to see comfortably while driving at night | Not Applicable, Excellent, Very Good, Good, Fair, Poor |
2.5. Statistical Methodology
This study was designed and powered to demonstrate superiority of the test lens compared with the control lens with respect to the four comfort endpoints. The sample size was calculated to achieve a statistical power of at least 90% for each hypothesis testing. The sample size calculation was based on data (on file) from four studies of the test lens and one study of the control lens. The target enrolment was approximately 360 subjects for a target sample size of 330 completed subjects with an even distribution between test and control groups (approximately 165 in each).
Analyses were conducted using all randomized subjects with observed data (no imputation for missing data). Item responses were converted into a binary variable as follows: top-two-box responses (Very Satisfied or Satisfied for “Comfort at the end of the day”, Strongly Agree or Agree for “I could wear these contact lenses comfortably for as long as I wanted to”, and Excellent or Very Good for “Ability to see comfortably while driving at night” and “Reduction in the feeling of tired eyes from using a computer or other digital device”) = 1, and neutral and negative responses = 0. The “Not Applicable” response was excluded from the analysis. The binary outcome variable was analyzed using a binomial generalized linear mixed model (GLMM) to model the probability of the positive responses (top-two-box responses) for each comfort endpoint separately. The model included lens type as a fixed effect controlling for age, gender, iris category (Dark and Light), and habitual lens type (habitual daily disposable and habitual daily wear reusable). The random intercept of site was included as appropriate to account for the site level variation.
For multiplicity adjustment, a gatekeeping approach combined with the truncated Hochberg procedure [30,31] was used to control the study overall Type I error rate at a two-sided 0.05 level. The four comfort endpoints were tested in the following manner: the end-of-day comfort endpoint was tested first and served as a gatekeeper for testing all-day comfort and reduced ocular fatigue from digital device use, and then these two comfort endpoints were used to gatekeep visual comfort while driving at night. If the hypothesis for end-of-day comfort was met, then all-day comfort and reduced ocular fatigue from digital device use were to be tested using the truncated Hochberg procedure – with confidence intervals for these endpoints adjusted accordingly.
2.6. Declaration of Helsinki and informed consent
The study was performed in accordance with ISO (International Standards Organization) 14155 standards for Good Clinical Practice and conformed with the tenets of the Declaration of Helsinki [32,33]. Prior informed consent was obtained from all subjects in order that all subjects would understand both the risks and benefits of participation and that participation was on a purely voluntary basis, as well as allowing subjects to ask questions about participation in the study. The research was reviewed and approved by Sterling Institutional Review Board (Atlanta, GA, approval ID 9778, 3/21/2022). The study, along with its predetermined outcomes of interest, was submitted to ClinicalTrials.gov (identifier: NCT05300763) before enrolment of subjects.
3. Results
3.1. Subject disposition and demographics
In total, 344 subjects were enrolled, of which 342 subjects were randomized and dispensed at least one pair of study lenses (Fig. 1) and 3 subjects discontinued after lens dispensing, resulting in a retention rate of 99.1%. Among the 342 randomized subjects (171 test subjects and 171 control subjects), 234 (68.4%) were female, 286 (83.6%) were White, and the average age was 29.7 (±5.53) years (Table 2).
Fig. 1.
Subject Accountability.
Table 2.
Demographics of all randomized subjects.
| Test group (n = 171) | Control group (n = 171) | Total (n = 342) | |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 29.5 (5.44) | 30.0 (5.63) | 29.7 (5.53) |
| Median | 30.0 | 30.0 | 30.0 |
| Range | 18.0–39.0 | 18.0–39.0 | 18.0–39.0 |
| Sex, n (%) | |||
| Female | 117 (68.4%) | 117 (68.4%) | 234 (68.4%) |
| Male | 54 (31.6 %) | 54 (31.6 %) | 108 (31.6 %) |
| Race, n (%) | |||
| White | 141 (82.5%) | 145 (84.8%) | 286 (83.6%) |
| Black or African American | 15 (8.8%) | 15 (8.8%) | 30 (8.8%) |
| Asian | 12 (7.0%) | 8 (4.7%) | 20 (5.8%) |
| Native Hawaiian or Other Pacific Islander | 3 (1.8%) | 1 (0.6%) | 4 (1.2%) |
| Multiple | 0 (0.0%) | 2 (1.2%) | 2 (0.6%) |
| Habitual lens replacement, n (%) | |||
| Daily Disposable | 118 (69.0%) | 116 (67.8%) | 234 (68.4%) |
| Daily Reusable | 53 (31.0%) | 55 (32.2%) | 108 (31.6%) |
| Iris category, n (%) | |||
| Dark Iris | 112 (65.5%) | 95 (55.6%) | 207 (60.5%) |
| Light Iris | 59 (34.5%) | 76 (44.4%) | 135 (39.5%) |
The subjects were enrolled at 19 investigational sites in the United States, with each site enrolling 9 to 22 subjects. Investigators enrolled habitual wearers of daily wear lenses (daily disposable and/or daily wear reusable) of any contact lens brand. The enrollment period was 4 weeks. Over two-thirds of subjects were habitual daily disposable contact lens wearers (234, 68.4%); the other subjects were habitual daily wear reusable contact lens wearers (108, 31.6%).
In total, three subjects were discontinued – two during the initial visit and one during the 2-week follow-up visit. A subject allocated to the control group was discontinued due to a non-ocular adverse event (headache/sinus pressure), and two subjects allocated to the test group were discontinued due to protocol deviations (dispensing error and no longer meeting eligibility criteria).
3.2. Lens comfort
The test lens was statistically superior to the control lens for all four comfort questions (Fig. 2, Fig. 3). The test group had approximately twice the odds of positive responses to comfort questions when compared with the control group (Table 3).
Fig. 2.
Odds ratio (test vs. control) with 95% confidence interval for top-two-box responses to “Comfort at the end of the day” (n = 340) and “Ability to see comfortably while driving at night” (n = 335) at 2 weeks. Superiority of the test lens is established if the lower limit of the confidence interval of the odds ratio is above 1.
Fig. 3.
Odds ratio (test vs. control) with alpha-adjusted confidence interval for top-two-box responses to “I could wear these contact lenses comfortably for as long as I wanted to” (n = 340) and “Reduction in the feeling of tired eyes from using a computer or other digital device” (n = 329) at 2 weeks. Superiority of the test lens is established if the lower limit of the confidence interval of the odds ratio is above 1.
Table 3.
Summaries of top-two-box responses and analysis for the test and control groups.
| Item | Responses, n (%) |
Statistical Analysis Results (Test vs. Control) |
||
|---|---|---|---|---|
| Test (N = 171) | Control (N = 171) | Odds Ratio (CI) | P-value | |
| Comfort at the End of the Day | 2.01 (1.25, 3.22)a | 0.0040 | ||
|
128 (75.3) | 104 (61.2) | ||
|
42 (24.7) | 66 (38.8) | ||
| I could wear these contact lenses comfortably for as long as I wanted to | 2.17 (1.30, 3.64)b | 0.0008 | ||
|
116 (68.2) | 87 (51.2) | ||
|
54 (31.8) | 83 (48.8) | ||
Reduction in the feeling of tired eyes from using a computer or other digital device
|
133 (78.2) | 106 (62.4) | 2.00 (1.18, 3.41)b | 0.0067 |
| 35 (20.6) | 55 (32.4) | |||
| 2 (1.2) | 9 (5.3) | |||
| Ability to see comfortably while driving at night | 1.77 (1.04, 3.02)a | 0.0358 | ||
|
138 (81.2) | 122 (71.8) | ||
|
30 (17.6) | 45 (26.5) | ||
|
2 (1.2) | 3 (1.8) | ||
CI: confidence interval.
95% confidence interval.
alpha-adjusted confidence interval based on the truncated Hochberg procedure for multiplicity adjustment.
The clinical data, represented by count and percentage of top-two-box responses for the test and control groups are summarized in Table 3. The table also includes results from the statistical analysis.
4. Discussion
Successful contact lens wear is often complicated with symptoms relating to both physical comfort and visual comfort. In this randomized controlled study, these comfort areas were explored with two questions each. For physical comfort, the questions ask the subjects to rate their experience with “comfort at the end of the day,” and “I could wear these contact lenses comfortably for as long as I wanted to.” The responses for both the test lens and control lens groups were compared and analyzed for differences in performance. For comfort at the end of the day, the odds ratio was 2.01, indicating that subjects (n = 171) wearing the test lens had two times the odds of responding “excellent” or “very good” than subjects wearing the control lens (control group n = 171 subjects). Similarly, the odds ratio for wearing the contact lenses comfortably for as long as they wanted to was 2.17, indicating a little more than two times the odds of responding positively with the test lens than the control lens.
For visual comfort, the questionnaire items asked the subjects to rate their experience with “reduction in the feeling of tired eyes from using a computer or other digital device,” and “ability to see comfortably while driving at night.” The odds ratio for the digital device question was 2.00, indicating that subjects with the test lens had two times the odds of having a positive response to that question than subjects in the control group. Likewise, the odds ratio for the ability to see comfortably while driving at night was 1.77, indicating that subjects with the test lens had nearly two times the odds of responding positively to that question than subjects in the control group. The test lens was statistically superior to the control with respect to the top-two-box responses for all four of these physical and visual comfort questions.
There are plausible reasons as to why the test lens outperformed the control lens. The test lens filters approximately 60% of blue-violet light within the range 380–450 nm, predominantly for shorter wavelengths, which may partly explain the findings of this study. Previous randomized, controlled, double-masked clinical studies have shown that, under glare conditions, the test lens reduces squinting, halo and starburst diameters, light scattering, and shortens photostress recovery times compared with an equivalent control lens [34,35]. The blue-violet filtering properties of the lens help explain perceptual benefits (e.g., visual comfort with night driving), but are unlikely to directly influence the physical comfort of the lens.
Alternatively, the tear-stabilizing technology in the test lens may contribute substantially to the improved physical comfort of the lens. Several studies have reported a relationship between tear-film instability and symptoms of ocular discomfort in contact lens wearers [8,[36], [37], [38]]. Discomfort is significantly correlated with tear meniscus volume [8,38], surface drying time [37], and tear evaporation rate [37], and is significantly associated with a shorter tear-film break-up time [36,38] and reduced surface coverage [36]. Data show that the test lens improves both tear evaporation rate and tear-film stability compared to the same lens without the new technologies. With the test lens, wearers had half the evaporation rate [39] and were 1.6 times (60%) more likely to have a long (>10 s) visual tear-film break-up time [40].
This study has a number of strengths that support its conclusions. The main strength of this study is its randomized study design, which minimizes the risk of potentially confounding factors [41,42]. Furthermore, the study had a large sample size, with subjects recruited from multiple investigational sites across the United States. Additionally, the control lens was Dailies Total1®, a popular daily disposable silicone hydrogel lens that was previously found to reduce symptoms of discomfort among symptomatic contact lens wearers [25] and to have superior comfort compared with several other daily disposable lenses [[26], [27], [28]]. Subjects were required to have been wearing and up-to-date contact lens prescription prior to enrolment, which reduced the likelihood they would prefer the study lens simply based on a new prescription during the study.
When interpreting the study findings, a few points should be considered. First, the study included only myopic subjects aged 18–39 years; therefore, the results may not necessarily be generalizable to other populations. Second, although subjects were masked to the identity of the contact lenses, investigators and other site personnel involved in data collection were not masked since they may have been able to detect a slight difference in lens color between the two lens types – the test lens was slightly more turquoise than the control. Third, since it was not the purpose of the study to examine the test lens technologies independently, it should be noted that their individual contributions to comfort are unknown. Similarly, other design factors, such as lens edge, may also have contributed to the better performance in the test group. In addition to the perceptual benefits noted [34,35], future studies could investigate the effect of the contact lens on various objective measures of vision such as disability glare, brightness perception, cortical excitability (e.g., fMRI), chromatic enhancement, and low-contrast visual acuity.
Technologies that stabilize the tear film and filter blue-violet light could provide benefits to many contact lens wearers. The increasing reliance on digital technology for work and leisure is likely contributing to an increased prevalence of symptoms of ocular and visual discomfort, especially for contact lens wearers [10]. Similarly, glare from the headlights of oncoming traffic is likely a common and increasing cause of discomfort for drivers, with more cars on the roads and a trend for car manufacturers to use brighter and bluer headlights [24,43]. The test lens offers a potential solution to improve ocular and visual comfort for contact lens wearers, even when using digital devices or driving at night.
5. Conclusion
In conclusion, the hypotheses were met, given that the test lens was statistically superior to the control lens in all four key physical and visual comfort questionnaire items. In the study sample of young, healthy, myopic, contact lens wearers, after 2 weeks of lens wear, the test lens was associated with significantly greater odds of subjects giving favorable responses to physical and visual comfort questions when compared with the control lens. Specifically, the test contact lens with technologies to stabilize the tear film and filter blue-violet light, was shown to be statistically superior to the control lens for end-of-day comfort, comfort throughout the day, comfortable vision while driving at night, and comfortable digital device use. This suggests that the novel lens may help fill common performance gaps that exist with current contact lens wear, potentially improving patient satisfaction with contact lens wear and increasing contact lens wear retention. When questioning patients about comfort, eye care professionals may wish to do so in relation to specific lifestyle needs, as well as discussing the possibility of trying new contact lens designs.
CRediT authorship contribution statement
John R. Buch: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Conceptualization. Patricia Martin: Writing – review & editing, Writing – original draft, Project administration, Methodology, Conceptualization. Jie Xu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Ethics statement
The study was performed in accordance with ISO 14155 standards for Good Clinical Practice and conformed with the tenets of the Declaration of Helsinki. Prior informed consent was obtained from all subjects. The research was reviewed and approved by Sterling Institutional Review Board, Atlanta, GA, US (ID: 9778, 3/21/2022). The study, along with its predetermined outcomes of interest, was submitted to ClinicalTrials.gov (ID: NCT05300763) before enrolment of subjects.
Data availability statement
The clinical data are included in the article.
Declaration of AI and AI-assisted technologies in the writing process
No AI or AI-assisted technologies were used in the drafting, editing, or review of this manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:John R. Buch, Patricia Martin, and Jie Xu report a relationship with Vision at Johnson & Johnson that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Maris Horne of Vision at Johnson & Johnson for clinical management of this study. The authors would also like to thank the 19 practitioners who took part in the study and Nathan Greenaway of Visioncare Research Ltd for helping with manuscript preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39995.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Riley C., Young G., Chalmers R. Prevalence of ocular surface symptoms, signs, and uncomfortable hours of wear in contact lens wearers: the effect of refitting with daily-wear silicone hydrogel lenses (senofilcon a) Eye Contact Lens. 2006;32(6):281–286. doi: 10.1097/01.icl.0000224522.04723.7a. [DOI] [PubMed] [Google Scholar]
- 2.Dumbleton K., Woods C.A., Jones L.W., Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39(1):93–99. doi: 10.1097/ICL.0b013e318271caf4. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers R.L., Begley C.G. Dryness symptoms among an unselected clinical population with and without contact lens wear. Cont Lens Anterior Eye. 2006;29(1):25–30. doi: 10.1016/j.clae.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Guillon M., Maissa C. Dry eye symptomatology of soft contact lens wearers and nonwearers. Optom. Vis. Sci. 2005;82(9):829–834. doi: 10.1097/01.opx.0000178060.45925.5d. [DOI] [PubMed] [Google Scholar]
- 5.Richdale K., Sinnott L.T., Skadahl E., Nichols J.J. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007;26(2):168–174. doi: 10.1097/01.ico.0000248382.32143.86. [DOI] [PubMed] [Google Scholar]
- 6.Sulley A., Young G., Hunt C. Prospective evaluation of new contact lens wearer retention rates. Cont Lens Anterior Eye. 2018;41:S4. [Google Scholar]
- 7.Buch J., Hofmann G., Ruston D. Getting into your comfort zone. Contact Lens Spectr. 2018;33:34–38. [Google Scholar]
- 8.Chen Q., Wang J., Shen M., Cui L., Cai C., Li M., et al. Tear menisci and ocular discomfort during daily contact lens wear in symptomatic wearers. Invest. Ophthalmol. Vis. Sci. 2011;52(5):2175–2180. doi: 10.1167/iovs.10-5780. [DOI] [PubMed] [Google Scholar]
- 9.Levin Lan S.F., Ver Hoeve J., Wu S.M. vol. 11. Elsevier; 2011. (Adler's Physiology of the Eye). [Google Scholar]
- 10.Altinbas E., Elibol A., Fıratlı G., Ayhan C., Celebi A.R. Assessment of risk factors on eye dryness in young adults using visual display device in both contact lens wearers and non-wearers. Int. Ophthalmol. 2023;43(2):441–450. doi: 10.1007/s10792-022-02441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen M., Begley C., Himebaugh N., Port N.L. Effect of contact lens wear and a near task on tearfilm break-up. Optom. Vis. Sci. 2010;87(5):350–357. doi: 10.1097/OPX.0b013e3181d951df. [DOI] [PubMed] [Google Scholar]
- 12.Craig J.P., Willcox M.D., Argüeso P., Maissa C., Stahl U., Tomlinson A., et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Invest. Ophthalmol. Vis. Sci. 2013;54(11):123–156. doi: 10.1167/iovs.13-13235. [DOI] [PubMed] [Google Scholar]
- 13.Eyesafe estimate based upon nielsen Q3 2019 total audience report. 2020. https://eyesafe.com/covid-19-screen-time-spike-to-over-13-hours-per-day/
- 14.JJV data on file . Urvey Fielded to 468 Contact Lens Wearing Patients in the US in April. 2022. s. [Google Scholar]
- 15.Tsubota K., Nakamori K. Dry eyes and video display terminals. N. Engl. J. Med. 1993;328(8):584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]
- 16.Patel S., Henderson R., Bradley L., et al. Effect of visual display unit use on blink rate and tear stability. Optom. Vis. Sci. 1991;68(11):888–892. doi: 10.1097/00006324-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Pucker A.D., Jones-Jordan L.A., Marx S., Powell D.R., Kwan J.T., Srinivasan S., et al. Clinical factors associated with contact lens dropout. Cont Lens Anterior Eye. 2019;42(3):318–324. doi: 10.1016/j.clae.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Pan A.P., Ma Y., Hu R., Cao X., Wu Y., Zhou K., et al. Simultaneous real-time analysis of tear film optical quality dynamics and functional visual acuity in dry eye disease. Eye Vis (Lond). 2023;10(1):1–2. doi: 10.1186/s40662-023-00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado-Codina C., Cornago M.N., Read M.L., Plowright A.J., Vega J., Orsborn G.N., et al. The association of comfort and vision in soft toric contact lens wear. Cont Lens Anterior Eye. 2021;44(4) doi: 10.1016/j.clae.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Sulley A., Young G., Hunt C. Factors in the success of new contact lens wearers. Cont Lens Anterior Eye. 2017;40(1):15–24. doi: 10.1016/j.clae.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Stringham J.M., Fuld K., Wenzel A.J. Action spectrum for photophobia. J. Opt. Soc. Am. Opt Image Sci. Vis. 2003;20(10):1852–1858. doi: 10.1364/josaa.20.001852. [DOI] [PubMed] [Google Scholar]
- 22.Bullough J.D. Spectral sensitivity for extrafoveal discomfort glare. J. Mod. Opt. 2009;56(13):1518–1522. [Google Scholar]
- 23.Zivcevska M., Lei S., Blakeman A., Goltz H.C., Wong A.M. A novel visual psychometric test for light-induced discomfort using red and blue light stimuli under binocular and monocular viewing conditions. Invest. Ophthalmol. Vis. Sci. 2018;59(3):1467–1474. doi: 10.1167/iovs.17-23526. [DOI] [PubMed] [Google Scholar]
- 24.Sivak M. University of Michigan; Ann Arbor, Transportation Research Institute: 2005. Blue Content of LED Headlamps and Discomfort Glare. [Google Scholar]
- 25.Arroyo-del Arroyo C., Novo-Diez A., Blanco-Vázquez M., Fernández I., López-Miguel A., González-García M.J. Does placebo effect exist in contact lens discomfort management? Cont Lens Anterior Eye. 2021;44(4) doi: 10.1016/j.clae.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Michaud L., Forcier P. Comparing two different daily disposable lenses for improving discomfort related to contact lens wear. Cont Lens Anterior Eye. 2016;39(3):203–209. doi: 10.1016/j.clae.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Diec J., Naduvilath T., Tilia D. Subjective ratings and satisfaction in contact Lens Wear. Optom. Vis. Sci. 2018;95(3):256–263. doi: 10.1097/OPX.0000000000001187. [DOI] [PubMed] [Google Scholar]
- 28.Pereira E.I., Lira M. Comfort, ocular dryness, and equilibrium water content changes of daily disposable contact lenses. Eye Contact Lens. 2018;44:S233–S240. doi: 10.1097/ICL.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food & Drug Administration Guidance document: IRB responsibilities for reviewing the qualifications of investigators, adequacy of research sites, and the determination of whether an IND/IDE is needed – guidance for IRBs, clinical investigators, and sponsors. 2013. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/irb-responsibilities-reviewing-qualifications-investigators-adequacy-research-sites-and
- 30.Dmitrienko A., Tamhane A.C., Wiens B.L. General multistage gatekeeping procedures. Biom. J. 2008;50(5):667–677. doi: 10.1002/bimj.200710464. [DOI] [PubMed] [Google Scholar]
- 31.Dmitrienko A., Kordzakhia G., Tamhane A.C. Multistage and mixture parallel gatekeeping procedures in clinical trials. J. Biopharm. Stat. 2011;21(4):726–747. doi: 10.1080/10543406.2011.551333. [DOI] [PubMed] [Google Scholar]
- 32.ISO 14155:2020: Clinical Investigation of Medical Devices for Human Subjects — Good Clinical Practice. Last accessed January 2024. Available from: 10.3403/30116062u. [DOI]
- 33.Declaration of Helsinki - Ethical principles for Medical Research Involving Human Subjects. Last accessed January 2024. Available from: 10.1001/jama.284.23.3043. [DOI]
- 34.Renzi-Hammond L.M., Buch J., Xu J., Hammond B.R. Reduction of glare discomfort and photostress recovery time through the use of a high-energy visible–filtering contact lens. Eye Contact Lens. 2022;48(12):516. doi: 10.1097/ICL.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renzi-Hammond L.M., Buch J., Xu J., Hammond B.R. The influence of HEV-filtering contact lenses on behavioral indices of glare. Eye Contact Lens. 2022;48(12):509. doi: 10.1097/ICL.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddireddy J.S., Vijay A.K., Tan J., Willcox M. The eyelids and tear film in contact lens discomfort. Cont Lens Anterior Eye. 2018;41(2):144–153. doi: 10.1016/j.clae.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Rohit A., Willcox M.D., Stapleton F. Lipid supplements and clinical aspects of tear film in habitual lens wearers. Optom. Vis. Sci. 2017;94(2):174–182. doi: 10.1097/OPX.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 38.Guillon M., Dumbleton K.A., Theodoratos P., Wong S., Patel K., Banks G., et al. Association between contact lens discomfort and pre-lens tear film kinetics. Optom. Vis. Sci. 2016;93(8):881–891. doi: 10.1097/OPX.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 39.Riederer D., DiStefano A., Scales C., Martin P. American Academy of Optometry; 2022. Material Dependence of Evaporative Water Loss from Hydrogel Contact Lenses. October 28; San Diego. [Google Scholar]
- 40.Buch J., Riederer D., Scales C., Xu J. Tear film dynamics of a new soft contact lens. Ophthalmic Physiol. Opt. 2023;43(5):1070–1078. doi: 10.1111/opo.13169. [DOI] [PubMed] [Google Scholar]
- 41.Wenzel A.J., Fuld K., Stringham J.M., Curran-Celentano J. Macular pigment optical density and photophobia light threshold. Vision Res. 2006;46(28):4615–4622. doi: 10.1016/j.visres.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Kowacs P.A., Piovesan E.J., Werneck L.C., Tatsui C.E., Lange M.C., Ribas L.C., et al. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21(3):184–188. doi: 10.1046/j.1468-2982.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 43.Fekete J., Sik-Lányi C., Schanda J. Spectral discomfort glare sensitivity under low photopic conditions. Ophthalmic Physiol. Opt. 2006;26(3):313–317. doi: 10.1111/j.1475-1313.2006.00359.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data are included in the article.