Abstract
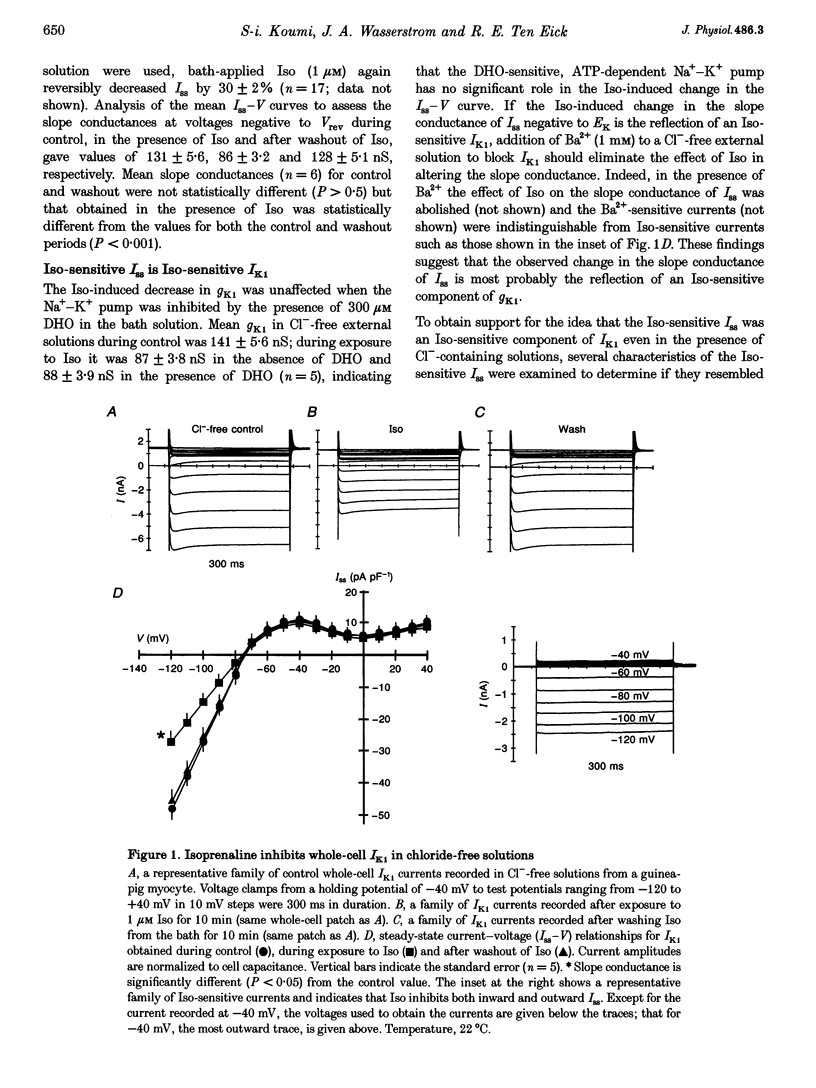
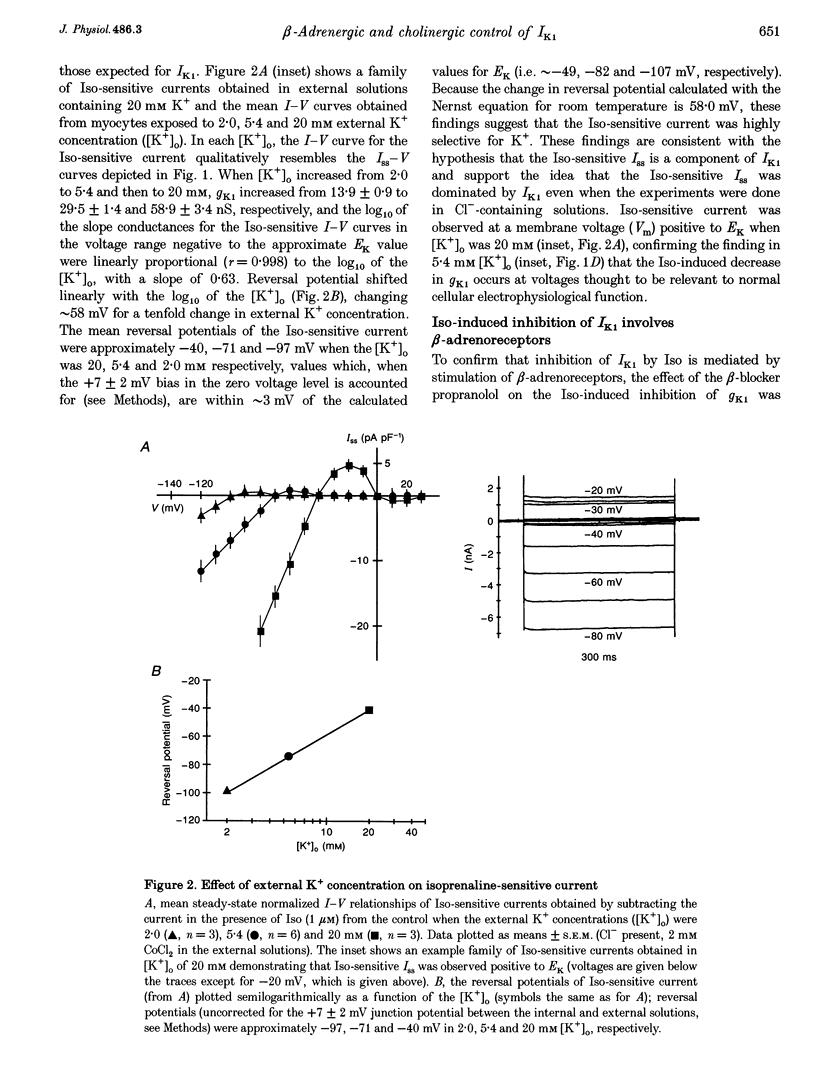
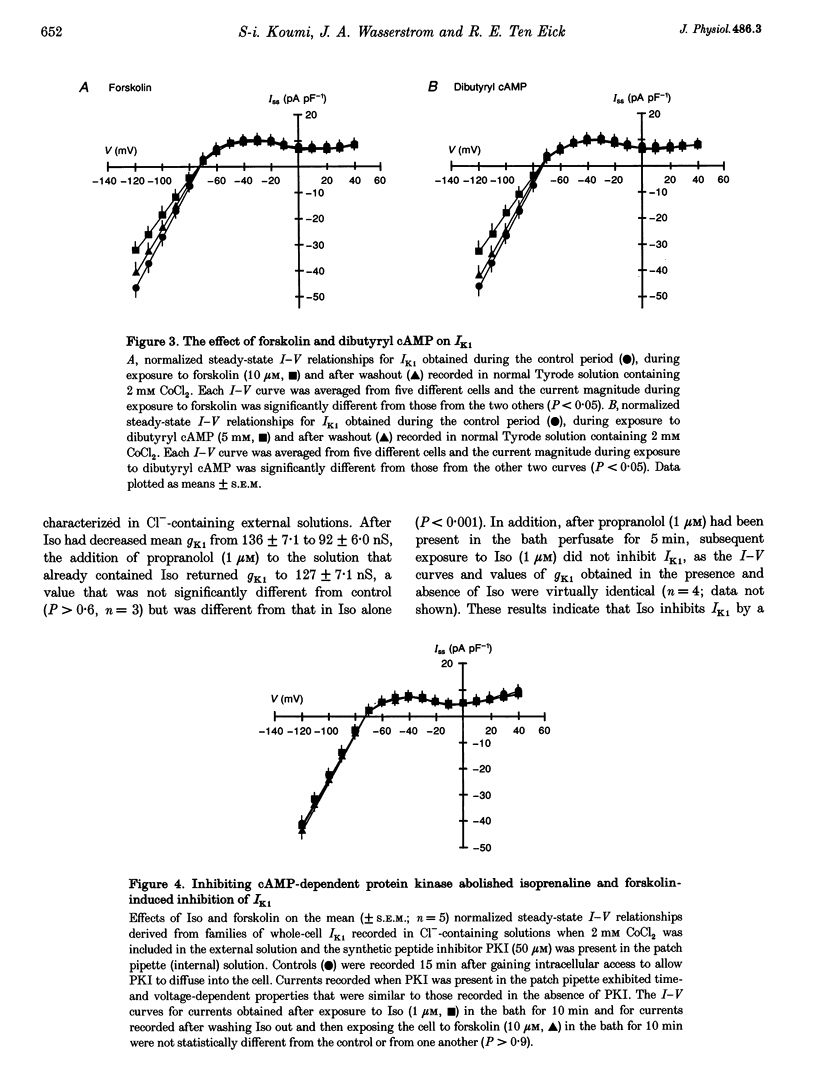
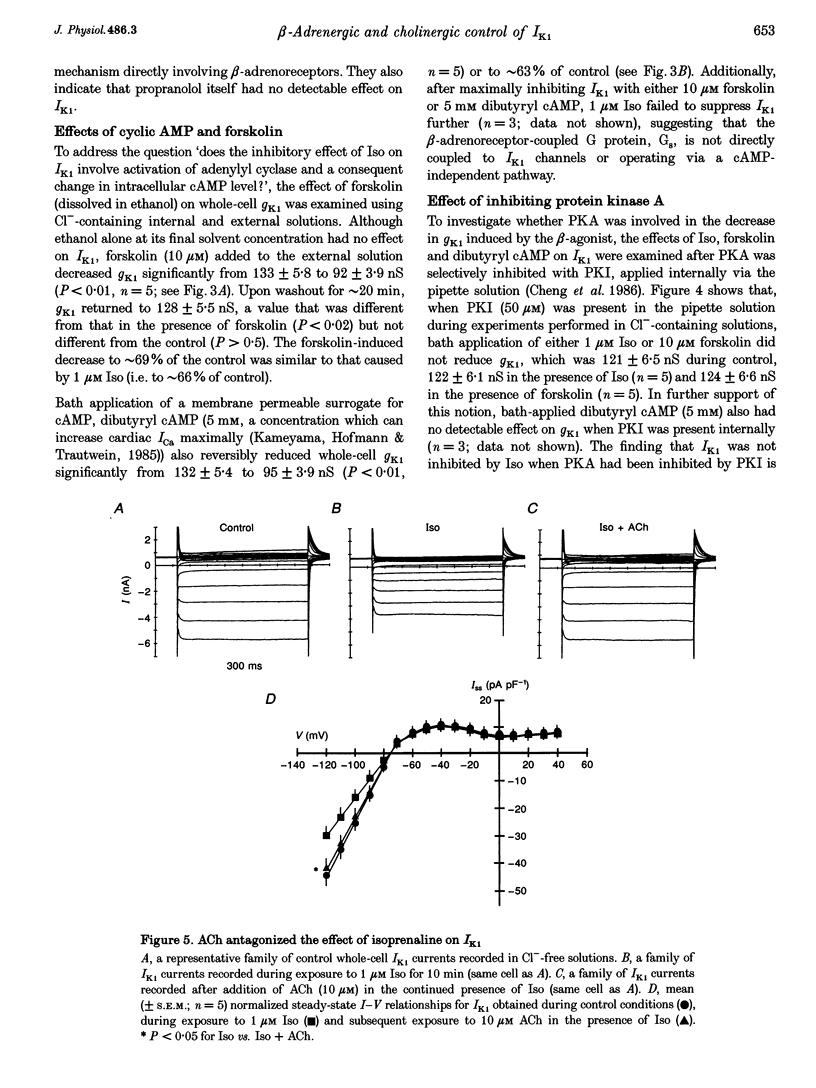
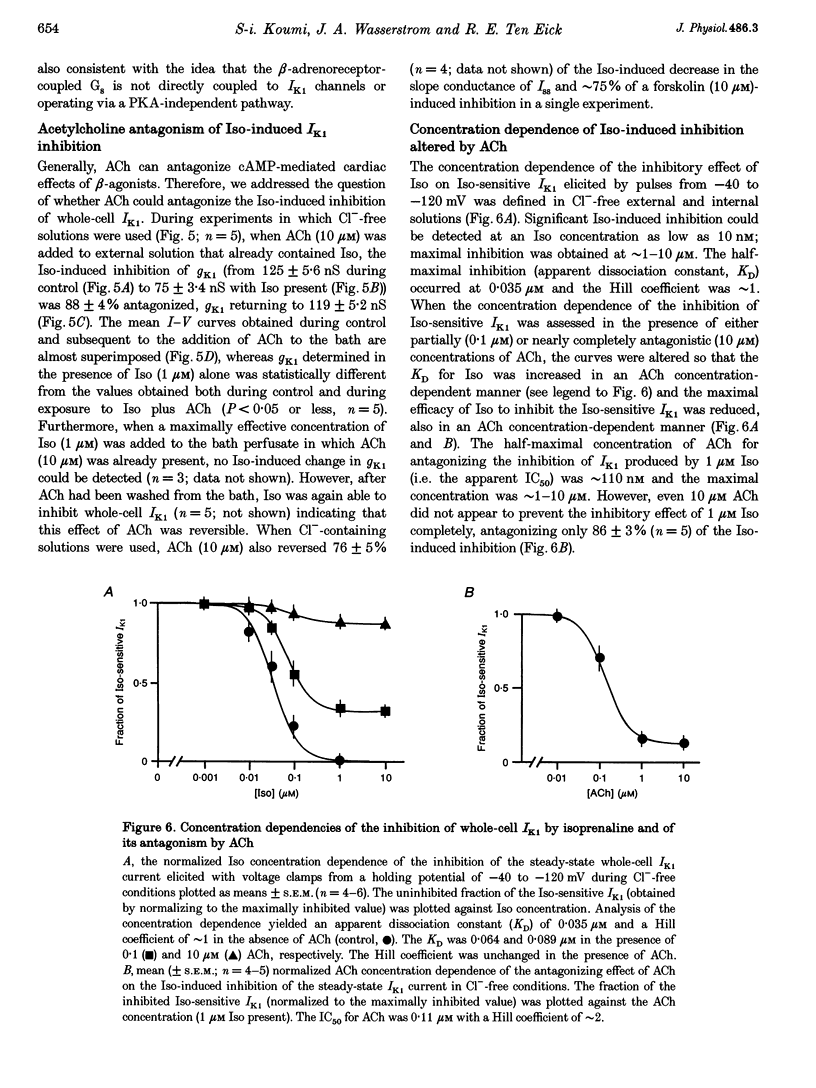
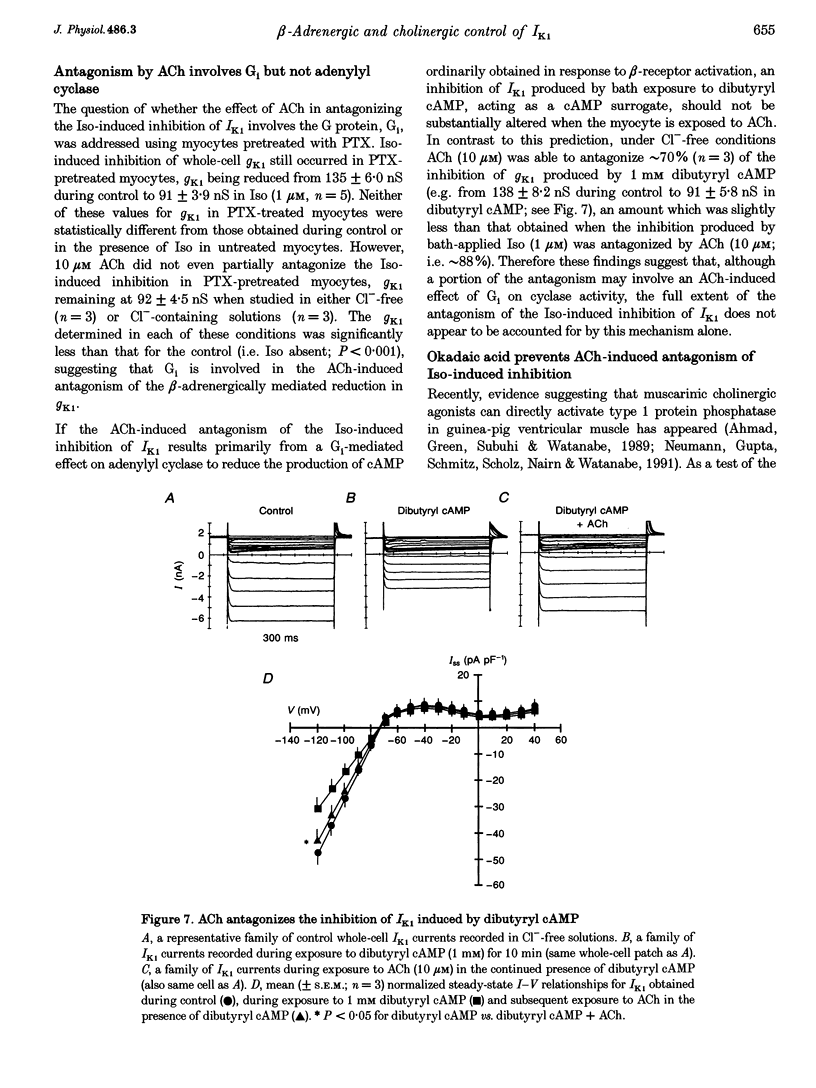
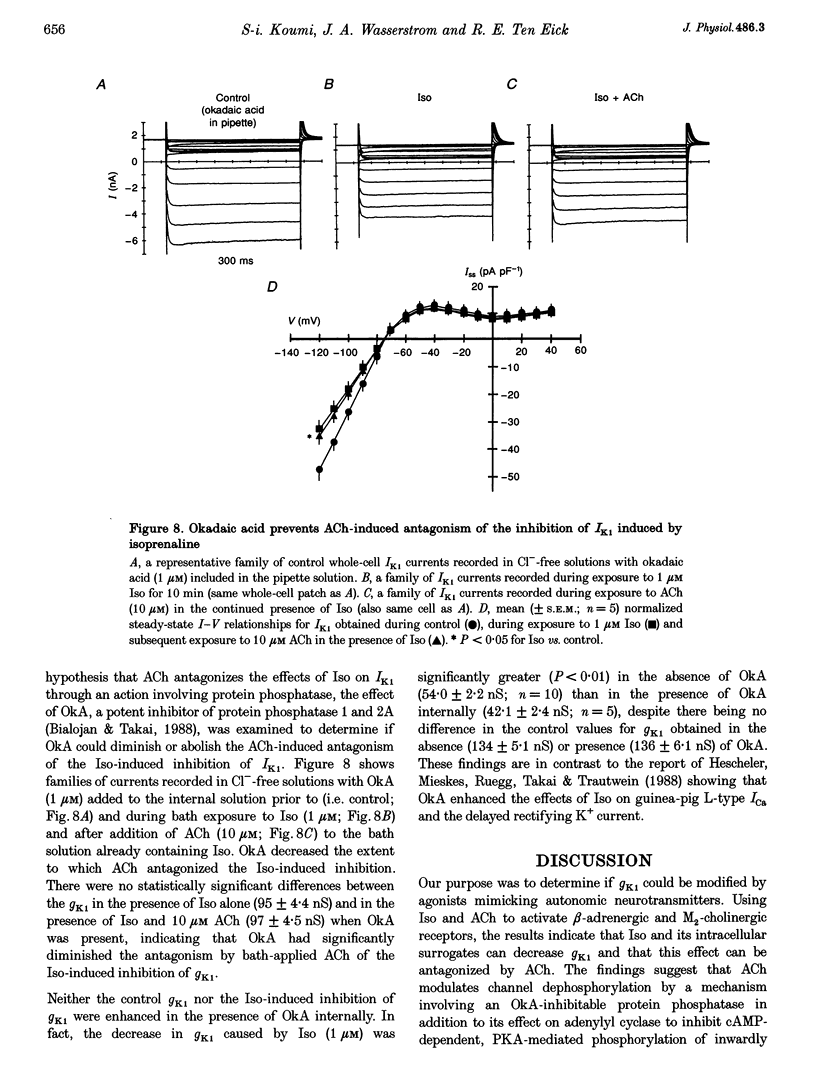
1. Whole-cell patch-clamp technique was used to study the beta-adrenergic and cholinergic regulation of the inwardly rectifying K+ conductance (gK1) in isolated guinea-pig ventricular myocytes. 2. In Cl(-)-free solutions or in the presence of 9-anthracenecarboxylic acid or Co2+, bath-applied isoprenaline (Iso) partially inhibited the steady-state whole-cell conductance (gss) calculated from the steady-state current (Iss)-voltage (Iss-V) curve at membrane voltages (Vm) negative to the equilibrium potential for potassium (EK). Iss was also inhibited at Vm positive to EK when the extracellular [K+] was 20 mM. The Iso-sensitive component of gss exhibited the characteristics of the inwardly rectifying K+ conductance (gK1). 3. The Iso-induced inhibition of gK1 was reversible, concentration dependent, blocked by propranolol, mimicked by both forskolin and dibutyryl cAMP, and prevented by including a cAMP-dependent protein kinase (PKA) inhibitor in the pipette solution. These findings suggest that PKA mediates the Iso-induced inhibition of gK1. 4. The apparent dissociation constant (KD) for the concentration dependence of Iso-induced inhibition was 0.035 microM and the Hill coefficient was approximately 1.0. A maximal Iso concentration (1 microM) inhibited gK1 by 40 +/- 4.1% (mean +/- S.E.M.; n = 13). 5. Bath application of acetylcholine (ACh, 0.1 microM or more) antagonized the Iso-induced (1 microM) inhibition of gK1; [ACh] > 1.0 microM antagonized 88 +/- 2.1% (n = 10) of the inhibition. ACh increased the KD for Iso to inhibit Iso-sensitive gK1 and also reduced the maximal Iso-induced inhibition. 6. ACh-induced antagonism could be abolished by pre-incubating myocytes with pertussis toxin (PTX), suggesting that a muscarinic receptor-coupled, PTX-sensitive G protein, Gi, is involved. 7. ACh (10 microM) also antagonized approximately 70% of the dibutyryl cyclic AMP (1 mM)-induced inhibition of gK1 (n = 3), suggesting that the ACh-induced antagonism involves more than simply inhibiting the Iso-mediated activation of adenylyl cyclase via the activated Gi. 8. Intracellularly applied okadaic acid (OkA, 1 microM) did not alter gK1 (control = 134 +/- 5.1 nS vs. OkA = 136 +/- 6.1 nS), but the Iso-induced decrease in gK1 was less (P < 0.001) with OkA present (42.1 +/- 2.4 nS, n = 5) than when absent (54.0 +/- 2.2 nS, n = 10). However, ACh (10 microM) failed to antagonize Iso-induced inhibition with OkA present, suggesting involvement of a protein phosphatase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., Green F. J., Subuhi H. S., Watanabe A. M. Autonomic regulation of type 1 protein phosphatase in cardiac muscle. J Biol Chem. 1989 Mar 5;264(7):3859–3863. [PubMed] [Google Scholar]
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bennett P., McKinney L., Begenisich T., Kass R. S. Adrenergic modulation of the delayed rectifier potassium channel in calf cardiac Purkinje fibers. Biophys J. 1986 Apr;49(4):839–848. doi: 10.1016/S0006-3495(86)83713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden P. A., Cranefield P. F., Gadsby D. C. Noradrenaline hyperpolarizes cells of the canine coronary sinus by increasing their permeability to potassium ions. J Physiol. 1983 Jun;339:185–206. doi: 10.1113/jphysiol.1983.sp014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Giles W., Nakajima T., Ono K., Shibata E. F. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J Physiol. 1989 Aug;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Smigel M. D., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and the inhibition of adenylate cyclase in S49 lymphoma cyc- and wild type membranes. J Biol Chem. 1984 Mar 25;259(6):3586–3595. [PubMed] [Google Scholar]
- Koumi S., Wasserstrom J. A., Ten Eick R. E. Beta-adrenergic and cholinergic modulation of inward rectifier K+ channel function and phosphorylation in guinea-pig ventricle. J Physiol. 1995 Aug 1;486(Pt 3):661–678. doi: 10.1113/jphysiol.1995.sp020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J. J., Lee H., Shibata E. F. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ Res. 1992 Jan;70(1):199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Rasmussen H. H., Singer D. H., Ten Eick R. E. Inhibition of Na-K pump current in guinea pig ventricular myocytes by dihydroouabain occurs at high- and low-affinity sites. Circ Res. 1989 Jun;64(6):1063–1069. doi: 10.1161/01.res.64.6.1063. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Gupta R. C., Schmitz W., Scholz H., Nairn A. C., Watanabe A. M. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ Res. 1991 Dec;69(6):1450–1457. doi: 10.1161/01.res.69.6.1450. [DOI] [PubMed] [Google Scholar]
- Nishiwaki S., Fujiki H., Suganuma M., Furuya-Suguri H., Matsushima R., Iida Y., Ojika M., Yamada K., Uemura D., Yasumoto T. Structure-activity relationship within a series of okadaic acid derivatives. Carcinogenesis. 1990 Oct;11(10):1837–1841. doi: 10.1093/carcin/11.10.1837. [DOI] [PubMed] [Google Scholar]
- Powell T., Twist V. W. Isoprenaline stimulation of cyclic AMP production by isolated cells from adult rat myocardium. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1218–1225. doi: 10.1016/s0006-291x(76)80260-4. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen F. M., Ono K., Noma A., Ehara T. Beta-adrenergic and muscarinic regulation of the chloride current in guinea-pig ventricular cells. J Physiol. 1991;440:225–241. doi: 10.1113/jphysiol.1991.sp018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromba C., Cohen I. S. A novel action of isoproterenol to inactivate a cardiac K+ current is not blocked by beta and alpha adrenergic blockers. Biophys J. 1990 Sep;58(3):791–795. doi: 10.1016/S0006-3495(90)82422-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. A combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 1990 Oct;417(2):142–148. doi: 10.1007/BF00370691. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


