Abstract
1. During whole-cell recordings from CA1 neurons of rat brain slices with electrodes containing only KMeSO4 and Hepes, brief anoxia (2-3 min) consistently evoked a hyperpolarization (delta V approximately 14 mV) and reduction in input resistance (delta R approximately -20%). 2. As in previous intracellular recordings, Dantrolene sodium (10 microM) suppressed the anoxic delta V and delta R, confirming the release of internal Ca2+ is a major component of the anoxic response. 3. To identify the relevant intracellular Ca2+ store, other blockers of Ca2+ release were applied either externally (in the bath) or internally, by addition to the contents of the recording electrode. 4. The anoxic hyperpolarization was abolished or much reduced by heparin (10-20 micrograms ml-1, internal), thapsigargin (10 microM, external), Ruthenium Red (50 microM, internal) and external procaine (0.5-2 mM), but not by internal procaine (0.5-1 mM) or ryanodine (10 microM, external). 5. The anoxic fall in resistance was also abolished or reduced by heparin, thapsigargin and external procaine, but not by ryanodine, internal procaine or Ruthenium Red. 6. In addition, external procaine (0.5-2 mM) eliminated the early (transient) depolarization and reduced the post-anoxic hyperpolarization by 60 +/- 22%. 7. None of these agents consistently changed the resting potential, but the input resistance was significantly increased by Dantrolene and external procaine. 8. In view of the marked effects of heparin and thapsigargin, but not ryanodine and internal procaine, we conclude that the anoxic response seen in such whole-cell recordings is initiated predominantly by Ca2+ release from an internal store that is InsP3 sensitive rather than Ca2+ sensitive. 9. Comparable but less pronounced effects of external procaine were seen during intracellular recordings with 3 M KCl-containing electrodes. The dose-dependent suppression of various features of the anoxic response by external procaine (EC50 approximately 0.2 mM) is presumed to be mediated by a superficial membrane trigger or modulating site.
Full text
PDF


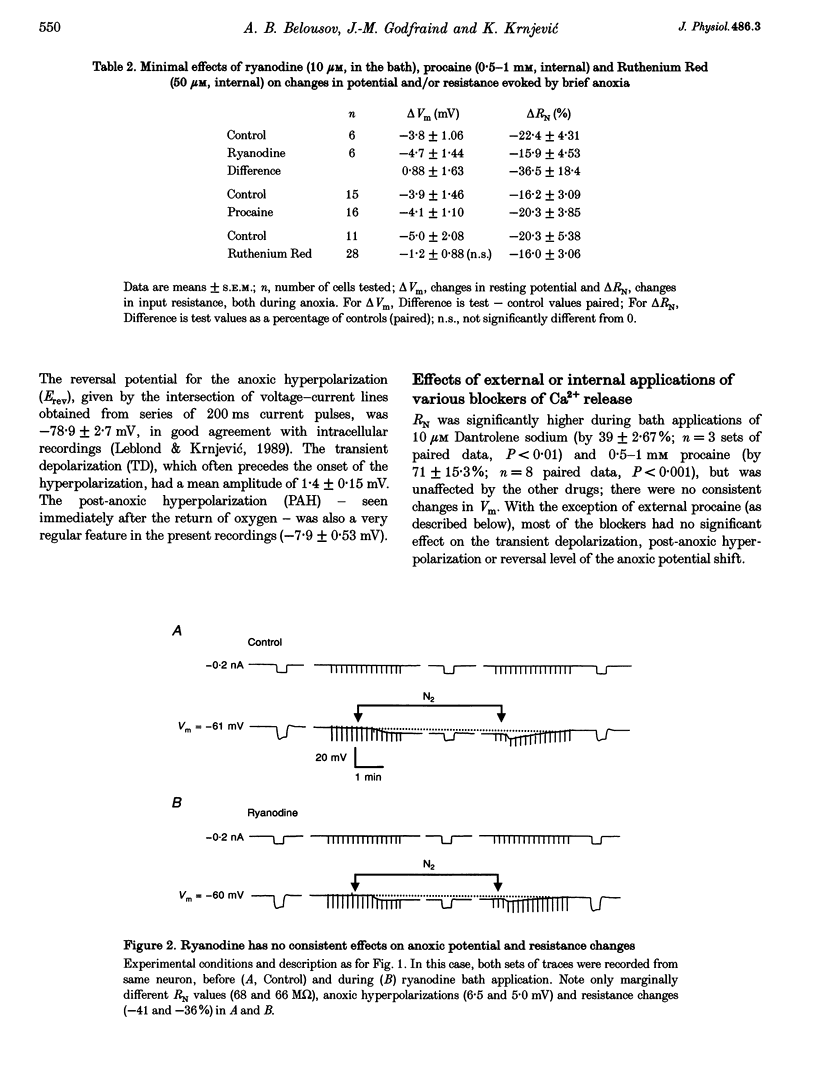
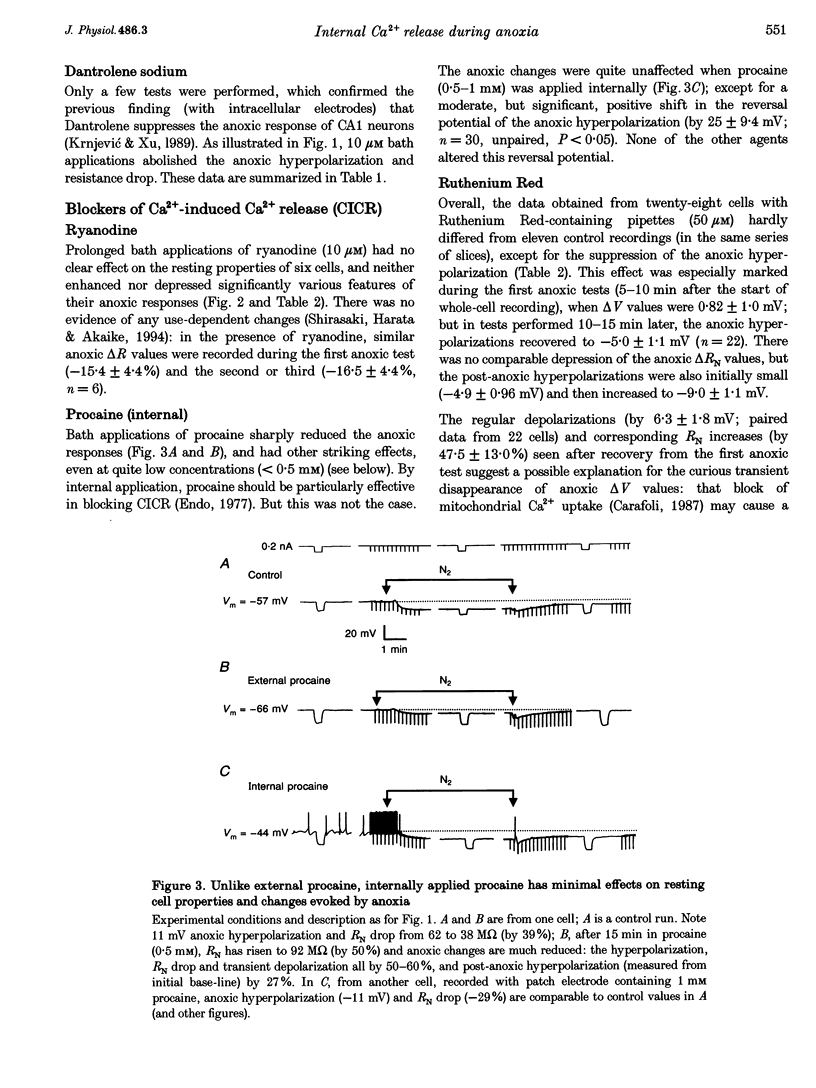
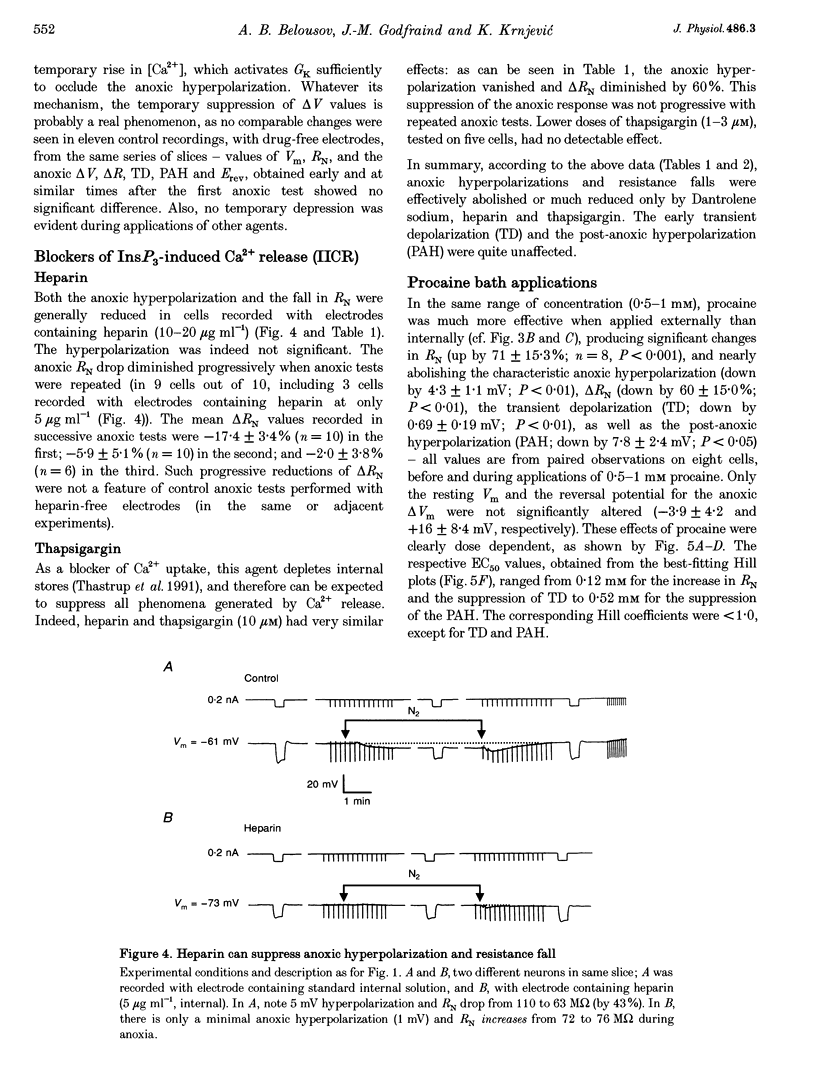
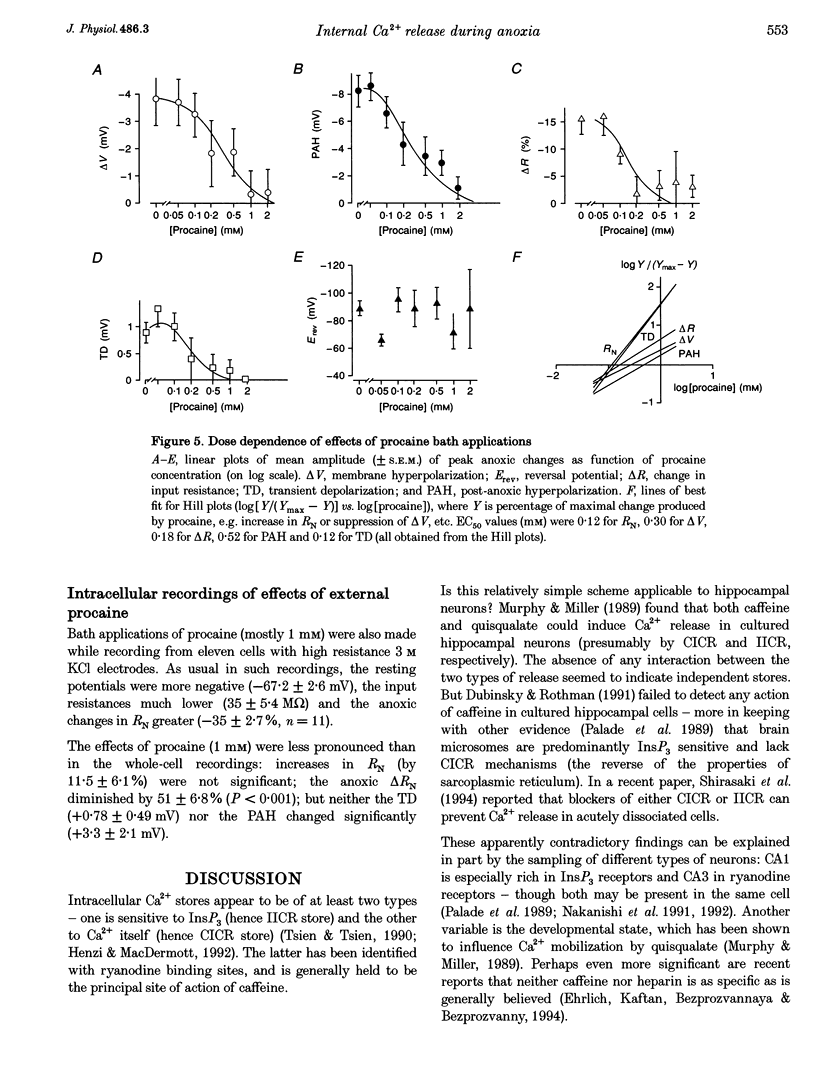



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Butterworth J. F., 4th, Cole L. R. Low concentrations of procaine and diethylaminoethanol reduce the excitability but not the action potential amplitude of hippocampal pyramidal cells. Anesth Analg. 1990 Oct;71(4):404–410. [PubMed] [Google Scholar]
- Butterworth J. F., 4th, Strichartz G. R. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990 Apr;72(4):711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Danko S., Kim D. H., Sreter F. A., Ikemoto N. Inhibitors of Ca2+ release from the isolated sarcoplasmic reticulum. II. The effects of dantrolene on Ca2+ release induced by caffeine, Ca2+ and depolarization. Biochim Biophys Acta. 1985 Jun 11;816(1):18–24. doi: 10.1016/0005-2736(85)90388-8. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Inhibition of the intracellular release of calcium by Dantrolene in barnacle giant muscle fibres. J Physiol. 1977 Feb;265(2):565–585. doi: 10.1113/jphysiol.1977.sp011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Rothman S. M. Intracellular calcium concentrations during "chemical hypoxia" and excitotoxic neuronal injury. J Neurosci. 1991 Aug;11(8):2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Kaftan E., Bezprozvannaya S., Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994 May;15(5):145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Erdemli G., Krnjević K. Tolbutamide suppresses slow and medium afterhyperpolarization in hippocampal slices. Neuroreport. 1994 Oct 27;5(16):2145–2148. doi: 10.1097/00001756-199410270-00039. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Excitatory amino acid-mediated cytotoxicity and calcium homeostasis in cultured neurons. J Neurochem. 1993 Apr;60(4):1202–1211. doi: 10.1111/j.1471-4159.1993.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993 Jan 15;259(5093):325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Hounsgaard J., Jahnsen H. Anoxia increases potassium conductance in hippocampal nerve cells. Acta Physiol Scand. 1982 Jul;115(3):301–310. doi: 10.1111/j.1748-1716.1982.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Sperelakis N. Stimulative and protective action of Sr2+ and Ba2+ on (Na+-K+)-ATPase from cultured heart cells. Biochim Biophys Acta. 1968 Nov 5;163(3):415–417. doi: 10.1016/0005-2736(68)90127-2. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Berggren P. O., Boynton A. L. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat liver cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992 Oct;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Krnjević K., Xu Y. Z. Dantrolene suppresses the hyperpolarization or outward current observed during anoxia in hippocampal neurons. Can J Physiol Pharmacol. 1989 Dec;67(12):1602–1604. doi: 10.1139/y89-258. [DOI] [PubMed] [Google Scholar]
- Leblond J., Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989 Jul;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- Lucas L. F., West C. A., Rigor B. M., Schurr A. Protection against cerebral hypoxia by local anesthetics: a study using brain slices. J Neurosci Methods. 1989 May;28(1-2):47–50. doi: 10.1016/0165-0270(89)90008-3. [DOI] [PubMed] [Google Scholar]
- Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992 Feb;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Mine T., Kojima I., Kimura S., Ogata E. Assessment of the role of Ca2+ mobilization from intracellular pool(s), using dantrolene, in the glycogenolytic action of alpha-adrenergic stimulation in perfused rat liver. Biochim Biophys Acta. 1987 Feb 18;927(2):229–234. doi: 10.1016/0167-4889(87)90139-x. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. Two distinct quisqualate receptors regulate Ca2+ homeostasis in hippocampal neurons in vitro. Mol Pharmacol. 1989 May;35(5):671–680. [PubMed] [Google Scholar]
- Nakanishi S., Kuwajima G., Mikoshiba K. Immunohistochemical localization of ryanodine receptors in mouse central nervous system. Neurosci Res. 1992 Oct;15(1-2):130–142. doi: 10.1016/0168-0102(92)90026-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Maeda N., Mikoshiba K. Immunohistochemical localization of an inositol 1,4,5-trisphosphate receptor, P400, in neural tissue: studies in developing and adult mouse brain. J Neurosci. 1991 Jul;11(7):2075–2086. doi: 10.1523/JNEUROSCI.11-07-02075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. Release of glutamate, aspartate, and gamma-aminobutyric acid from isolated nerve terminals. J Neurochem. 1989 Feb;52(2):331–341. doi: 10.1111/j.1471-4159.1989.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Alderson B., Volpe P. Pharmacologic differentiation between inositol-1,4,5-trisphosphate-induced Ca2+ release and Ca2+- or caffeine-induced Ca2+ release from intracellular membrane systems. Mol Pharmacol. 1989 Oct;36(4):673–680. [PubMed] [Google Scholar]
- Segal M., Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol. 1992 Mar;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T., Harata N., Akaike N. Metabotropic glutamate response in acutely dissociated hippocampal CA1 pyramidal neurones of the rat. J Physiol. 1994 Mar 15;475(3):439–453. doi: 10.1113/jphysiol.1994.sp020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Nahorski S. R. Characterisation and distribution of inositol polyphosphate and Ryanodine receptors in the rat brain. J Neurochem. 1993 May;60(5):1605–1614. doi: 10.1111/j.1471-4159.1993.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Waite M., Sisson P. Effect of local anesthetics on phospholipases from mitochondria and lysosomes. A probe into the role of the calcium ion in phospholipid hydrolysis. Biochemistry. 1972 Aug 1;11(16):3098–3105. doi: 10.1021/bi00766a025. [DOI] [PubMed] [Google Scholar]
- Zhang L., Krnjević K. Whole-cell recording of anoxic effects on hippocampal neurons in slices. J Neurophysiol. 1993 Jan;69(1):118–127. doi: 10.1152/jn.1993.69.1.118. [DOI] [PubMed] [Google Scholar]



