Summary
Cardiovascular diseases (CVDs) are a major global health issue, causing significant morbidity and mortality worldwide. Early diagnosis and continuous monitoring of physiological signals are crucial for managing cardiovascular diseases, necessitating the development of lightweight and cost-effective wearable devices. These devices should incorporate portable energy storage systems, such as lithium-ion batteries (LIBs). To enhance the durability and consistency of the monitoring systems, there is a need to develop LIBs with high energy density. Silicon-based materials hold great promise for future LIBs anodes due to their high theoretical capacity and cost-efficiency. Despite their potential, silicon-based materials encounter challenges like substantial volume fluctuations and sluggish kinetics. Transition metal carbide, MXene, features a two-dimensional structure, offering advantages in silicon-based anode materials. This review initially presents the potential of silicon-based anodes and then addresses their challenges. Subsequently, the advantages of MXene are systematically reviewed, including unique structure, abundant surface functional groups, excellent electrical conductivity, and excellent ion transport performance. Next, the detailed discussion covers recent advancements in Si/Ti3C2Tx MXene anode materials for LIBs, with a focus on their synthesis methods. Finally, the challenges and future perspectives of synthesizing Si/Ti3C2Tx nanocomposites are examined, aiming to provide a foundational resource for designing advanced materials for high-energy LIBs.
Subject areas: Health sciences, Natural sciences, Applied sciences
Graphical abstract

Health sciences; Natural sciences; Applied sciences
Introduction
Cardiovascular diseases (CVDs) are a major global health concern, causing significant morbidity and mortality.1-2 Early diagnosis and continuous monitoring are crucial for effective CVD management. Traditional diagnostic and monitoring methods, such as clinical examinations and static medical devices, have their advantages but are limited in terms of real-time monitoring and convenience. Wearable devices have emerged as an important tool to address this issue due to their characteristics of being lightweight, user-friendly, and cost-effective.3-4 Wearable devices can monitor various physiological parameters related to cardiovascular health, such as heart rate, heart rhythm, blood oxygen saturation, and activity levels. These devices continuously record data, which they can wirelessly transmit to cloud services or smartphone applications, allowing healthcare providers or users to view and analyze the data in real time.5 Multifunctional wearable devices require high-performance lithium-ion batteries (LIBs) to supply power. To improve the durability and consistency of wearable device monitoring systems, it is necessary to develop LIBs with high energy density.6
Energy storage mainly relies on devices like supercapacitors and batteries.7 LIBs are preferred for their high energy density, long life, and low environmental impact, but improving capacity and charging is the key. Graphite, the common negative electrode, has low capacity (∼372 mAh·g−1), while silicon offers much higher capacity (4,200 mAh·g−1) but faces issues like poor conductivity and volume changes. To solve this, silicon is combined with materials like carbon and MXene to improve performance. Silicon offers much higher capacity than graphite for LIB anodes, with a theoretical capacity of 4,200 mAh·g−1, abundant resources, and a suitable working potential. However, its use is limited by poor cycling stability and rate capability due to volume changes during charge/discharge, which damage the electrode and degrade performance.8,9,10,11,12,13,14
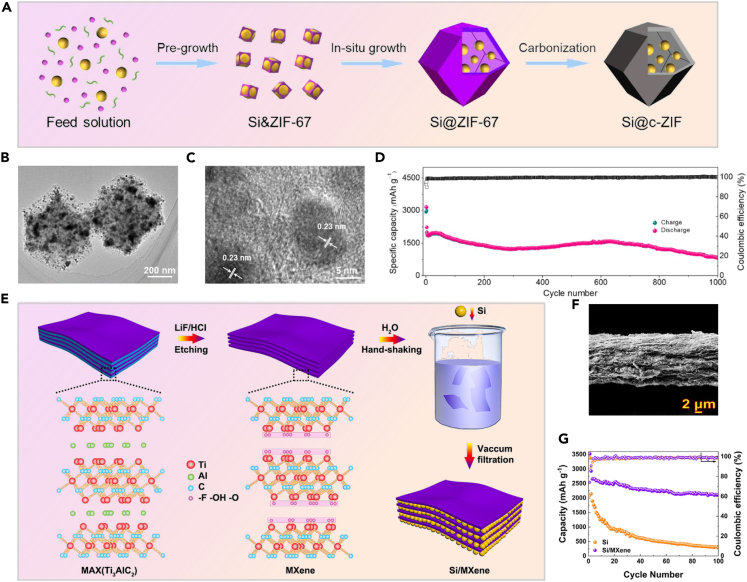
To reduce silicon’s volume changes during charging, researchers add inert or low-expansion materials like Sn, Ag, TiN, SiC, and C, which improve conductivity.15,16,17,18,19 Composite silicon reduces charge-discharge issues, but exposed silicon still causes solid electrolyte interface (SEI) film growth. Encapsulating silicon electrodes forms SEI films on a protective layer, improving capacity and cycling performance.20,21,22,23,24,25,26,27,28,29,30,31,32,33,34 As shown in Figures 1A–1C, Gao et al. introduced an in situ encapsulation technique for Si nanoparticles, resulting in the formation of a metal-organic framework (MOF) carbon shell.35 Si nanoparticles are evenly dispersed in a porous carbon shell from MOF, allowing effective lithium ion diffusion and reducing electrolyte exposure. After 1,000 cycles, the composite electrode retains a reversible capacity of 820 mAh·g−1 at 5 A·g−1.While carbon coatings improve silicon anode conductivity, they still fall short of MXene’s superior conductivity, which enhances overall battery performance. MXene’s functional groups (–OH, –F, and –O) form strong bonds with silicon, boosting mechanical stability and electrochemical performance. Its excellent conductivity and lithium-ion diffusion rate compensate for silicon’s limitations. In Figure 1E, Tian et al. demonstrated this by preparing Si/MXene paper using single-layer MXene mixed with silicon nanoparticles.36 The cross-sectional scanning electron microscopy (SEM) image (Figure 1F) of Si/MXene depicts silicon nanoparticles randomly dispersed within parallel MXene layers, enhancing ion transport throughout cycling. Figure 1G shows that, compared to pure silicon particles, Si/MXene exhibits improved cycling performance over multiple cycles at 0.2 A·g−1.
Figure 1.
MXene has higher conductivity and stability compared to carbon coating
(A) Steps for creating Si@c-ZIF, from solution to final product; (B and C) TEM images of Si@c-ZIF at different magnifications; (D) Cycling tests of Si@c-ZIF starting at 500 mA g−1 and increasing to 5000 mA·g−135 Copyright 2020 Elsevier; (E) Schematic of Si/MXene composite; (F) Cross-sectional SEM image; (G) Cycling stability comparison between Si/MXene and pure Si anodes at 200 mA·g−136 Copyright 2019 American Chemical Society.
Advantages of MXene
MXene, a two-dimensional (2D) transition metal carbide/nitride, has the formula Mn+1XnTx, derived from the precursor MAX with the formula Mn+1AXn, where A denotes a group III or IV element (Al, Sn, Si, Ge, etc.). To date, over 70 types of MAX precursors have been reported, yielding more than 30 types of MXene.37,38,39,40,41,42 MXene, initially identified by researchers at Drexel University in 2011, has since become a focal point of interest within the scientific community.43 The structure of MXene is similar to graphene, but with an oxide layer between the metal atoms, enabling interlayer delamination of the metal carbide. This delamination can be achieved through various chemical treatments, such as acid treatment.44 MXene’s unique properties, such as its 2D layered structure, abundant surface functional groups, excellent conductivity, and ion transport capabilities, make it an ideal electrode material.
Unique 2D layered structure
To understand MXene’s properties, it’s important to examine its 2D layered structure. The precursor MAX phase has alternating MX and A layers, and after etching to remove the A layers, the MXene retains a similar structure to the original MAX phase. In Ti3AlC2, the etched MXene preserves the alternating Al and Ti3C2 layers, with Ti atoms densely packed and C atoms in body-centered positions. This layered structure gives MXene high surface area, flexibility, and numerous active sites.37,45,46,47
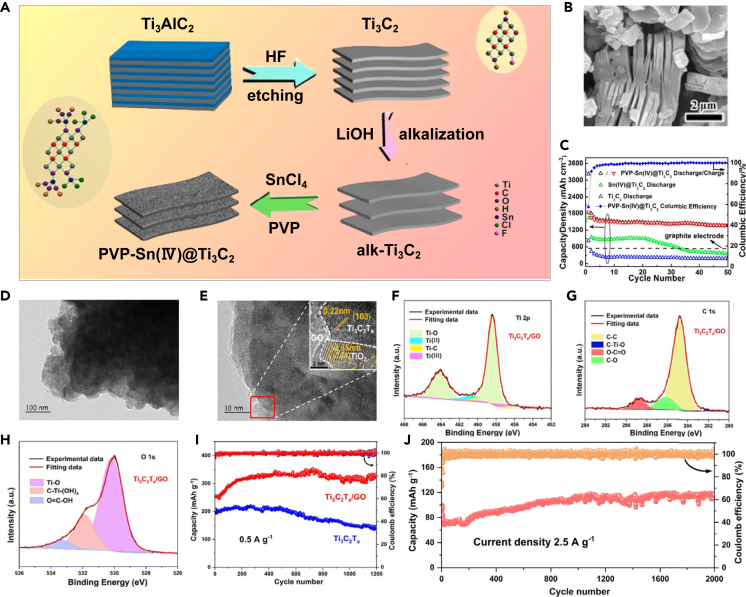
Luo et al. utilized Sn4+ to modify the complex chemical surface microenvironment of Ti3C2Tx MXene.48 As shown in Figures 2A–2C, with the aid of polyvinylpyrrolidone (PVP) as a surfactant, PVP-Sn(IV)@Ti3C2 composite material was prepared via intermolecular electrostatic attraction, and it served as the negative electrode for LIBs. The 2D layered structure of Ti3C2Tx MXene enhances the electrode material’s electron and ion conductivity, manages Sn(IV)’s volume changes during electrochemical reactions, and boosts the overall capacity. Due to the synergistic interaction and the “pillar effect” of Sn(IV) within the Ti3C2Tx MXene framework, the PVP-Sn(IV)@Ti3C2 composite material achieved a volumetric capacity of 1375 mAh·cm−3 at a current density of 216.5 mA cm−3 and a mass capacity of 635 mAh·g−1 at 100 mA g−1.
Figure 2.
MXene has a unique layered structure and a rich variety of functional groups
(A) Production steps for PVP-Sn(IV)@ Ti3C2 nanocomposites; (B) SEM images of Ti3C2 nanosheets; (C) Cycling performance at 0.1 A·g−148 Copyright 2016 American Chemical Society; (D and E) TEM images of Ti3C2Tx/GO; (F–H) High-resolution XPS spectra of Ti3C2Tx/GO (Ti 2p, C 1s, O 1s); (I) Cycle properties of Ti3C2Tx and Ti3C2Tx/GO composites; (J) Cycling stability of Ti3C2Tx/GO composites at 2.5 A·g−149 Copyright 2023 Royal Society of Chemistry.
Abundant surface functional groups
MXene materials have surface groups like –OH, –F, and –O, enabling tunable properties, better material compatibility, and functionalization for applications in catalysis, sensing, and adsorption. These groups also improve dispersibility and stability, making MXene easier to use in coatings, films, and composites.50,51,52,53
As depicted in Figures 2D and 2E, Wang et al. utilized a high-energy ball milling self-assembly technique to process Ti3C2Tx MXene nanosheets and graphene oxide (GO) under vacuum conditions. GO serves as a link between Ti3C2Tx MXene nanosheets in the composite, buffering the mechanical shear force during ball milling, preventing structural damage to the nanosheets, and improving structural stability during lithium processing.49 Figures 2F–2H demonstrate the chemical bonds formed by the abundant functional groups between GO and Ti3C2Tx MXene, which can construct Ti3C2Tx/GO composite materials with strong interfacial interactions, effectively enhancing ion and electron transport at heterogeneous interfaces, improving their electrochemical behavior. In Figures 2I and 2J, after 500 cycles at 0.5 A·g−1, the Ti3C2Tx/GO composite electrode exhibits significantly greater capacity compared to Ti3C2Tx MXene alone. At 2.5 A·g−1, the reversible capacity after 2,000 cycles is 116.5 mAh·g−1, with an outstanding capacity retention rate of 116.6%.
Excellent conductivity
MXene materials exhibit outstanding quasi-metallic conductivity, with an electrical conductivity higher than that of graphene. The theoretical electron mobility can reach 106 cm2/(V·s), whereas graphene is only around 2 × 105 cm2/(V·s). The unique structure and chemical composition of MXene impart excellent conductivity.54,55,56,57,58,59,60,61,62,63,64,65,66
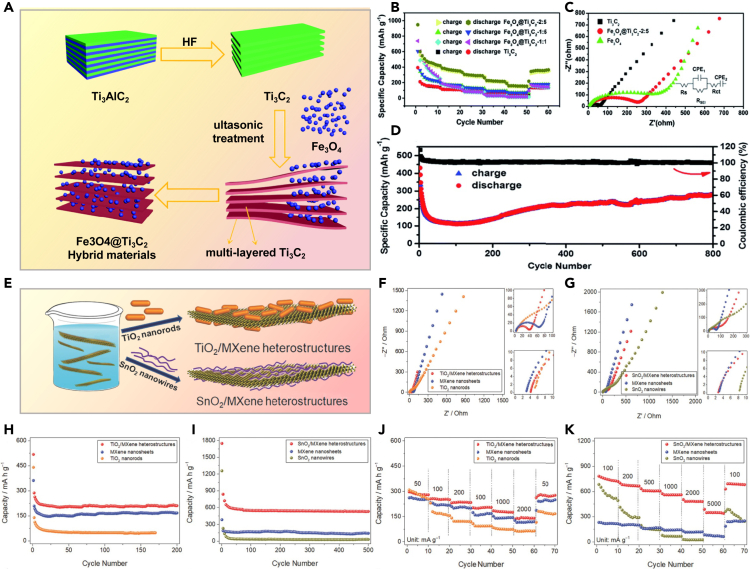
Wang et al. synthesized a series of Fe3O4@Ti3C2 composite materials through simple ultrasonication.67 Benefiting from Ti3C2Tx MXene’s excellent conductivity and Fe3O4’s remarkable lithium storage performance, the electrochemical properties of the Fe3O4@Ti3C2 composite materials outperformed those of pure Fe3O4 or Ti3C2Tx MXene alone, as shown in Figures 3A–3C. In Figure 3D, using the Fe3O4@Ti3C2 composite material at a 2:5 mass ratio as the negative electrode maintained a reversible capacity of 278.3 mAh·g−1 after 800 cycles at 5C. Ti3C2Tx MXene, acting as both the electrochemically active material and conductive matrix, remains pivotal in Ti3C2Tx-based lithium-ion battery electrodes, making it an attractive choice.
Figure 3.
MXene exhibits excellent conductivity and outstanding ion transport properties
(A) Schematic showing the preparation process of Fe3O4@Ti3C2 hybrids; (B) Fe3O4@Ti3C2 and Ti3C2 electrodes’ specific capacities across various charge/discharge rates, including 0.5C, 1C, 2C, 5C, 10C, and 0.5C; (C) EIS diagrams comparing Ti3C2, Fe3O4, and Fe3O4@ Ti3C2-2:5 electrodes, with an inset in Figure 4C displaying an equivalent circuit diagram model; (D) Long-term cycling performance of Fe3O4@ Ti3C2-2:5 at 5C67 Copyright 2018 Royal SOC Chemistry; (E) Schematic illustrating the straightforward self-assembly of TMO nanostructures (TiO2 nanorods and SnO2 nanowires) on MXene nanosheets in THF via van der Waals interactions; (F, H, and J) Nyquist plots, cycling performance (at a current density of 500 mA g−1), and rate capabilities of TiO2 nanorods, MXene nanosheets, and TiO2/MXene heterostructures; (G, I, and K) Nyquist plots, cycling behavior (current density = 1000 mA g−1), and rate capabilities of SnO2 nanowires, MXene nanosheets, and SnO2/MXene heterostructures68 Copyright 2018 WILEY-VCH.
Excellent ion transport performance
The 2D structure of MXene materials allows ions to diffuse and transport rapidly on its plane, thereby enhancing the rate of ion transport. Additionally, MXene materials typically possess abundant surface functional groups, which can adsorb ions and provide additional ion transport pathways, further facilitating ion transport.46,69,70,71,72,73,74,75,76
In Figure 3E, Liu et al. demonstrated the assembly of TiO2 nanorods and SnO2 nanowires onto MXene nanosheets through van der Waals forces.68 The nanostructures serve as insulators to prevent MXene nanosheet restacking, thus preserving active sites. Simultaneously, this structure facilitates rapid lithium-ion transport, enhancing the material’s electrochemical performance. Results in Figures 3F–3K show that the obtained TiO₂/MXene and SnO₂/MXene heterostructures exhibit superb cycling stability and rate capability.
MXene greatly improves silicon anodes in LIBs by mitigating volume expansion with its conductivity and stability. Combining MXene with silicon enhances structural stability, ion transport, and electrochemical performance, improving cycle stability and capacity retention. It shows strong potential for future high-performance LIBs. Although MXene shows great potential for high-performance LIBs, it faces challenges in practical use. Its current preparation process is complex and costly, making large-scale production difficult. To overcome this, developing safer, more eco-friendly synthesis methods, such as using hydrochloric acid and sodium fluoride instead of hydrofluoric acid, can reduce risks and simplify production. Additionally, MXene is highly prone to oxidation, which degrades its performance, so it must be stored in sealed, dry, and light-protected conditions.
Synthesis of Si/MXene composite materials
Silicon is a promising negative electrode material for lithium-ion batteries due to its high capacity, but its volume changes during charge/discharge limit its use in commercial batteries. Researchers are exploring composite materials to improve the stability and cycling performance of Si electrodes. MXene, with its excellent conductivity and stability, offers a solution. By physically dispersing nanoscale Si particles on MXene or embedding them between layers, volume expansion can be reduced and conductivity improved. Chemical modifications can further enhance Si-MXene interaction, preventing particle aggregation and improving cycling stability and performance.
Physical composite
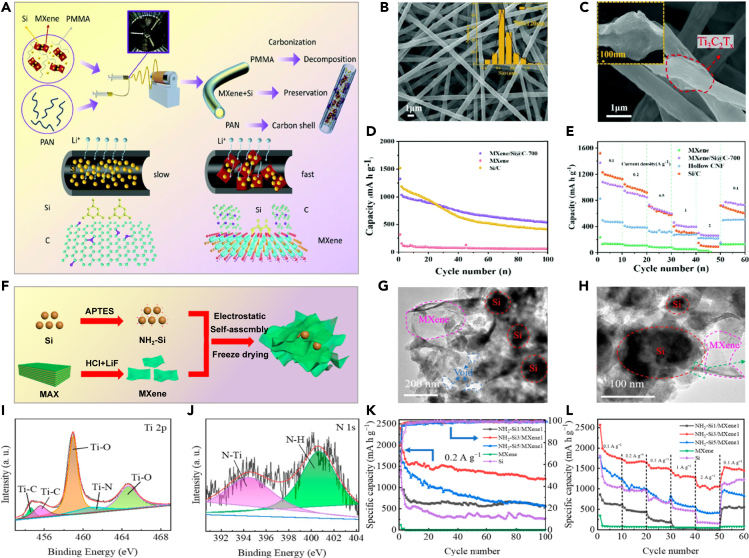
Through physical synthesis methods, Si nanoparticles are uniformly dispersed, resulting in high specific capacity and rate performance. As shown in Figure 4A, Liu et al. prepared MXene-Si-CNT composite materials through simple ball milling. Transmission electron microscopic (TEM) images in Figure 4B reveal the close integration of silicon particles with the surfaces of MXene and carbon nanotubes (CNTs).77 Figure 4C shows silicon particles bond with MXene and CNTs, enhancing the cycling stability of the composite material. Figure 4D demonstrates that the MXene-Si-CNT (MSC) composite, containing 60wt % silicon, outperforms others, maintaining about 80% capacity after 200 cycles. As depicted in Figures 4E–4H, Kong et al. synthesized Si/MXene composites via ultrasonic mixing, with silicon nanoparticles coating the Ti3C2 substrate surface and integrating into the multilayer structure.78 The preparation of Ti3C2 in this method is simpler than that of graphene, resulting in improved electrochemical performance. At 0.2 A·g−1, the reversible capacity remains at 188 mAh·g−1 after 150 cycles, attributed to enhanced Si-Ti3C2 contact.
Figure 4.
Mechanical ball milling and ultrasonic mixing are used to prepare composites
(A) Illustration of the synthesis steps for MSC composites; schematic depiction of the mechanochemical process involving ball-milling MXene with Si and CNT to yield MSC composites; (B) TEM images showing the MSC-60 composite, comprising MXene, Si, and CNT; (C) High-resolution XPS spectra of Si 2p after Ar+ ion sputtering; (D) Cycling performance and Coulombic efficiency of various composites77 Copyright 2019 American Chemical Society; (E) Schematic of Si@Ti3C2 nanocomposite preparation; (F) X-ray diffraction (XRD) patterns of Ti3C2, Si nanoparticles and Si@Ti3C2 nanocomposite; (G) SEM images of Si@Ti3C2 nanocomposite; (H) Cycling performance at 0.2 A·g−178 Copyright 2018 Elsevier.
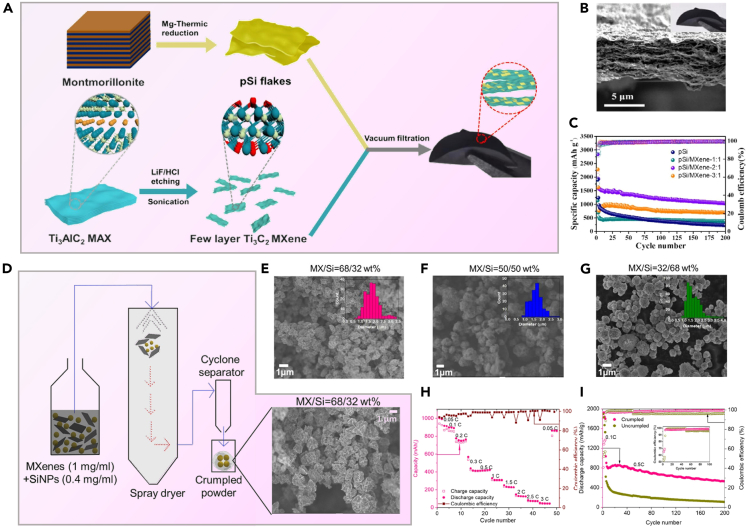
Zhang et al. used vacuum filtration to create flexible, self-supporting, binder-free porous silicon (pSi)/MXene-2:1 composite film (pSi/MXene 2:1 films) as LIB anodes, as shown in Figure 5A.79 Figure 5B shows that pSi has a flake-like structure, derived from layered montmorillonite, which facilitates the reduction of ion transport distance. Furthermore, the pSi/MXene-2:1 film exhibits remarkable mechanical flexibility, with its structure containing voids that can accommodate volume expansion during cycling. Leveraging these structural benefits, Figure 5C illustrates the consistent cycling stability of the pSi/MXene-2:1 film anode over 200 cycles, maintaining a capacity of 1039.3 mAh·g−1 at 500 mA g−1. Sarang et al. used the spray drying technique to prepared Si/MXene composite materials with a core-shell structure. The wrinkled particles for the anode, depicted in Figure 5D,80 and SEM images in Figures 5E–5G demonstrate that different MXene/Si ratios all exhibit Si nanoparticles encapsulated by wrinkled MXene. Figures 5H and 5I showcase the impressive rate performance and cycling stability of the composite material at an electrode loading of 1.5 mg cm−2. By the 200th cycle, the composite attains a total specific capacity of 550 mAh·g−1 under a total current density of 1.7 A·g−1.
Figure 5.
Vacuum filtration and spray drying are used to prepare composites
(A) Schematic illustration for the preparation of pSi/MXene-2:1 film; (B) cross-section SEM of pSi/MXene-2:1 film; (C) Cycling performance comparison of pSi/MXene films with varied mass ratios and pSi anodes at 0.5 A·g−179 Copyright 2023 Elsevier; (D) Schematic of spray dryer setup for MXene-SiNP crumpling, with SEM image of crumpled product.; (E–G) SEM images depict three spray-dried mixtures: MX/Si ratios of 68/32, 50/50, and 32/68 by weight. The inset histograms illustrate the distribution of crumpled particle sizes; (H) Rate performance of MX/Si = 32/68 electrode containing 5 wt % PVDF; (I) The galvanostatic cycling performance of both crumpled and non-crumpled MX/Si = 32/68 electrodes was compared. The inset provides a magnified view of the Coulombic efficiency versus cycle number for the initial 50 cycles80 Copyright 2021 American Chemical Society.
Doping silicon/MXene composites with other elements is a common strategy to improve properties like conductivity, chemical stability, and ion transport. This optimization enhances their effectiveness in energy storage and conversion applications, such as in LIBs. Jiang et al. employed a simple coaxial electrospinning method to craft core-shell MXene/Si@C nanofibers, as illustrated in Figure 6A.81 These nanofibers provide several distinctive structural benefits that enhance the performance of silicon particles. Figures 6B and 6C demonstrate that MXene nanosheets function as conductive substrates, effectively linking silicon particles and carbon shells to create a conductive network. This network facilitates rapid charge transfer and lithium-ion migration. The sturdy carbon shell and MXene nanosheets collaboratively manage the significant volume expansion of silicon during charge and discharge cycles, ensuring the structural stability of the electrode. Figures 6D and 6E illustrate that the resulting negative electrode material demonstrates excellent electrochemical performance, achieving a high capacity of 1083 mAh·g−1 at 0.1 A·g−1 and impressive rate performance of 301.1 mAh·g−1 at a current density of 2 A·g−1. In Figures 6F–6L, Yang et al. developed a stable Si/Ti3C2Tx MXene composite material using a straightforward freeze-drying method. They bonded silicon nanoparticles to –NH2 groups, purportedly imparting a positive charge to the silicon surface, which allows for electrostatic self-assembly with negatively charged MXene nanosheets.82 Anchored on the surface or within the gaps of MXene nanosheets, silicon nanoparticles enhance the composite material’s conductivity. The NH2-Si/MXene composite features a stable porous structure, providing extra space for silicon expansion and ample channels for charge carrier transport. After 100 cycles at 200 mA g−1, this composite material achieves a discharge capacity of 1203.3 mAh·g−1, and at a current density of 2 A·g−1, it maintains a capacity of 1046.1 mAh·g−1.
Figure 6.
Coaxial electrospinning and freeze drying are used to prepare composites
(A) A schematic illustrating the preparation process of core–shell MXene/Si@C nanofibers; (B and C) SEM images of MXene/Si@C-700; (D) Cycling performance of MXene/Si@C-700, Si/C and Mxene; (E) Comparison of the rate performance among MXene/Si@C-700, Si/C nanofibers, hollow Carbon Nanofibers (CNFs), and MXene81 Copyright 2021 Royal Society of Chemistry; (F) Schematic illustrating the synthesis process of NH2-Si/MXene composites; (G and H) TEM images of NH2-Si3/MXene1 composites; (I and J) corresponding high-resolution XPS spectra of Ti 2p and N 1s; (K) Cycling stability comparison among NH2-Si1/MXene1, NH2-Si3/MXene1, NH2-Si5/MXenel, MXene, and Si negative electrodes at 200 mA g−1; (L) Rate capacity comparison of NH2-Si1/MXenel, NH2-Si3/MXenel, NH2-Si5/MXenel, MXene, and Si negative electrodes at different specific currents82 Copyright 2023 MDPI.
Despite the advantages of physical methods, such as simplicity of process, ease of operation, and suitability for large-scale production, they also face certain challenges. For instance, high-energy ball milling consumes a significant amount of energy, which increases production costs, and physical methods cannot achieve precise control over the material.
Chemical composite
Chemical synthesis offers finer control over the structure, shape, and composition of composite materials compared to physical synthesis. Additionally, chemical methods can achieve nano-level composites of Si and MXene, enhancing lithium-ion diffusion rates and thereby improving battery performance and cycle life.
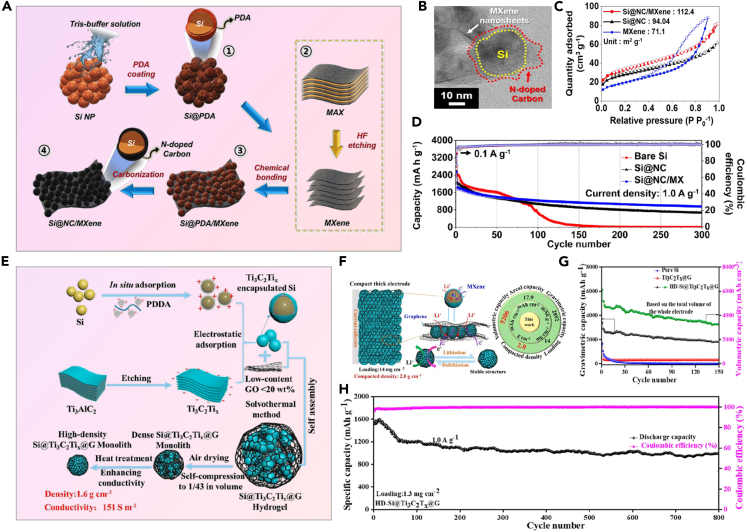
As shown in Figures 7A–7D, Li et al. first modified silicon with hexadecyl trimethyl ammonium bromide. Subsequently, they compounded it with Ti3C2Tx MXene nanosheets and synthesized a three-dimensional Si/Ti3C2Tx MXene composite material via a hydrothermal reaction in an alkaline environment.83 Compared to physical synthesis, chemical synthesis provides more precise control over the structure, morphology, and composition of composite materials. The outer MXene layer facilitates quick electron transfer and ion migration while also buffering volume changes. Crucially, the dual MXene wrapping design helps to form a stable SEI film by preventing direct contact between Si and the electrolyte. The composite material initially achieves a capacity of 574 mAh·g−1 at 5 A·g−1. After 200 cycles at 0.5 A·g−1, it retains a high capacity of 1422 mAh·g−1, indicating excellent cycling stability and rate performance. In Figures 7E–7I, Yang et al. prepared silicon-distributed Si/MXene composite materials using a simple and effective electrostatic assembly method.84 The incorporation of silicon nanoparticles prevents Ti3C2 nanosheets from restacking, yielding a composite material with mesopores and significantly increased surface area compared to pure Ti3C2, thus providing more active sites. X-ray photoelectron spectroscopy (XPS) analysis reveals that the structure of Ti3C2 remains preserved after Si NP deposition. After 200 cycles at 1 A·g−1, the capacity remains at 1342.8 mAh·g−1. Even at a high current density of 3 A·g−1, the capacity remains stable at 1515.8 mAh·g−1.
Figure 7.
Hydrothermal and electrostatic assembly are used to prepare composites
(A) Synthesis process schematic for SiNP@MX1/MX2; (B) TEM images of SiNP@MX1/MX2; (C) Rate performance at different current densities; (D) Extended cycling stability at 0.5 A·g−183 Copyright 2020 American Chemical Society; (E) Schematic illustration of the fabrication of Si@Ti3C2 superstructures; (F) N2 adsorption/desorption isotherms and corresponding pore-size distribution profile (inset) of Si@Ti3C2 and Ti3C2; (G) The corresponding high-resolution XPS spectra of O 1s; (H) Cycling performance of pristine Si and different Si@Ti3C2 at a current density of 1 A·g−1; (I) Rate-capability assessment of different Si@Ti3C2 configurations84 Copyright 2020 Elsevier.
Jo et al. chemically bonded Polydopamine (PDA)-coated Si nanoparticles with MXene, followed by heat treatment under inert atmosphere to prepare Si@N-doped carbon/Mxene (Si@NC/MXene) composite material, as shown in Figure 8A.85 The magnified TEM image in Figure 8B reveals the close interaction between few-layer MXene nanosheets and NC-coated Si nanoparticles through chemical bonding, which results in a higher surface area for the Si@NC/MX composite material compared to Si@NC composite material, as depicted in Figure 8C. This composite material features pore structures smaller than 10 nm, attributed to the interlayer spacing of MXene nanosheets. The presence of Si@NC nanoparticles on the MXene nanosheets prevents restacking and enhances the surface area. Consequently, the composite material exhibits a high reversible capacity of 953 mAh·g−1 after 300 cycles at 1 A·g−1, as shown in Figure 8D. In Figure 8E, Liu et al. devised a novel method to create a 3D high-density structure (HD-Si@Ti3C2Tx@G) by employing dual encapsulation for silicon structure and dense structure engineering.86 The process illustrated in Figure 8F shows positively charged silicon (Si) encased within negatively charged Ti3C2Tx MXene, forming a dense structure alongside graphene. This configuration effectively counters Si’s volume expansion during cycling. The 3D network of Ti3C2Tx MXene and graphene in the HD-Si@Ti3C2Tx@G electrode ensures efficient electron transfer, electrolyte wettability, and structural stability, addressing challenges such as sluggish ion and electron kinetics and instability in thick silicon-based electrodes. The dense structure also enhances electrode’s density and volumetric performance. As a result, the compact HD-Si@Ti3C2Tx@G electrode achieves notable volumetric and areal capacities. As depicted in Figures 8G and 8H, due to its high conductivity (151 S/m) and 3D double-encapsulated Si structure, the HD-Si@Ti3C2Tx@G anode maintains 3240 mAh·g−1 after 150 cycles at 0.1 A·g−1 and 984.9 mAh·g−1 after 800 cycles at 1.0 A·g−1.
Figure 8.
Anneal and heat treatment and self assembly are used to prepare composites
(A) Fabrication process of Si@NC/MXene composites; (B) TEM images of Si@NC/MXene composites; (C) N2 adsorption/desorption isotherms for MXene, Si@NC, and Si@NC/MX composites; (D) Cycling stability of electrodes with bare Si, Si@NC, and Si@NC/MX composites85 Copyright 2021 Elsevier; (E) Synthesis process of HD-Si@Ti3C2Tx@G monolith; (F) Microstructure and lithium storage mechanism of the dense electrode; (G) Cycling stability at 0.1 A·g−1; (H) Extended cycling durability at 1.0 A·g−1 for HD-Si@Ti3C2Tx @G anodes with different mass loads86 Copyright 2022 American Chemical Society.
Chemical methods for making Si/MXene composites offer good structural control but have challenges like complexity, environmental impact from by-products, and potential impurities that can affect material performance.
In situ growth of SiO2 on reduced MXene surface
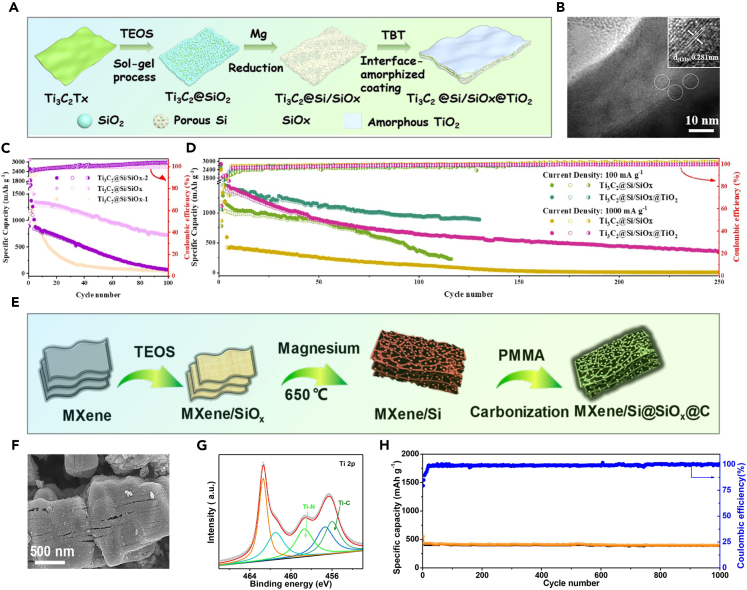
Silicon has a high capacity of 4200 mAh·g−1, but its 400% volume expansion during cycling harms performance. To improve stability, nanoscale Si particles and carbon coatings are used. Ti3C2Tx MXene has also been added as a buffer for Si anodes, but traditional synthesis methods are complex and not suited for large-scale production. Jiang et al. introduced a simpler approach using magnesium thermal reduction to prepare Si/Ti3C2Tx MXene, followed by coating with TiO2 to form a sandwich-like Ti3C2@Si/SiOx@TiO2 composite, as shown in Figures 9A and 9B.87 The Ti3C2@Si/SiOx@TiO2 composite achieved 939 mAh·g−1 after 100 cycles at 500 mA g−1 and 365 mAh·g−1 after 250 cycles at 1000 mA g−1, with a Coulombic efficiency over 99.6%., as shown in Figures 9C and 9D.
Figure 9.
Magnesium thermal reduction are used to prepare composites
(A) The formation stages of the Ti3C2@Si/SiOx@TiO2 composite; (B) HRTEM images of Ti3C2@Si/SiOx@TiO2; (C) Cycling performance of Ti3C2@Si/SiOx-1, Ti3C2@Si/SiOx, and Ti3C2@Si/SiOx-2 anodes at 500 mA g−1; (D) Cycling performance of Ti3C2@Si/SiOx@TiO2 and Ti3C2@Si/SiOx anodes at various current densities87 Copyright 2020 American Chemical Society; (E) Preparation process of MXene/Si@SiOx@C nanohybrids; (F) SEM images of MXene/Si@SiOx@C-2; (G) Ti 2p XPS spectrum of MXene/Si@SiOx@C-2; (H) Cycling performance of MXene/Si@SiOx@C-2 over 1000 cycles at 10 C88 Copyright 2019 American Chemical Society.
Zhang et al. created Si/MXene composite materials through magnesium thermal reduction,88 converting MXene/SiO2 to MXene/Si. Urea formed a nitrogen-doped carbon layer around Si, resulting in a porous structure. This anode, with high lithium capacity and strong Ti-N bonds, retained 76.4% capacity after 1,000 cycles at 10 C, as seen in Figures 9E–9H.
When using the magnesiothermic reduction method to reduce silicon dioxide, magnesium as a reducing agent has a very high reduction capability, which can effectively reduce the silicon source to elemental silicon, ensuring high purity of silicon in the composite material. Additionally, magnesium is a relatively inexpensive and readily available reducing agent, giving the magnesiothermic reduction method a cost advantage suitable for large-scale production. Despite its many advantages, the magnesiothermic reduction method also has notable drawbacks. It typically requires high temperatures, which pose certain safety hazards. Moreover, the process produces by-products such as MgO, which need to be further processed and removed; otherwise, they can affect the purity and performance of the composite material.
Summary and outlook
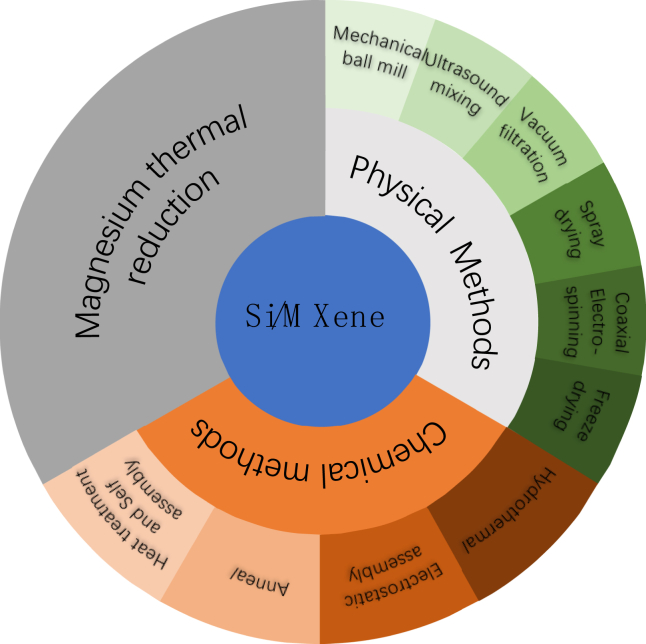
Early diagnosis and continuous monitoring are crucial for effectively managing cardiovascular diseases (CVD). Currently, wearable devices play a key role in the diagnosis and treatment of CVD by real-time monitoring of various cardiovascular health-related physiological parameters, such as heart rate, heart rhythm, blood oxygen saturation, blood pressure, and activity levels. Continuous technological advancements in these devices, coupled with their widespread adoption, are expected to significantly enhance the prevention, monitoring, and management of CVD, thereby providing strong support for global cardiovascular health. However, as wearable devices incorporate more functions, the demand for high-performance LIBs increases. Flexible Si/MXene anodes are promising for wearable devices that monitor physiological parameters. These anodes combine silicon’s high capacity with MXene’s excellent conductivity and flexibility, making them ideal for stretchable electronics. While silicon’s volume changes during charging reduce battery life, MXene’s structure helps accommodate this expansion and maintain electrical contact. This combination enhances electrochemical performance and ensures the flexibility needed for wearables, leading to more durable and efficient power sources for reliable physiological monitoring. This paper reviews recent progress on Si/MXene composites for improving lithium-ion battery performance, discussing key factors affecting their efficiency and proposing strategies to address challenges (Figure 10). It suggests focusing on: Optimizing Si/MXene structures at the nanoscale and surface functionalization for better electrochemical properties and stability; Developing simpler, low-cost methods for composite preparation and large-scale production; Studying interfacial reactions and stability with solid electrolytes to improve charge efficiency and cycle life; Exploring dopants like NH2 and C to enhance performance through optimized doping.
Figure 10.
Overview of Si/MXene synthesis methods
Physical methods for synthesizing Si/MXene composites include electrostatic assembly, ultrasonic mixing, and vacuum filtration, which ensure uniform dispersion of Si within MXene, stabilizing electrode materials and reducing degradation. These methods help create densely packed Si nanostructures for advanced LIBs, supporting flexible battery designs. Chemical methods, like annealing and hydrothermal processes, control the structure, size, and surface properties of the composites, optimizing electrochemical performance. Si/MXene composites can also be made by reducing in situ grown SiO2 on MXene, improving stability and mitigating volume expansion. Although still in early stages, Si/MXene electrodes show potential, with research focusing on refining production, improving interfaces, and exploring doping modifications for better performance and scalability. Table 1 outlines the synthesis techniques and evaluations of Si/MXene negative electrodes for LIBs.
Table 1.
Comparison of electrochemical properties of Si/MXene composites synthesized by different methods
| Anodes | Method | Specific Capacity (mAh/g) | Cycling Stability (mAh/g) | Rate Capacity (mAh/g) | Reference | |
|---|---|---|---|---|---|---|
| Si/MXene | Physical Methods |
Mechanical ball mill | 3486.2 | 980.8 after 500 cycles at 0.5A/g | 632.4 at 5 A/g | Liu et al.77 |
| Ultrasound mixing | 1195 | 188 after 150 cycles at 0.2A/g | – | Kong et al.78 | ||
| Vacuum filtration | 2843.5 | 555.5 after 500 cycles at 1A/g | 840.3 at 5 A/g | Zhang et al.79 | ||
| Spray drying | 2610 | 550 after 200 cycles at 1.7A/g | – | Sarang et al.80 | ||
| Coaxial electrospinning | 1377.8 | 540 after 100 cycles at 0.1A/g | 301.1 at 2 A/g | Jiang et al.81 | ||
| Freeze drying | 2395.1 | 1203.3 after 100 cycles at 0.2A/g | 1046.1 at 2 A/g | Yang et al.82 | ||
| Chemical methods | Hydrothermal | 3257 | 1422 after 200 cycles at 0.5A/g | 574 at 5 A/g | Li et al.83 | |
| Electrostatic assembly | 3502.3 | 1342.8 after 200 cycles at 1A/g | 1515.8 at 3 A/g | Yang et al.84 | ||
| Anneal | 2554 | 953 after 300 cycles at 1A/g | 849 at 1 A/g | Jo et al.85 | ||
| Heat treatment and Self assembly | 3388.9 | 984.9 after 800 cycles at 1A/g | 1033.6 at 2 A/g | Liu et al.86 | ||
| Magnesium thermal reduction |
2517 | 939 after 100 cycles at 0.5A/g | – | Jiang et al.87 | ||
| 1674 | 390 after 1000 cycles at 42A/g | 398 at 42 A/g | Zhang et al.88 | |||
Acknowledgments
The work was supported by the National Natural Science Foundation of China (52002170), the Central Guidance Fund Project for Local Scientific and Technological Development in Qinghai Province (2024ZY013), the Natural Science Foundation of Jiangsu Province (BK20190687), the State Key Laboratory of Materials- Oriented Chemical Engineering (no. KL19-03), the Foundation of Key Laboratory of Flexible Electronics of Zhejiang Province (no. 2023FE011), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_1429).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jiwei Hou, Email: jwhou@njtech.edu.cn.
Shan Jiang, Email: jiangshan@xidian.edu.cn.
Leichao Meng, Email: leichao5166@163.com.
Yi Zhang, Email: zhangy@njtech.edu.cn.
References
- 1.Khraishah H., Alahmad B., Ostergard R.L., AlAshqar A., Albaghdadi M., Vellanki N., Chowdhury M.M., Al-Kindi S.G., Zanobetti A., Gasparrini A., Rajagopalan S. Climate change and cardiovascular disease: implications for global health. Nat. Rev. Cardiol. 2022;19:798–812. doi: 10.1038/s41569-022-00720-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu S., An S., Li W., Lichtenstein A.H., Gao J., Kris-Etherton P.M., Wu Y., Jin C., Huang S., Hu F.B., Gao X. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X., Kim D.-H., Lu N. Introduction: Wearable devices. Chem. Rev. 2024;124:6145–6147. doi: 10.1021/acs.chemrev.4c00271. [DOI] [PubMed] [Google Scholar]
- 4.Lin W., Tang C., Wang F., Zhu Y., Wang Z., Li Y., Wu Q., Lei S., Zhang Y., Hou J. Building low-cost, high-performance flexible photodetector based on tetragonal phase VO2 (A) nanorod networks. Materials. 2023;16:6688. doi: 10.3390/ma16206688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayoumy K., Gaber M., Elshafeey A., Mhaimeed O., Dineen E.H., Marvel F.A., Martin S.S., Muse E.D., Turakhia M.P., Tarakji K.G., Elshazly M.B. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat. Rev. Cardiol. 2021;18:581–599. doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S., Cheng T., Zhang Y.Z., Wu X., Xiao S., Lai W.-Y. Deformable lithium-ion batteries for wearable and implantable electronics. Appl. Phys. Rev. 2022;9 [Google Scholar]
- 7.Zhu Y., Wang Z., Zhu X., Feng Z., Tang C., Wang Q., Yang Y., Wang L., Fan L., Hou J. Optimizing performance in supercapacitors through surface decoration of bismuth nanosheets. ACS Appl. Mater. Interfaces. 2024;16:16927–16935. doi: 10.1021/acsami.3c17699. [DOI] [PubMed] [Google Scholar]
- 8.Chan C.K., Peng H., Liu G., McIlwrath K., Zhang X.F., Huggins R.A., Cui Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008;3:31–35. doi: 10.1038/nnano.2007.411. [DOI] [PubMed] [Google Scholar]
- 9.Goriparti S., Miele E., De Angelis F., Di Fabrizio E., Proietti Zaccaria R., Capiglia C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources. 2014;257:421–443. [Google Scholar]
- 10.Liu J., Kopold P., van Aken P.A., Maier J., Yu Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a Silicon anode for lithium-ion batteries. Angew. Chem. Int. Ed. 2015;54:9632–9636. doi: 10.1002/anie.201503150. [DOI] [PubMed] [Google Scholar]
- 11.Magasinski A., Dixon P., Hertzberg B., Kvit A., Ayala J., Yushin G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010;9:353–358. doi: 10.1038/nmat2725. [DOI] [PubMed] [Google Scholar]
- 12.Song T., Xia J., Lee J.-H., Lee D.H., Kwon M.-S., Choi J.-M., Wu J., Doo S.K., Chang H., Park W.I., et al. Arrays of sealed Silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010;10:1710–1716. doi: 10.1021/nl100086e. [DOI] [PubMed] [Google Scholar]
- 13.Huang K., Hou J., Zhang Q., Ou G., Ning D., Hussain N., Xu Y., Ge B., Liu K., Wu H. Ultrathin two-dimensional metals with fully exposed (111) facets. Chem. Commun. 2017;54:160–163. doi: 10.1039/c7cc07923k. [DOI] [PubMed] [Google Scholar]
- 14.Wu H., Chan G., Choi J.W., Ryu I., Yao Y., McDowell M.T., Lee S.W., Jackson A., Yang Y., Hu L., Cui Y. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012;7:310–315. doi: 10.1038/nnano.2012.35. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu L.Y., Hewitt K.C., Turner R.L., Bonakdarpour A., Abdo A.A., Christensen L., Eberman K.W., Krause L.J., Dahn J.R. The electrochemical reaction of Li with amorphous Si-Sn alloys. J. Electrochem. Soc. 2003;150:149–156. [Google Scholar]
- 16.Hwang S.-M., Lee H.-Y., Jang S.-W., Lee S.-M., Lee S.-J., Baik H.-K., Lee J.-Y. Lithium insertion in SiAg powders produced by mechanical alloying. Electrochem. Solid State Lett. 2001;4:97–100. [Google Scholar]
- 17.Kim I., Kumta P.N., Blomgren G.E. Si/TiN nanocomposites-Novel anode materials for Li-ion batteries. Electrochem. Solid State Lett. 2000;3:493–496. [Google Scholar]
- 18.Kim I.-s., Blomgren G.E., Kumta P.N. Si–SiC nanocomposite anodes synthesized using high-energy mechanical milling. J. Power Sources. 2004;130:275–280. [Google Scholar]
- 19.Yang X., Wen Z., Xu X., Lin B., Lin Z. High-performance silicon/carbon/graphite composites as anode materials for lithium ion batteries. J. Electrochem. Soc. 2006;153:1341–1344. [Google Scholar]
- 20.Chen W., Kuang S., Wei H., Wu P., Tang T., Li H., Liang Y., Yu X., Yu J. Dual carbon and void space confined SiOx/C@void@Si/C yolk-shell nanospheres with high-rate performances and outstanding cyclability for lithium-ion batteries anodes. J. Colloid Interface Sci. 2022;610:583–591. doi: 10.1016/j.jcis.2021.11.099. [DOI] [PubMed] [Google Scholar]
- 21.Gao P., Huang X., Zhao Y., Hu X., Cen D., Gao G., Bao Z., Mei Y., Di Z., Wu G. Formation of Si hollow structures as promising anode materials through reduction of silica in AlCl3–NaCl molten salt. ACS Nano. 2018;12:11481–11490. doi: 10.1021/acsnano.8b06528. [DOI] [PubMed] [Google Scholar]
- 22.Guo S., Hu X., Hou Y., Wen Z. Tunable synthesis of yolk–shell porous silicon@carbon for optimizing Si/C-based anode of lithium-ion batteries. ACS Appl. Mater. Interfaces. 2017;9:42084–42092. doi: 10.1021/acsami.7b13035. [DOI] [PubMed] [Google Scholar]
- 23.He D., Li P., Wang W.A., Wan Q., Zhang J., Xi K., Ma X., Liu Z., Zhang L., Qu X. Collaborative design of hollow nanocubes, in situ cross-linked binder, and amorphous Void@SiO@C as a three-pronged strategy for ultrastable lithium storage. Small. 2020;16 doi: 10.1002/smll.201905736. [DOI] [PubMed] [Google Scholar]
- 24.He R., Wang Y., Zhang C., Liu Z., He P., Hong X., Yu R., Zhao Y., Wu J., Zhou L., et al. Sequential and dendrite-free Li plating on Cu foil enabled by an ultrathin yolk–shell SiO/C@C layer. Adv. Energy Mater. 2023;13 [Google Scholar]
- 25.Hu L., Luo B., Wu C., Hu P., Wang L., Zhang H. Yolk-shell Si/C composites with multiple Si nanoparticles encapsulated into double carbon shells as lithium-ion battery anodes. J. Energy Chem. 2019;32:124–130. [Google Scholar]
- 26.Li B., Jiang Y., Jiang F., Cao D., Wang H., Niu C. Bird's nest-like nanographene shell encapsulated Si nanoparticles – Their structural and Li anode properties. J. Power Sources. 2017;341:46–52. [Google Scholar]
- 27.Liu N., Wu H., McDowell M.T., Yao Y., Wang C., Cui Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012;12:3315–3321. doi: 10.1021/nl3014814. [DOI] [PubMed] [Google Scholar]
- 28.Lu B., Ma B., Deng X., Wu B., Wu Z., Luo J., Wang X., Chen G. Dual stabilized architecture of hollow Si@TiO2@C nanospheres as anode of high-performance Li-ion battery. Chem. Eng. J. 2018;351:269–279. [Google Scholar]
- 29.Mu G., Mu D., Wu B., Ma C., Bi J., Zhang L., Yang H., Wu F. Pomegranate-like shell structured Si@C with tunable inner-space as an anode material for lithium-ion battery. J. Power Sources. 2019;441 [Google Scholar]
- 30.Yao Y., McDowell M.T., Ryu I., Wu H., Liu N., Hu L., Nix W.D., Cui Y. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 2011;11:2949–2954. doi: 10.1021/nl201470j. [DOI] [PubMed] [Google Scholar]
- 31.Yoon T., Bok T., Kim C., Na Y., Park S., Kim K.S. Mesoporous silicon hollow nanocubes derived from metal–organic framework template for advanced lithium-ion battery anode. ACS Nano. 2017;11:4808–4815. doi: 10.1021/acsnano.7b01185. [DOI] [PubMed] [Google Scholar]
- 32.Yu C., Du Y., He R., Ma Y., Liu Z., Li X., Luo W., Zhou L., Mai L. Hollow SiOx/C microspheres with semigraphitic carbon coating as the “lithium host” for dendrite-free lithium metal anodes. ACS Appl. Energy Mater. 2021;4:3905–3912. [Google Scholar]
- 33.Zhang L., Wang C., Dou Y., Cheng N., Cui D., Du Y., Liu P., Al-Mamun M., Zhang S., Zhao H. A yolk–shell structured silicon anode with superior conductivity and high tap density for full lithium-ion batteries. Angew. Chem. Int. Ed. 2019;58:8824–8828. doi: 10.1002/anie.201903709. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Hu G., Yu Q., Liu Z., Yu C., Wu L., Zhou L., Mai L. Polydopamine sacrificial layer mediated SiOx/C@C yolk@shell structure for durable lithium storage. Mater. Chem. Front. 2020;4:1656–1663. [Google Scholar]
- 35.Gao R., Tang J., Yu X., Tang S., Ozawa K., Sasaki T., Qin L.-C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy. 2020;70 [Google Scholar]
- 36.Tian Y., An Y., Feng J. Flexible and freestanding Silicon/MXene composite papers for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces. 2019;11:10004–10011. doi: 10.1021/acsami.8b21893. [DOI] [PubMed] [Google Scholar]
- 37.Anasori B., Lukatskaya M.R., Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017;2 [Google Scholar]
- 38.Gogotsi Y., Anasori B. The Rise of MXenes. ACS Nano. 2019;13:8491–8494. doi: 10.1021/acsnano.9b06394. [DOI] [PubMed] [Google Scholar]
- 39.Hu M., Zhang H., Hu T., Fan B., Wang X., Li Z. Emerging 2D MXenes for supercapacitors: status, challenges and prospects. Chem. Soc. Rev. 2020;49:6666–6693. doi: 10.1039/d0cs00175a. [DOI] [PubMed] [Google Scholar]
- 40.Naguib M., Barsoum M.W., Gogotsi Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021;33 doi: 10.1002/adma.202103393. [DOI] [PubMed] [Google Scholar]
- 41.Jin X., Wang S., Sang C., Yue Y., Xu X., Mei C., Xiao H., Lou Z., Han J. Patternable nanocellulose/Ti3C2Tx flexible films with tunable photoresponsive and electromagnetic interference shielding performances. ACS Appl. Mater. Interfaces. 2022;14:35040–35052. doi: 10.1021/acsami.2c11567. [DOI] [PubMed] [Google Scholar]
- 42.Wei Y., Zhang P., Soomro R.A., Zhu Q., Xu B. Advances in the Synthesis of 2D MXenes. Adv. Mater. 2021;33 doi: 10.1002/adma.202103148. [DOI] [PubMed] [Google Scholar]
- 43.Feng A., Yu Y., Wang Y., Jiang F., Yu Y., Mi L., Song L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017;114:161–166. [Google Scholar]
- 44.Lei J.-C., Zhang X., Zhou Z. Recent advances in MXene: Preparation, properties, and applications. Front. Physiol. 2015;10:276–286. [Google Scholar]
- 45.Wang J., An Y., Shen H., Man Q., Feng J. Flexible and ultralight MXene paper as a current collector for microsized porous silicon anode in high-energy lithium-ion batteries. 2D Mater. 2023;10 [Google Scholar]
- 46.An Y., Tian Y., Liu C., Xiong S., Feng J., Qian Y. One-step, vacuum-assisted construction of micrometer-sized nanoporous silicon confined by uniform two-dimensional n-doped carbon toward advanced Li Ion and MXene-based Li metal batteries. ACS Nano. 2022;16:4560–4577. doi: 10.1021/acsnano.1c11098. [DOI] [PubMed] [Google Scholar]
- 47.Hong S., Fu D., Hou J., Zhou D., Wang B., Sun Y., Liu P., Liu K. Robust photoluminescence energy of MoS2/graphene heterostructure against electron irradiation. Sci. China Mater. 2018;61:1351–1359. [Google Scholar]
- 48.Luo J., Tao X., Zhang J., Xia Y., Huang H., Zhang L., Gan Y., Liang C., Zhang W. Sn4+ ion decorated highly conductive Ti3C2 MXene: Promising lithium-ion anodes with enhanced volumetric capacity and cyclic performance. ACS Nano. 2016;10:2491–2499. doi: 10.1021/acsnano.5b07333. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Yuan K., Bai H., Xuan P., Xu N., Yin C., Li K., Hao P., Zhou Y., Dong B. MXene/graphene oxide heterojunction as a high performance anode material for lithium ion batteries. RSC Adv. 2023;13:26239–26246. doi: 10.1039/d3ra04775j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan X., Si C., Zhou J., Sun Z. MXene and MXene-based composites: synthesis, properties and environment-related applications. Nanoscale Horiz. 2020;5:235–258. [Google Scholar]
- 51.Ren C.E., Hatzell K.B., Alhabeb M., Ling Z., Mahmoud K.A., Gogotsi Y. Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 2015;6:4026–4031. doi: 10.1021/acs.jpclett.5b01895. [DOI] [PubMed] [Google Scholar]
- 52.Kajiyama S., Szabova L., Iinuma H., Sugahara A., Gotoh K., Sodeyama K., Tateyama Y., Okubo M., Yamada A. Enhanced Li-ion accessibility in MXene titanium carbide by steric chloride termination. Adv. Energy Mater. 2017;7 [Google Scholar]
- 53.Aslam M.K., Niu Y., Xu M. MXenes for non-lithium-ion (Na, K, Ca, Mg, and Al) batteries and supercapacitors. Adv. Energy Mater. 2021;11 [Google Scholar]
- 54.Anasori B., Shi C., Moon E.J., Xie Y., Voigt C.A., Kent P.R.C., May S.J., Billinge S.J.L., Barsoum M.W., Gogotsi Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016;1:227–234. doi: 10.1039/c5nh00125k. [DOI] [PubMed] [Google Scholar]
- 55.Deshmukh K., Kovářík T., Khadheer Pasha S.K. State of the art recent progress in two dimensional MXenes based gas sensors and biosensors: A comprehensive review. Coord. Chem. Rev. 2020;424 [Google Scholar]
- 56.Dillon A.D., Ghidiu M.J., Krick A.L., Griggs J., May S.J., Gogotsi Y., Barsoum M.W., Fafarman A.T. Highly conductive optical quality solution-processed films of 2D titanium carbide. Adv. Funct. Mater. 2016;26:4162–4168. [Google Scholar]
- 57.Huang Z.-Q., Xu M.-L., Macam G., Hsu C.-H., Chuang F.-C. Large-gap topological insulators in functionalized ordered double transition metal carbide MXenes. Phys. Rev. B. 2020;102 [Google Scholar]
- 58.Kamysbayev V., Filatov A.S., Hu H., Rui X., Lagunas F., Wang D., Klie R.F., Talapin D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science. 2020;369:979–983. doi: 10.1126/science.aba8311. [DOI] [PubMed] [Google Scholar]
- 59.Khazaei M., Arai M., Sasaki T., Chung C.-Y., Venkataramanan N.S., Estili M., Sakka Y., Kawazoe Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013;23:2185–2192. [Google Scholar]
- 60.Khazaei M., Ranjbar A., Arai M., Sasaki T., Yunoki S. Electronic properties and applications of MXenes: a theoretical review. J. Mater. Chem. C. 2017;5:2488–2503. [Google Scholar]
- 61.Kunitski M., Eicke N., Huber P., Köhler J., Zeller S., Voigtsberger J., Schlott N., Henrichs K., Sann H., Trinter F., et al. Double-slit photoelectron interference in strong-field ionization of the neon dimer. Nat. Commun. 2019;10:1. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao H., Guo X., Wan P., Yu G. Conductive MXene nanocomposite organohydrogel for flexible, healable, low-temperature tolerant strain sensors. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 63.Mathis T.S., Maleski K., Goad A., Sarycheva A., Anayee M., Foucher A.C., Hantanasirisakul K., Shuck C.E., Stach E.A., Gogotsi Y. Modified MAX phase synthesis for environmentally stable and highly conductive Ti3C2 MXene. ACS Nano. 2021;15:6420–6429. doi: 10.1021/acsnano.0c08357. [DOI] [PubMed] [Google Scholar]
- 64.Lv P., Li Y.-L., Wang J.-F. Monolayer Ti2C MXene: manipulating magnetic properties and electronic structures by an electric field. Phys. Chem. Chem. Phys. 2020;22:11266–11272. doi: 10.1039/d0cp00507j. [DOI] [PubMed] [Google Scholar]
- 65.Xu H., Yin X., Li X., Li M., Liang S., Zhang L., Cheng L. Lightweight Ti2CTx MXene/poly(vinyl alcohol) composite foams for electromagnetic wave shielding with absorption-dominated feature. ACS Appl. Mater. Interfaces. 2019;11:10198–10207. doi: 10.1021/acsami.8b21671. [DOI] [PubMed] [Google Scholar]
- 66.Aghamohammadi H., Eslami-Farsani R., Castillo-Martinez E. Recent trends in the development of MXenes and MXene-based composites as anode materials for Li-ion batteries. J. Energy Storage. 2022;47 [Google Scholar]
- 67.Wang Y., Li Y., Qiu Z., Wu X., Zhou P., Zhou T., Zhao J., Miao Z., Zhou J., Zhuo S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A. 2018;6:11189–11197. [Google Scholar]
- 68.Liu Y.-T., Zhang P., Sun N., Anasori B., Zhu Q.-Z., Liu H., Gogotsi Y., Xu B. Self-assembly of transition metal oxide nanostructures on MXene nanosheets for fast and stable lithium storage. Adv. Mater. 2018;30 doi: 10.1002/adma.201707334. [DOI] [PubMed] [Google Scholar]
- 69.Li S., Fan Z., Wu G., Shao Y., Xia Z., Wei C., Shen F., Tong X., Yu J., Chen K., et al. Assembly of nanofluidic MXene fibers with enhanced ionic transport and capacitive charge storage by flake orientation. ACS Nano. 2021;15:7821–7832. doi: 10.1021/acsnano.1c02271. [DOI] [PubMed] [Google Scholar]
- 70.Natarajan B., Stein I.Y., Lachman N., Yamamoto N., Jacobs D.S., Sharma R., Liddle J.A., Wardle B.L. Aligned carbon nanotube morphogenesis predicts physical properties of their polymer nanocomposites. Nanoscale. 2019;11:16327–16335. doi: 10.1039/c9nr03317c. [DOI] [PubMed] [Google Scholar]
- 71.Tang Q., Zhou Z., Shen P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012;134:16909–16916. doi: 10.1021/ja308463r. [DOI] [PubMed] [Google Scholar]
- 72.VahidMohammadi A., Rosen J., Gogotsi Y. The world of two-dimensional carbides and nitrides (MXenes) Science. 2021;372 doi: 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]
- 73.Wang T., Yao K., Hua Y., Shankar E.G., Shanthappa R., Yu J.S. Rational design of MXene-MoS2 heterostructure with rapid ion transport rate as an advanced anode for sodium-ion batteries. Chem. Eng. J. 2023;457 [Google Scholar]
- 74.Wu W., Fang H., Ma H., Wu L., Zhang W., Wang H. Boosting transport kinetics of ions and electrons simultaneously by Ti3C2Tx (MXene) addition for enhanced electrochromic performance. Nano-Micro Lett. 2020;13:20. doi: 10.1007/s40820-020-00544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong D., Huang S., Fang D., Yan D., Li G., Yan Y., Chen S., Liu Y., Li X., Von Lim Y., et al. Porosity engineering of MXene membrane towards polysulfide inhibition and fast lithium ion transportation for lithium–sulfur Batteries. Small. 2021;17 doi: 10.1002/smll.202007442. [DOI] [PubMed] [Google Scholar]
- 76.Yang R., Hu Q., Yang S., Zeng Z., Zhang H., Cao A., Gui X. Anchoring oxidized MXene nanosheets on porous carbon nanotube sponge for enhancing ion transport and pseudocapacitive performance. ACS Appl. Mater. Interfaces. 2022;14:41997–42006. doi: 10.1021/acsami.2c10659. [DOI] [PubMed] [Google Scholar]
- 77.Liu S., Zhang X., Yan P., Cheng R., Tang Y., Cui M., Wang B., Zhang L., Wang X., Jiang Y., et al. Dual bond enhanced multidimensional constructed composite silicon anode for high-performance lithium ion batteries. ACS Nano. 2019;13:8854–8864. doi: 10.1021/acsnano.9b02129. [DOI] [PubMed] [Google Scholar]
- 78.Kong F., He X., Liu Q., Qi X., Sun D., Zheng Y., Wang R., Bai Y. Enhanced reversible Li-ion storage in Si@Ti3C2 MXene nanocomposite. Electrochem. Commun. 2018;97:16–21. [Google Scholar]
- 79.Zhang Z., Ying H., Huang P., Zhang S., Zhang Z., Yang T., Han W.-Q. Porous Si decorated on MXene as free-standing anodes for lithium-ion batteries with enhanced diffusion properties and mechanical stability. Chem. Eng. J. 2023;451 [Google Scholar]
- 80.Sarang K., Zhao X., Holta D., Cao H., Arole K., Flouda P., Oh E.-S., Radovic M., Green M.J., Lutkenhaus J.L. Carbon additive-free crumpled Ti3C2Tx MXene-encapsulated silicon nanoparticle anodes for lithium-ion batteries. ACS Appl. Energy Mater. 2021;4:10762–10773. [Google Scholar]
- 81.Jiang R., Yuan H., Wei X., Wang H., Shin H.-J., Lan J., Yu Y., Yang X. Constructing robust and freestanding MXene/Si@C core–shell nanofibers via coaxial electrospinning for high performance Li-ion batteries. Mater. Chem. Front. 2021;5:8218–8228. [Google Scholar]
- 82.Yang H., Jiang T., Zhou Y. Enhanced lithium storage performance in Si/MXene porous composites. INORGA. 2023;11:279. [Google Scholar]
- 83.Li X., Chen Z., Li A., Yu Y., Chen X., Song H. Three-dimensional hierarchical porous structures constructed by two-stage MXene-wrapped Si nanoparticles for Li-ion batteries. ACS Appl. Mater. Interfaces. 2020;12:48718–48728. doi: 10.1021/acsami.0c15527. [DOI] [PubMed] [Google Scholar]
- 84.Yang Q., Wang Z., Xia Y., Wu G., Chen C., Wang J., Rao P., Dong A. Facile electrostatic assembly of Si@MXene superstructures for enhanced lithium-ion storage. J. Colloid Interface Sci. 2020;580:68–76. doi: 10.1016/j.jcis.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 85.Jo D.Y., Kim J.K., Oh H.G., Kang Y.C., Park S.-K. Chemically integrating MXene nanosheets with n-doped c-coated Si nanoparticles for enhanced Li storage performance. Scripta Mater. 2021;199 [Google Scholar]
- 86.Liu Z., Lu D., Wang W., Yue L., Zhu J., Zhao L., Zheng H., Wang J., Li Y. Integrating dually encapsulated Si architecture and dense structural engineering for ultrahigh volumetric and areal capacity of lithium storage. ACS Nano. 2022;16:4642–4653. doi: 10.1021/acsnano.1c11298. [DOI] [PubMed] [Google Scholar]
- 87.Jiang M., Zhang F., Zhu G., Ma Y., Luo W., Zhou T., Yang J. Interface-amorphized Ti3C2@Si/SiOx@TiO2 anodes with sandwiched structures and stable lithium storage. ACS Appl. Mater. Interfaces. 2020;12:24796–24805. doi: 10.1021/acsami.0c05116. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y., Mu Z., Lai J., Chao Y., Yang Y., Zhou P., Li Y., Yang W., Xia Z., Guo S. MXene/Si@SiOx@C layer-by-layer superstructure with autoadjustable function for superior stable lithium storage. ACS Nano. 2019;13:2167–2175. doi: 10.1021/acsnano.8b08821. [DOI] [PubMed] [Google Scholar]