Abstract
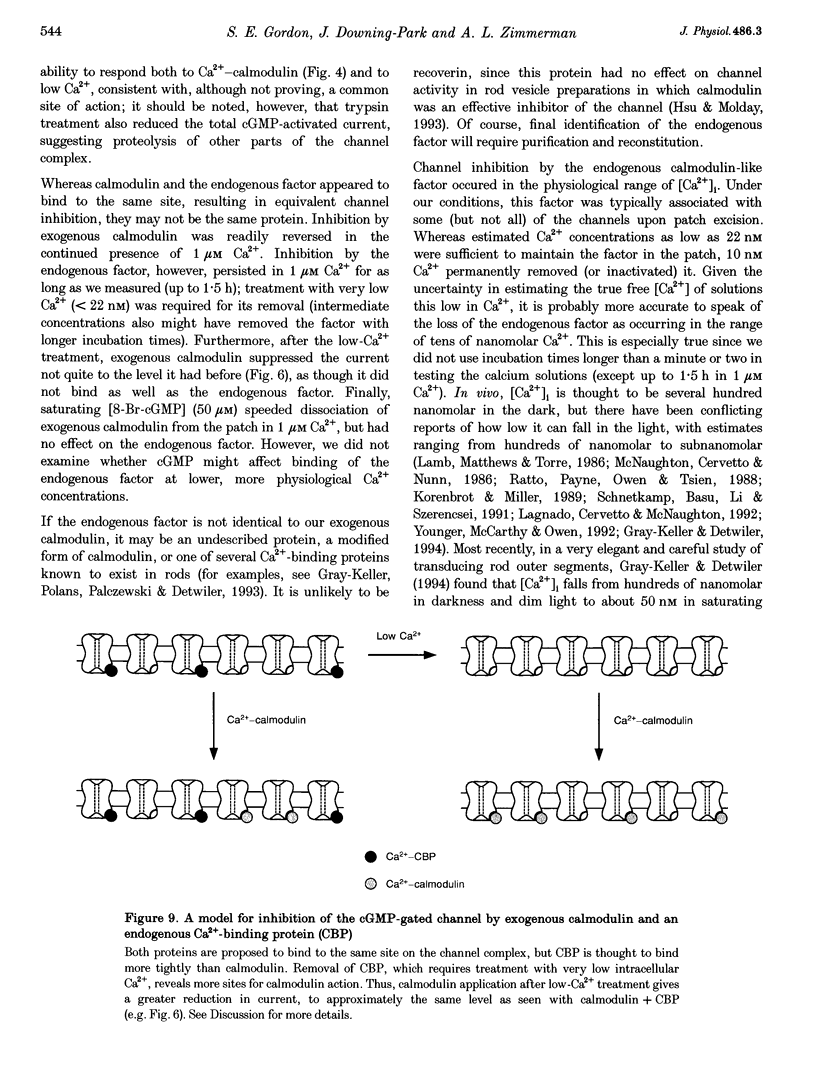
1. Outer segment patches excised in the light were used to investigate the effects of exogenous calmodulin and an endogenous inhibitory factor on the cGMP-gated channel of frog rods. 2. Calmodulin shifted to the right the dose-response relation for activation of the channels by 8-Br-cGMP, but did not change the maximum current or the form of the relation. Reversal of this effect by removal of calmodulin was accelerated by brief exposure to saturating [8-Br-cGMP]. Inhibition by calmodulin required calcium and gave as much as a 5-fold decrease in current for an [8-Br-cGMP] functionally comparable to the presumed physiological [cGMP]. 3. Exposure to low [Ca2+]i (tens of nanomolar) appeared to irreversibly remove or inactivate an endogenous channel inhibitory factor from the patches, increasing the current at low [8-Br-cGMP]. Like calmodulin, this factor slowed the voltage-dependent channel-gating kinetics and did not change the maximum current. However, unlike calmodulin, the endogenous factor remained stably associated with the patches at high [Ca2+]i (1 microM), even with exposure to saturating [8-Br-cGMP]. 4. After the low-Ca2+ treatment increased the current, calmodulin reduced the current to about the same level as it had before the low-Ca2+ treatment, giving a larger fractional suppression. Furthermore, patches with high initial sensitivity to 8-Br-cGMP had small low-Ca2+ effects and large calmodulin effects, while the reverse was true for patches with low initial agonist sensitivity. 5. Application of trypsin to the intracellular surface of the patch prevented the responses to calmodulin and to low [Ca2+]i, suggesting involvement of a cytoplasmic portion of the channel. However, trypsin also reduced the total agonist-induced patch current. 6. Our results are consistent with a model in which calmodulin and an endogenous calcium-binding protein compete for the same site, inhibiting channel opening or cGMP binding. The tight association of the endogenous factor with the channel even at relatively low [Ca2+]i suggests that in the transducing rod it may inhibit the channels most of the time in darkness and in dim light, preventing any potential inhibitory effects of calmodulin. The endogenous factor would be expected to leave the channel only in bright or prolonged light, when the [Ca2+]i is thought to be very low.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chader G., Johnson M., Fletcher R., Besinger R. Cyclic nucleotide phosphodiesterase of the bovine retina: activity, subcellular distribution and kinetic parameters. J Neurochem. 1974 Jan;22(1):93–99. doi: 10.1111/j.1471-4159.1974.tb12183.x. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Illing M., Molday L. L., Hsu Y. T., Yau K. W., Molday R. S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Yau K. W. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994 Apr 7;368(6471):545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Gray-Keller M. P. Some unresolved issues in the physiology and biochemistry of phototransduction. Curr Opin Neurobiol. 1992 Aug;2(4):433–438. doi: 10.1016/0959-4388(92)90176-l. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fain G. L., Matthews H. R. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990 Sep;13(9):378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Gordon S. E., Brautigan D. L., Zimmerman A. L. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992 Oct;9(4):739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M. P., Polans A. S., Palczewski K., Detwiler P. B. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 1993 Mar;10(3):523–531. doi: 10.1016/0896-6273(93)90339-s. [DOI] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Miller D. L. Cytoplasmic free calcium concentration in dark-adapted retinal rod outer segments. Vision Res. 1989;29(8):939–948. doi: 10.1016/0042-6989(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Morelli A., Damonte G., Panfoli I., Pepe I. Proteins of rod outer segments of toad retina: binding with calmodulin and with GTP. Biochem Biophys Res Commun. 1989 Aug 30;163(1):363–369. doi: 10.1016/0006-291x(89)92144-x. [DOI] [PubMed] [Google Scholar]
- Nagao S., Yamazaki A., Bitensky M. W. Calmodulin and calmodulin binding proteins in amphibian rod outer segments. Biochemistry. 1987 Mar 24;26(6):1659–1665. doi: 10.1021/bi00380a026. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Koutalos Y., Yau K. W. Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol. 1995 Apr 1;484(Pt 1):69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 1990;30(12):1923–1948. doi: 10.1016/0042-6989(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P. P., Basu D. K., Li X. B., Szerencsei R. T. Regulation of intracellular free Ca2+ concentration in the outer segments of bovine retinal rods by Na-Ca-K exchange measured with fluo-3. II. Thermodynamic competence of transmembrane Na+ and K+ gradients and inactivation of Na(+)-dependent Ca2+ extrusion. J Biol Chem. 1991 Dec 5;266(34):22983–22990. [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Volotovsky I. D., Baranova L. A., Khovratovich V. I. Specific cGMP binding by retinal rod axoneme and its modulation by calcium ions and calmodulin. Exp Eye Res. 1991 Apr;52(4):389–392. doi: 10.1016/0014-4835(91)90033-b. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Calcium and light adaptation in retinal photoreceptors. Curr Opin Neurobiol. 1991 Aug;1(2):252–257. doi: 10.1016/0959-4388(91)90086-m. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Karpen J. W., Baylor D. A. Hindered diffusion in excised membrane patches from retinal rod outer segments. Biophys J. 1988 Aug;54(2):351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



