Summary
Background
Emergence of SARS-CoV-2 variants that escape neutralising antibodies hampers the development of vaccines and therapeutic antibodies against SARS-CoV-2. IGHV3-53/3-66-derived public antibodies, which are generally specific to the prototype virus and are frequently induced in infected or vaccinated individuals, show minimal affinity maturation and high potency against prototype SARS-CoV-2.
Methods
Monoclonal antibodies isolated from a Delta breakthrough infection case were analysed for cross-neutralising activities against SARS-CoV-2 variants. The broadly neutralising antibody K4-66 was further analysed in a hamster model, and the effect of somatic hypermutations was assessed using the inferred germline precursor.
Findings
Antibodies derived from IGHV3-53/3-66 showed broader neutralising activity than antibodies derived from IGHV1-69 and other IGHV genes. IGHV3-53/3-66 antibodies neutralised the Delta variant better than the IGHV1-69 antibodies, suggesting that the IGHV3-53/3-66 antibodies were further maturated by Delta breakthrough infection. One IGHV3-53/3-66 antibody, K4-66, neutralised all Omicron subvariants tested, including EG.5.1, BA.2.86, and JN.1, and decreased the viral load in the lungs of hamsters infected with Omicron subvariant XBB.1.5. The importance of somatic hypermutations was demonstrated by the loss of neutralising activity of the inferred germline precursor of K4-66 against Beta and Omicron variants.
Interpretation
Broadly neutralising IGHV3-53/3-66 antibodies have potential as a target for the development of effective vaccines and therapeutic antibodies against newly emerging SARS-CoV-2 variants.
Funding
This work was supported by grants from AMED (JP23ym0126048, JP22ym0126048, JP21ym0126048, JP23wm0125002, JP233fa627001, JP223fa627009, JP24jf0126002, and JP22fk0108572), and the JSPS (JP21H02970, JK23K20041, and JPJSCCA20240006).
Keywords: SARS-CoV-2, Neutralising antibody, Variant, Public antibody
Research in context.
Evidence before this study
SARS-CoV-2 variants are resistant to neutralising antibodies induced by vaccination based on the prototype virus. The antibody response to the original antigen used for the first vaccination was considerably intense, and a phenomenon known as “immune imprinting” limits the induction of neutralising antibodies against a variant antigen used as a booster. Immune imprinting, together with escape mutations in the Spike protein, have made it difficult to develop vaccines that induce antibodies effective against newly emerging SARS-CoV-2 variants. The vaccine-induced neutralising antibodies contain “public antibodies”, which have the same specificity, use the same immunoglobulin germline gene, and are isolated from different individuals with a high frequency. Antibodies using the IGHV3-53/3-66 gene is one of the representative public antibodies in individuals vaccinated or infected with SARS-CoV-2. The IGHV3-53/3-66 public antibodies target the receptor binding domain and strongly neutralise the prototype virus, while most of them don’t neutralise SARS-CoV-2 Omicron variants, which acquired many mutations in the Spike protein.
Added value of this study
Analysis of monoclonal antibodies isolated from an individual who was vaccinated twice and infected with the Delta variant revealed that antibodies using the IGHV3-53/3-66 gene showed better cross-reactivity against SARS-CoV-2 variants than those using the IGHV1-69 gene. The importance of somatic hypermutation for broadly neutralising activity, as well as the potent neutralisation of the Delta variant by IGHV3-53/3-66 antibodies compared with that of IGHV1-69 antibodies, suggested that Delta breakthrough infection resulted in the maturation of prototype virus-specific IGHV3-53/3-66 antibodies to broadly neutralising antibodies. Acquisition of broadly neutralising activity via activation of specific public antibodies has not been previously reported.
Implications of all the available evidence
The original SARS-CoV-2 vaccines were very effective against prototype SARS-CoV-2, but showed significantly decreased efficacy against Omicron variants. Although Omicron variant-based vaccines have recently been used, Omicron subvariants have acquired mutations that confer escape from antibodies induced by early Omicron subvariants. Additionally, the phenomenon of immune imprinting limits the induction of new antibodies against Omicron variants. Our findings suggest that prototype-specific public antibodies induced by the original vaccine evolved to broadly neutralising antibodies during Delta breakthrough infection. This implies that prototype-specific public antibodies, which are primed by vaccination, have the potential to acquire broad neutralising activity. Boosting certain lineages of public antibodies may be a potent strategy to gain broadly neutralising activities against emerging SARS-CoV-2 variants. Further studies on the boosting of specific public antibodies will be required for the development of vaccines that induce broadly neutralising antibodies.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variants strongly evade the humoral immunity induced by vaccination and infection.1,2 Furthermore, the Omicron lineage is continuously evolving, and recent subvariants, such as BQ.1.1, XBB.1.5, EG.5.1, BA.2.86, and JN.1, acquired higher resistance to antibodies compared with earlier Omicron subvariants, such as BA.1, BA.2, and BA.5.3, 4, 5, 6, 7, 8, 9, 10, 11 Mutations in the receptor binding domain (RBD) of the Spike (S) protein have made the virus more evasive to antibodies induced by SARS-CoV-2 vaccination and infection, as well as therapeutic monoclonal antibodies. Analysis of monoclonal antibodies (mAbs) from vaccinated or infected individuals revealed the induction of public antibodies.12, 13, 14 Public antibodies have the same specificity, use the same immunoglobulin germline gene, and are frequently isolated from different individuals. The IGHV3-53/3-66 public antibodies, representative public antibodies against SARS-CoV-2, were potent neutralising antibodies targeting the RBD, while most of them didn’t neutralise SARS-CoV-2 Omicron variants, which acquired many mutations in the S protein. The potent neutralising activity and high prevalence of these public antibodies have strongly driven the evolution of RBD mutations, with most public antibodies failing to neutralise recent variants.1, 2, 3, 4, 5, 6, 7
Due to the decreased efficacy of the original vaccines against SARS-CoV-2, which were developed based on the Wuhan strain, the antigens used for newer vaccines were replaced with Omicron variants.1, 2, 3, 4, 5,15 The antigenicity of Omicron variant-based vaccines, which are genetically closer to recent Omicron subvariants than to the Wuhan strain, may be advantageous for the induction of protective immunity against recent Omicron subvariants; however, recent subvariants carry mutations in the RBD that allow escape from the neutralising antibodies induced by early Omicron subvariants.3, 4, 5, 6, 7,15 Furthermore, the antibody response to infection with the Omicron variant showed considerable intensity toward the original antigen used for the first vaccination; this phenomenon, known as “immune imprinting”, may limit the induction of neutralising antibodies against a new antigen used as a booster.4,15 Immune imprinting, together with escape mutations in the RBD, make it difficult to develop vaccines that induce antibodies effective against newly emerging variants.
Emergence of neutralisation-resistant variants increased breakthrough infection (BTI) cases in vaccinated individuals.2,15 BTI increased the potency and breadth of the neutralising antibody response, and this so-called “hybrid immunity” induced after BTI was reportedly more effective than infection-induced or vaccine-induced immunity.15, 16, 17 In this study, we isolated mAbs from an individual who received two doses of the Pfizer-BioNTech mRNA vaccine (BNT162b2) and became infected with the Delta variant. Analysis of the mAbs isolated suggested that maturation of public antibodies, which are frequently induced by vaccination, are important to elicit broadly neutralising antibodies.
Methods
Ethics
All study protocols involving specimens from human subjects were reviewed and approved by the institutional ethical review boards of Kyushu Medical Center (20C120), Nagoya City University Hospital (60-20-0070), and Kumamoto University (2013, 461, and 2339). All study participants provided written informed consent for the collection and analysis of samples. Protocols for the experiments using hamsters were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (PA19-75).
Sample collection from patients with COVID-19
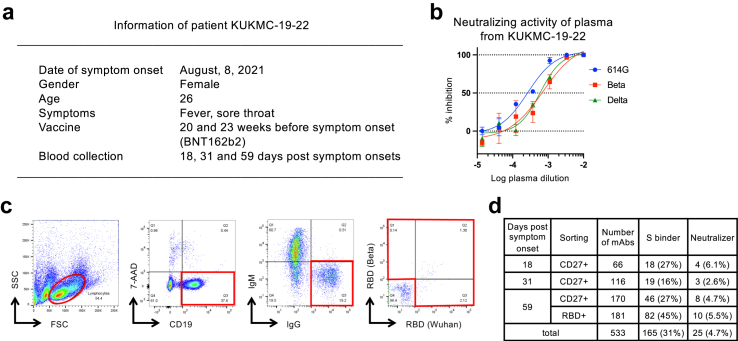
Peripheral blood samples for antibody isolation were obtained from patient KUKMC-19–22 (Fig. 1a). Plasma samples were obtained from seven patients with Delta BTI, whose information is summarised in Supplementary Table S1.
Fig. 1.
Isolation of mAbs from the patient with Delta BTI, KUKMC-19–22. (a) Summary of patient information for KUKMC-19–22, who donated blood samples for isolation of mAbs by single-cell sorting and construction of recombinant IgGs. (b) Neutralising activity of plasma from KUKMC-19–22 at 18 days post symptom onset, analysed using pseudoviruses carrying S proteins from variants 614G, Beta, and Delta. (c) Single-cell sorting of IgG+IgM− B cells, with or without RBD probes from the Wuhan strain and Beta variant. (d) Summary of mAbs isolated from KUKMC-19–22. The supernatants from the transfected cells were analysed for reactivity to the Wuhan strain S protein using flow cytometry and neutralising activity against the 614G variant.
Cells
The 293, 293T (RRID: CVCL_0063),18 and 293FT/DSP1-7/ACE2/TMPRSS219 cells were maintained in Dulbecco’s modified Eagle’s medium (Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% heat-inactivated foetal bovine serum (FBS). Vero E6 cells (RRID: CVCL_0574) expressing transmembrane serine protease 2 (VeroE6/TMPRSS2) were maintained in Eagle’s minimal essential medium containing l-glutamine (Fujifilm, Tokyo, Japan) and supplemented with 5% FBS. ExpiCHO-S cells (Thermo Fisher Scientific, Waltham, MA, USA; RRID:CVCL_5J31) were maintained in ExpiCHO expression medium (Thermo Fisher Scientific). All cell lines used are tested for mycoplasma and are maintained mycoplasma-free.
Isolation of mAbs from the Delta BTI case
Peripheral blood samples were obtained from KUKMC-19–22, who was vaccinated twice with BNT162b2 and infected with SARS-CoV-2 (Fig. 1a). Isolation of mAbs was performed as previously described.20 Briefly, magnetically enriched B cells were single-cell sorted, and immunoglobulin genes were amplified using RNA from 7AAD−CD19+IgM−IgG+RBD+ cells or 7AAD−CD19+IgM−IgG+CD27+ cells. S protein RBDs (amino acid residues P322 to N536) from Wuhan and Beta variants carrying a C-terminal 6-histidine tag and a biotinylation tag were used as probes for sorting. The RBDs were expressed in 293 cells and purified using a COSMOGEL His-Accept Ni2+-NTA affinity column (Nacalai Tesque) and a Superdex 75 Increase 10/300 GL gel filtration column (Cytiva, Tokyo, Japan). Purified proteins were biotinylated in accordance with the manufacturer’s instructions of the BirA500 biotinylation kit (Avidity LLC, Aurora, CO, USA).
Immunoglobulin (Ig) heavy chain (H-chain) and light chain (L-chain) expression plasmids were constructed from the sorted cells as previously described.20 The nucleotide sequences of the Ig variable regions were analysed using IMGT vquest (http://imgt.org/IMGT_vquest/vquest). The Genbank accession numbers of the neutralising antibody sequences are PP154178–PP154223.
Plasmids of inferred germline precursor of K4-66 H-chain and L-chain were constructed based on the IGHV3-53∗04 and IGKV1-33∗01 genes using PCR mutagenesis. Introduction of F28I/L and A96 G/V mutations was also performed by PCR mutagenesis.
Production and purification of recombinant IgG
Recombinant IgG was produced in transfected 293T cells and purified using Protein A, as previously described.20,21 Large-scale production of mAbs was performed by transient transfection of ExpiCHO cells.20
Previously isolated mAbs 9–105 and 10–121 were also purified from transfected ExpiCHO cells.20 The therapeutic mAbs, casirivimab, imdevimab,22 bamlanivimab,23 sotrovimab,24 and bebtelovimab25 were purified from ExpiCHO cells transfected with expression plasmids carrying the respective antibody genes synthesised on the basis of amino acid sequences.
Concentration of purified IgG was determined by sandwich ELISA using anti-human IgG (γ-chain specific) antibody (SIGMA, Saint Louis, MO, USA) for the capture and anti-human IgG (γ-chain specific)-alkaline phosphatase (SIGMA) for the detection. Purity of mAbs were analysed by SAS-PAGE (Supplementary Fig. S1).
Neutralisation assay using pseudovirus
The neutralising activity of antibodies was determined using an HIV-1-based pseudovirus with SARS-CoV-2 S.5,20,26 Briefly, 293T cells were transfected with psPAX2-IN/HiBiT, pWPI-Luc2, and a plasmid expressing S. After 48 h or 72 h, the culture supernatants were stored at −80 °C as a pseudovirus stock. The median tissue culture infectious dose (TCID50) of each pseudovirus was determined by infection of 293FT/DSP1-7/ACE2/TMPRSS2 cells with serially diluted viruses. The neutralisation assay was performed in triplicate. Serially diluted antibody and pseudovirus (400 TCID50) were incubated for 1 h and transferred to 293FT/DSP1-7/ACE2/TMPRSS2 cells plated at 2 × 104 cells/well in 96-well plates 1 day before infection. After incubation for 2 h, the medium was changed to fresh medium, and incubation was continued for an additional 48 h. Luciferase activity was measured using an EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) and luciferase assay system (Promega, Madison, WI, USA) or britelite plus (PerkinElmer). Relative light units were compared to calculate the reduction in infectivity and 50% of the maximal inhibitory concentration (IC50) or 50% neutralisation titre (NT50) was calculated using Prism 8. Plasmids expressing S variants were constructed by mutagenesis of pSARS-CoV-2-S-D614G-19del, which has the D614G mutation and a 19-amino acid deletion in the cytoplasmic tail, as previously described.20 Plasmids expressing S derived from variants BQ.1.1, XBB, XBB.1.5, XBB.1.16, EG.5.1, BA.2.86, and JN.1 were constructed by insertion of the S gene into pCAGGS.5,6,8, 9, 10, 11
Analysis of mAb binding activity
The binding activity of mAbs was analysed by flow cytometry and surface plasmon resonance (SPR), as previously described.20 Cells expressing SARS-CoV-2 S were stained with mAbs and analysed using a FACSCanto II system (BD Biosciences, San Diego, CA, USA). The reactivity of antibodies was analysed using FlowJo (BD Biosciences). SPR experiments were performed using a Biacore T200 (Cytiva), CM5 sensor chip, Human Antibody Capture Kit (Cytiva), and RBD proteins from the Wuhan strain and XBB variant (Thermo Fisher Scientific). KD values were determined using BIAevaluation version 4.1 software (Cytiva).
Neutralisation of authentic virus
Plaque assays were performed as previously described.20 Briefly, VeroE6/TMPRSS2 cells were plated in a 6-well or 12-well plate 1 day before the experiment. Serially diluted mAb was mixed with virus, incubated for 1 h, and added to VeroE6/TMPRSS2 cells. After incubation for 2 h, 1 ml plaque-forming unit (PFU) buffer containing 1× minimum essential medium, 3% FBS, and 1.5% carboxymethyl cellulose (Merck, Darmstadt, Germany) was overlaid. After further incubation for 3 days, cells were fixed and stained with 0.1% methylene blue (Nacalai Tesque) to visualise the plaques. IC50 values were calculated using Prism 8. Prototype SARS-CoV-2 virus (JPN/NGS/IA-1/2020, GISAID ID: EPI_ISL_481251) was provided by Nagasaki University.27 Delta (hCoV-19/Japan/TKYTK1734/2021, EPI_ISL_2378732), Omicron BA.1 (hCoV-19/Japan/TKYX00012/2021, EPI_ISL_8559478), BA.2 (hCoV-19/Japan/TKYS02037/2022, EPI_ISL_9397331), and BA.5 (hCoV-19/Japan/TKYS14631/2022, EPI_ISL_12812500) viruses were isolated at Tokyo Metropolitan Institute of Public Health.7,28 BQ.1.1 (hCoV-19/Japan/TY41-796/2022, EPI_ISL_15579783) and XBB.1.5 (hCoV-19/Japan/23-018/2022, EPI_ISL_16889601) viruses were provided by the National Institute of Infectious Disease.28
Evaluation of mAb therapeutic efficacy in Syrian hamsters
The effects of mAbs in vivo were examined as previously described.3,29, 30, 31 Under isoflurane anaesthesia, five 6-week-old male Syrian hamsters per group were intranasally inoculated with 103 PFU (in 30 μl) of XBB.1.5 (hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022, GISAID ID: EPI_ISL_16026423).3 On Day 1 post infection, the hamsters were intraperitoneally injected with 1 mL of 5 mg/kg K4-66 mAb or Normal Human IgG (Sigma–Aldrich). The animals were euthanised on Day 4 post infection, and virus titres in the nasal turbinates and lungs were determined using plaque assays on VeroE6/TMPRSS2 cells and quantification by PCR. A total of ten hamsters were used for the experiment to estimate the effect of K4-66 administration. The weights were measured on day 0 so that we could calculate the amount of antibody for injection. No method of randomisation was used to determine how the animals were allocated to the experimental groups and processed in this study. However, covariates, including sex and age, were identical in the groups. Sample sizes were determined based on previous in vivo virus challenge experiments.29, 30, 31 Thus, the same effects as therapeutic antibodies in the previous studies can be detected by this experiment. The study was not blinded due to the limited staff availability. No animals were excluded from the analysis. The lack of randomisation and non-blinded experiment may affect the comparison between groups.
Excised animal tissues were fixed in 4% paraformaldehyde in PBS and processed for paraffin embedding. The tissue sections were stained with haematoxylin and eosin, and were stained with a rabbit polyclonal antibody against SARS-CoV-2 nucleocapsid protein (Prospec, Ness-Ziona, Israel). The scores were determined based on the extent of inflammatory cell infiltration and viral antigen-positive cells by using the following scoring system: 0, no change; 1, mild; 2, slight; 3, moderate; 4, severe.
Cryo-EM sample preparation and data collection
XBB.1.5 S protein ectodomain and human ACE2 were expressed and purified as previously described.7,32 Briefly, the expression plasmids encoding the H-chain of K4-66 and L-chain of K4-66 were co-transfected into HEK293 GnTI (−) cells for cryoEM analysis or Expi293 GnTI (−) cells for X-ray crystallographic analysis. Proteins in the cell-culture supernatant were purified with cOmplete His-Tag Purification Resin (Roche, Mannheim, Germany) affinity column, followed by Superdex 75 Increase 10/300 size-exclusion chromatography (Cytiva) with a running buffer containing of 0.1 M Imidazole pH8.0, 150 mM NaCl.
The purified XBB.1.5 S protein was incubated at 37 °C for 1 h before cryo-EM grid preparation. The purified Fab K4-66 was mixed with the S protein solution at a molar ratio of 1:1.5 (spike: Fab). A 3 μl aliquot of the S-Fab mixture was applied to glow-discharged grids covered with a holey carbon film (Quantifoil R0.6/1 Au 300 mesh, Quantifoil Micro Tools GmbH), followed by rapid freezing in liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific) set to 25 °C and 100% humidity.
The grids were maintained at liquid nitrogen temperature, and the cryo-EM dataset was collected on a Glacios Cryo-TEM operated at an accelerating voltage of 200 kV, equipped with a Falcon4 direct electron detector (Thermo Fisher Scientific) at the Institute for Life and Medical Sciences, Kyoto University. Images were collected as movies in electron event representation (EER) format at a nominal magnification of 150,000× (corresponding to a calibrated pixel size of 0.925 Å per pixel) with a total electron dose of 52 e−/Å2.
Cryo-EM image processing
The EER movie frames were initially lossless compressed and summed into 52-frame TIFF stacks, and a gain reference was generated using the “relion_estimate_gain” algorithm with RELION4.0.0.33 Subsequent single particle analysis was conducted using the software package cryoSPARC v4.1.1.34 A total of 5469 movies were subjected to motion correction and gain normalization using the “Patch Motion Correction” job, followed by contrast transfer function (CTF) estimation using the “Patch CTF estimation” job. The 5415 images were then selected with the “Manually Curate Exposure” job by excluding images with poor CTF fits. Particle coordinates were initially identified with the “Blob Picker” job, and 2D class averages were generated with the “2D classification” jobs. Using these 2D class averages as templates, particles were re-picked with the “Template Picker” job, resulting in 1,222,638 particles. These particles were then subjected to two rounds of “2D classification” and two rounds of “Ab-Initio Reconstruction” to remove junk particles, assess the heterogeneity of the S-Fab complex structure, and obtain an initial 3D volume. A total of 60,704 selected particles were used for 3D reconstruction with imposed C3 symmetry using the “Non-Uniform Refinement” job, yielding a 3D cryo-EM map at an estimated overall resolution of 3.76 Å. After the symmetry expansion around the C3 axis, “Local refinement,” “3D classification” without alignment, and another “Local refinement” job were performed using a mask covering an RBD-Fab region to better resolve the complex interface, resulting in a reconstruction at 5.27 Å resolution. The image processing workflow is depicted in Supplementary Fig. S6. Cryo-EM consensus and locally refined maps have been deposited on the Electron Microscopy Data Bank (EMDB) under the accession number EMD-60274.
Crystallization and data collection
Crystals of Fab K4-66 were obtained by sitting-drop vapor diffusion at 20 °C in a drop containing 0.4 μl each of Fab K4-66 (10 mg/ml) and the crystallisation solution (0.1 M HEPES pH 7.0–7.5, 16.5–19.0% polyethylene glycol 20000). Crystals were soaked briefly in cryoprotectant mixture of precipitant and 100% ethylene glycol in a ratio of 6.5:3.5 ratio and flash frozen in liquid nitrogen. X-ray diffraction data were collected from a beamline BL41XU at SPring-8 (Hyogo, Japan), and crystals were diffracted, at 1.0 Å wavelength, to 2.83 Å. All diffraction data were indexed, integrated, scaled, and merged using XDS.35
Structure determination and refinement
Crystal structure of Fab K4-66 was determined using CCP4 v8.0,36 by the molecular replacement method with Molrep,37 using polyalanine model of the previously reported crystal structure of Fab (PDB: 1AIF) as a search model. The initial protein models were fitted manually using Coot.38 The structure was then refined using Refmac539 and phenix.refine.40 All crystal structure figures were generated by PyMOL (https://pymol.org/2/). The atomic coordinate for the crystal structure of Fab K4-66 determined in this study are available in the Protein Data Bank (www.rcsb.org) under accession code 9II9. Source data are provided with this paper.
Statistics
IC50 values, SHM rates, virus titres, and pathological scores were compared using non-parametric Mann–Whitney test in Prism 9 (GraphPad, Boston, MA). Correlation between neutralising and binding activities was examined using nonlinear fitting (semilog line) in Prism 9.
Role of funders
The funders played no role in the design, conduct, interpretation, or reporting of this study.
Results
Isolation of mAbs from the Delta BTI case
We isolated mAbs from one patient, KUKMC-19–22, who was infected with SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine (Fig. 1a). This infection occurred at the peak of the Delta wave in Japan, and was presumed to be a BTI with the Delta variant. The plasma sample from KUKMC-19–22 effectively neutralised the Beta variant (NT50: 1:1500), which is resistant to neutralisation compared with 614G, at a level close to those against the prototype 614G virus and the Delta variant (NT50: 1:3800 and 1:1,700, respectively) (Fig. 1b). This suggested that cross-reactive neutralising antibodies had been induced in KUKMC-19–22.
Single-cell sorting of IgG+IgM− peripheral B cells from KUKMC-19–22 was performed using RBD probes from prototype and Beta variant viruses, or CD27 instead of RBD probes, at 18, 31, and 59 days post symptom onset (DPSO) (Fig. 1c). Next, recombinant antibodies produced from the amplified Ig genes were screened for reactivity to the S protein of the SARS-CoV-2 Wuhan strain using flow cytometry. S-binding mAbs were examined for neutralising activity using a pseudovirus carrying the 614G S protein. Among the 533 mAbs isolated, 166 mAbs (31% of total mAbs) were reactive to the S protein, and 25 mAbs (4.7%) neutralised SARS-CoV-2 pseudovirus (Fig. 1d). Germline gene usage of S-binding and neutralising mAbs are summarized in Supplementary Fig. S2. Twenty three lineages were identified in S-binding mAbs (Supplementary Table S2). RBD probes increased S binders (45%) compared with the CD27 staining (27%) at 59 DPSO, while the frequency of neutralising mAbs were at the same level (5.0% and 4.7%, respectively).
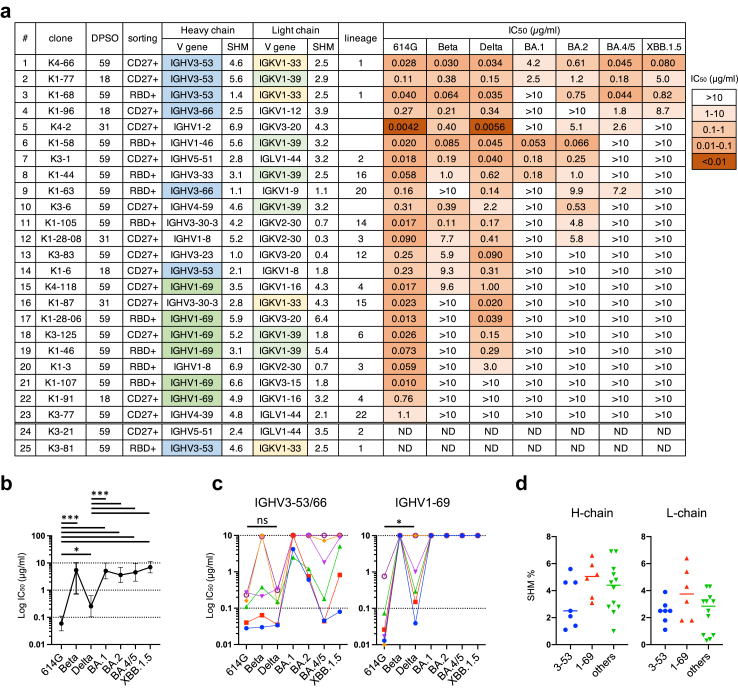
Two of these 25 mAbs, K3-21 and K3-81, were excluded from further analysis because they shared identical amino acid sequences with K3-1 and K4-66, respectively. The remaining mAbs were examined for neutralising activity against 614G, Beta, and Delta variants, and Omicron subvariants BA.1, BA.2, BA.4/5, and XBB.1.5 (Fig. 2a). Only two of the 23 mAbs analysed, K4-66 and K1-77, neutralised all of these variants. The neutralisation potencies of the mAbs isolated against Omicron subvariants BA.1 (geometric mean of IC50 [95% confidence interval, CI]: 5.1 μg/ml [2.6, 9.9]), BA.2 (3.6 μg/ml [1.9, 6.9]), BA.4/5 (4.5 μg/ml [2.2, 9.5]), XBB.1.5 (7.0 μg/ml [4.3, >10]), and the Beta variant (1.7 μg/ml [0.68, 4.2]) were significantly lowered compared with those against 614G (0.059 μg/ml [0.032, 0.11]) and the Delta variant (0.26 μg/ml [0.10, 0.63]) (Fig. 2b). Half of the mAbs did not neutralise any of the Omicron subvariants tested. The neutralisation potency of mAbs against the Delta variant was also lower than that against 614G (P = 0.011 [Mann–Whitney test]) (Fig. 2b). Among the 23 mAbs analysed, 19 mAbs neutralised 614G better than the Delta variant, and three mAbs had a slightly higher neutralisation potency against the Delta variant than they had against 614G. Only one mAb, K3-83, showed clear neutralisation potency against the Delta variant (IC50: 0.090 μg/ml) better than 614G (IC50: 0.25 μg/ml). These findings demonstrated that the immune response in this Delta BTI case was mainly specific to the prototype virus, which was used for the vaccine, even 2 months post infection with the Delta variant. This is consistent with previous studies showing a strong immune imprinting effect of vaccination with the prototype strain.4,15
Fig. 2.
Characterisation of mAbs isolated from the Delta BTI case. (a) Characteristics of neutralising mAbs, of which 23 mAbs were purified by Protein A and examined for neutralising activities against pseudoviruses carrying S proteins from the 614G, Beta, and Delta variants and Omicron subvariants BA.1, BA.2, BA.4/5, and XBB.1.5. Sorting was performed with RBD probes (RBD+) or CD27 (CD27+). Lineages of mAbs are summarized in Supplementary Table S2. DPSO: Days post symptom onset; SHM: Somatic hypermutation %; ND: not done. (b) Plot of IC50 values of 23 mAbs against SARS-CoV-2 variants. P values calculated using the Mann–Whitney test; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. (c) IC50 values of mAbs using IGHV3-53/3-66 (n = 6, left) and IGHV1-69 (n = 6, right) against the SARS-CoV-2 614G and Delta variants. P values calculated using the Mann–Whitney test; ∗P < 0.05, and ns: not significant. (d) Rates of SHM (%) of mAbs using IGHV3-53/3-66 (n = 7, blue), IGHV1-69 (n = 6, red), or other genes (n = 12, green).
Greater breadth of IGHV3-53/3-66 antibodies compared with IGHV1-69 antibodies
Two major public antibody groups featuring usage of IGHV3-53/3-66 and IGHV1-69 were identified in the isolated neutralising mAbs (Fig. 2a). The IGHV3-53/3-66 group comprised seven mAbs, including three mAbs in lineage 1 (K4-66, K1-68, and K3-81). The IGHV1-69 group comprised six mAbs, including two mAbs in lineage 4 (K4–118 and K1–91). The IGHV3-53/3-66 mAbs, which neutralised a median of 5.5 variants, showed a greater neutralisation breadth than other mAbs, including the IGHV1-69 mAbs (median: 3 variants; P = 0.0062 [Mann–Whitney test]). Omicron subvariants were highly resistant to the IGHV1-69 mAbs (Fig. 2c), and subvariant XBB.1.5 was only neutralised by the IGHV3-53/3-66 mAbs (Fig. 2a). In addition, while each mAb in the IGHV3-53/3-66 group neutralised both 614G and Delta variants at the same level, the IGHV1-69 mAbs showed significantly increased IC50 values against the Delta variant (P = 0.031 [Mann–Whitney test]; Fig. 2c). Four of the six IGHV1-69 mAbs were highly potent against 614G, with IC50 values (0.010–0.026 μg/ml) that were lower than those of all of the IGHV3-53/3-66 mAbs (IC50: 0.028–0.27 μg/ml), indicating that these IGHV1-69 mAbs were highly specific to the prototype virus. Binding activities of these mAbs against variants were correlated with the neutralisation activities (Supplementary Fig. S3). The high specificity of the IGHV1-69 mAbs to the prototype virus is consistent with previous studies showing a strong immune imprinting effect of vaccination with the prototype strain,4,15 while the IGHV3-53/3-66 mAbs were exceptional ones. The relatively high somatic hypermutation (SHM) rate of several IGHV1-69 mAbs may be due to the maturation to the vaccine antigen, although there was no statistically significant difference in the SHM rates of H-chains and L-chains between the IGHV1-69 and IGHV3-53/3-66 mAbs (Fig. 2d). Among the IGHV3-53/3-66 mAbs, K4-66, K1-77, and K3-81 (which has an amino acid sequence identical to K4-66) showed higher rates of H-chain SHM (4.6%–5.6%) compared with other IGHV3-53/3-66 mAbs (1.1%–2.5%) (Fig. 2a–d). K4-66 and K1-77 showed the widest breadth among the isolated mAbs (Fig. 2a), suggesting that the high SHM rate may be critical for the wide breadth of IGHV3-53/3-66 mAbs.
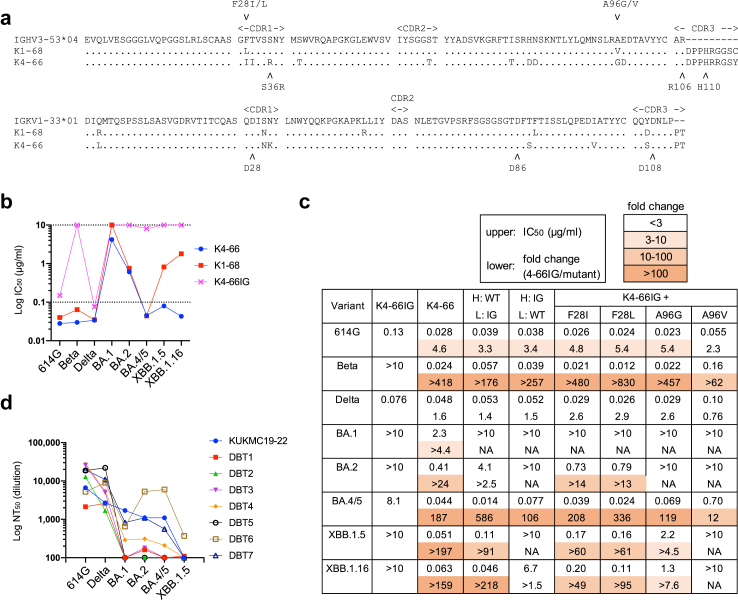
To analyse the effect of mutations in K4-66 on cross-neutralising activity, we examined the neutralisation breadth of the inferred germline precursor mAb, K4-66IG, in which the VH and VK regions of K4-66 were replaced with the germline sequences (Fig. 3a). K4-66IG potently neutralised 614G and the Delta variant, but showed very weak or no neutralising activity against the Beta variant and Omicron subvariants BA.1, BA.2, BA.4/5, XBB.1.5, and XBB.1.16 (Fig. 3b). This contrasted with the wide breadth of neutralisation by K4-66 and K1-68, and further demonstrated the importance of SHM for neutralisation breadth. The mAb with H-chain from WT K4-66 and L-chain from the inferred germline clearly showed a better neutralisation potency against BA.4/5, XBB.1.5, and XBB.1.16 than the mAb with H-chain from the inferred germline and L-chain from WT, suggesting the importance of mutations in H-chain for the wide breadth (Fig. 3c and Supplementary Fig. S4a). On the other hand, the Beta variant was potently neutralised by mAbs with either H-chain or L-chain from WT, indicating that the mutations in L-chain also enhance the neutralisation breadth of K4-66. Both K4-66 and K1-68 had mutations in the IGHV3-53 gene at positions 28 and 96 (Fig. 3a). Introduction of F28I, F28L, A96G, and A96V into K4-66IG enhanced neutralisation potency of most variants tested (Fig. 3c and Supplementary Fig. S4b). The effect of F28I/L mutations on neutralisation was significant against the Beta, BA.2, and BA.4/5, while the effect of A96 G/V was weak compared with that of the F28I/L mutations. Neutralising potency of K4-66IG mutants against the Delta variant was not enhanced, although the patient, who K4-66 was isolated from, was infected with the Delta variant (Fig. 3c and Supplementary Fig. S4).
Fig. 3.
Neutralisation breadth of the K4-66 lineage antibodies. (a) Amino acid sequences of K4-66 lineage antibodies K1-68 and K4-66 aligned with those of their germline genes, IGHV3-53∗04 and IGKV1-33∗01. (b) IC50 values of K4-66, K1-68, and K4-66IG, the inferred germline precursor of K4-66 against SARS-CoV-2 variants. (c) Neutralising activities of K4-66 mutants against pseudoviruses carrying S proteins from the 614G, Beta, and Delta variants and Omicron subvariants BA.1, BA.2, BA.4/5, XBB.1.5, and XBB.1.16. IC50 values (μg/mL) and the fold change (IC50 value of 4-66IG/IC50 value of mutant) are shown in the upper and lower rows, respectively. K4-66IGK (H-chain from WT K4-66 and L-chain from the inferred germline-reverted K4-66 [H: WT, L: IG]), K4-66IGH (H-chain from the inferred germline-reverted K4-66 and L-chain from WT K4-66 [H: IG, L: WT]), and K4-66IG mutants containing F28I, F28L, A96G, and A96V in the H-chain were compared with K4-66IG and K4-66. NA; not available. (d) NT50 values of plasma samples from patient KUKMC-19–22 and seven other patients with Delta BTI (see Supplementary Table S1) against SARS-CoV-2 variants.
The neutralising activities of K4-66 and K1-68 against the BA.1 subvariant were very weak compared with their activities against other Omicron subvariants (Fig. 3b). The comparatively low neutralising activity against BA.1 was also observed in plasma samples from other Delta BTI cases (Fig. 3d and Supplementary Table S1). The mean NT50 titre of plasma samples against BA.1 was 483 (standard deviation: 567), whereas titres against BA.2 and BA.4/5 were 1040 (1770) and 1030 (2040), respectively. The eight BTI samples in this study were insufficient to estimate the difference among neutralising activities against variants. However, the low neutralisation potency against the BA.1 subvariant among Delta BTI cases may be partially attributable to usage of IGHV3-53/3-66 for vaccine-induced antibodies, which maturated similarly to K4-66 following Delta BTI.
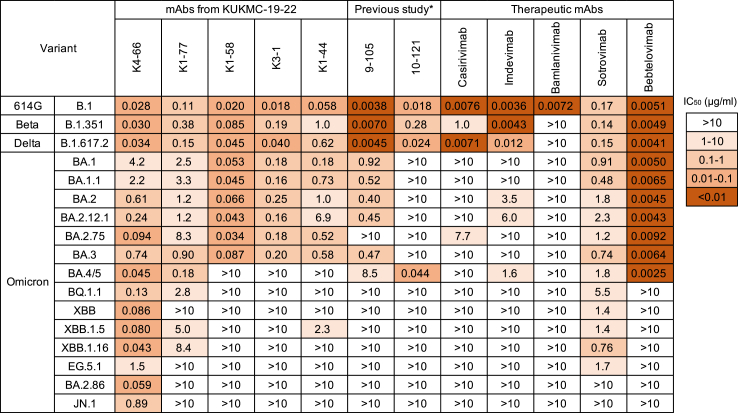
Cross-neutralisation potency of mAbs against a range of variants
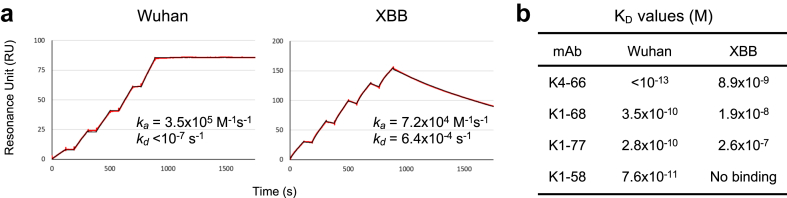
Five mAbs from KUKMC-19–22 that neutralised BA.1, namely K4-66, K1-77, K1-58, K3-1, and K1-44 (Fig. 2a), were further analysed for cross-neutralising activity against 17 viral variants (Fig. 4). Two mAbs derived from IGHV3-53, K4-66 and K1-77, neutralised 17 and 13 variants, respectively, while the other three mAbs neutralised nine or 10 variants, indicating a wide neutralisation breadth of the IGHV3-53 mAbs. While K4-66, K1-77, and sotrovimab (which was isolated from a patient infected with SARS-CoV) were cross-reactive to most of the variants tested, their respective potencies against the 614G variant were low (IC50: 0.028, 0.11, and 0.17 μg/mL) compared with those of the other therapeutic mAbs (0.0036–0.0076 μg/mL) and previously isolated mAbs 9–105 and 10–12120 (0.0038 and 0.018 μg/mL, respectively). Additionally, neutralisation breadth was correlated with the affinity (Fig. 5). K4-66 showed an extremely low KD value (<10−13 M) against the RBD from the Wuhan strain. The affinity of K4-66 against the RBD from Omicron subvariant XBB was also high (8.9 × 10−9 M), although the KD value decreased compared with that against the Wuhan strain. K1-68, a mAb in the same lineage as K4-66, and K1-77 both showed significantly low affinity against XBB compared with K4-66.
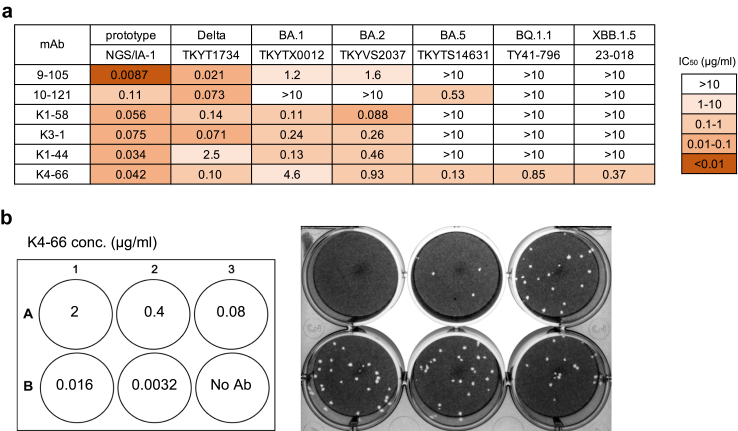
Fig. 4.
Neutralising activity of mAbs against various SARS-CoV-2 variants. IC50 values (μg/ml) of mAbs against pseudoviruses carrying S from 614G, Beta, and Delta variants, and various Omicron subvariants. In addition to five mAbs from patient KUKMC-19–22, two mAbs isolated from two convalescent patients infected with prototype SARS-CoV-2 (previous study), and five therapeutic mAbs were examined for neutralising activities.
Fig. 5.
Binding affinity of mAbs against the Wuhan strain and Omicron subvariant XBB RBDs. (a) Representative SPR sensorgrams of K4-66 binding affinity to Wuhan and XBB RBDs. Mean ka and kd values from three replicates are also shown. (b) Mean KD values of binding affinity of four mAbs to Wuhan and XBB RBDs. Binding affinity was determined by SPR analysis using Biacore T200.
The IGHV3-53-derived mAbs previously isolated from convalescent patients recovering from infection with prototype SARS-CoV-2, 9–105 and 10–121, potently neutralised variants that arose before the emergence of Omicron, but poorly neutralised Omicron subvariants (Fig. 4). In particular, Omicron subvariants that emerged after BQ.1.1 were completely resistant to 9–105 and 10–121. Omicron subvariants were also resistant to the therapeutic mAbs casirivimab, imdevimab, and bamlanivimab (Fig. 4). Bebtelovimab showed strong neutralisation potency against early Omicron subvariants that arose before BQ.1.1, but did not neutralise those that arose more recently, after BQ.1.1. Sotrovimab showed a wide breadth of neutralisation compared with other therapeutic mAbs, but did not neutralise BA.2.86 and JN.1. By contrast, K4-66 neutralised all variants tested (Fig. 4 and Supplementary Fig. S5). K4-66 potently neutralised recent Omicron subvariants BQ.1.1, XBB, XBB.1.5, XBB.1.16, and BA.2.86 (IC50: 0.043–0.13 μg/mL), but was weaker against EG.5.1 and JN.1 (IC50: 1.5 and 0.89 μg/mL, respectively). The broadly neutralising activity of K4-66 was also observed against authentic SARS-CoV-2 variants (Fig. 6). Consistent with our results using pseudoviruses, K4-66 neutralised authentic Omicron subvariants BA.1, BA.2, BA.5, BQ1.1, and XBB.1.5.
Fig. 6.
Neutralising activity of mAbs against authentic SARS-CoV-2 variants. (a) IC50 values of mAb neutralisation of authentic variants as determined by plaque assay. (b) Representative results of neutralisation by K4-66. Serially diluted K4–66 was mixed with the Omicron subvariant BA.5 (TKYTS14631), and added to VeroE6/TMPRSS2 cells for plaque formation.
Suppression of viral replication by K4-66 in hamsters infected with Omicron subvariant XBB.1.5
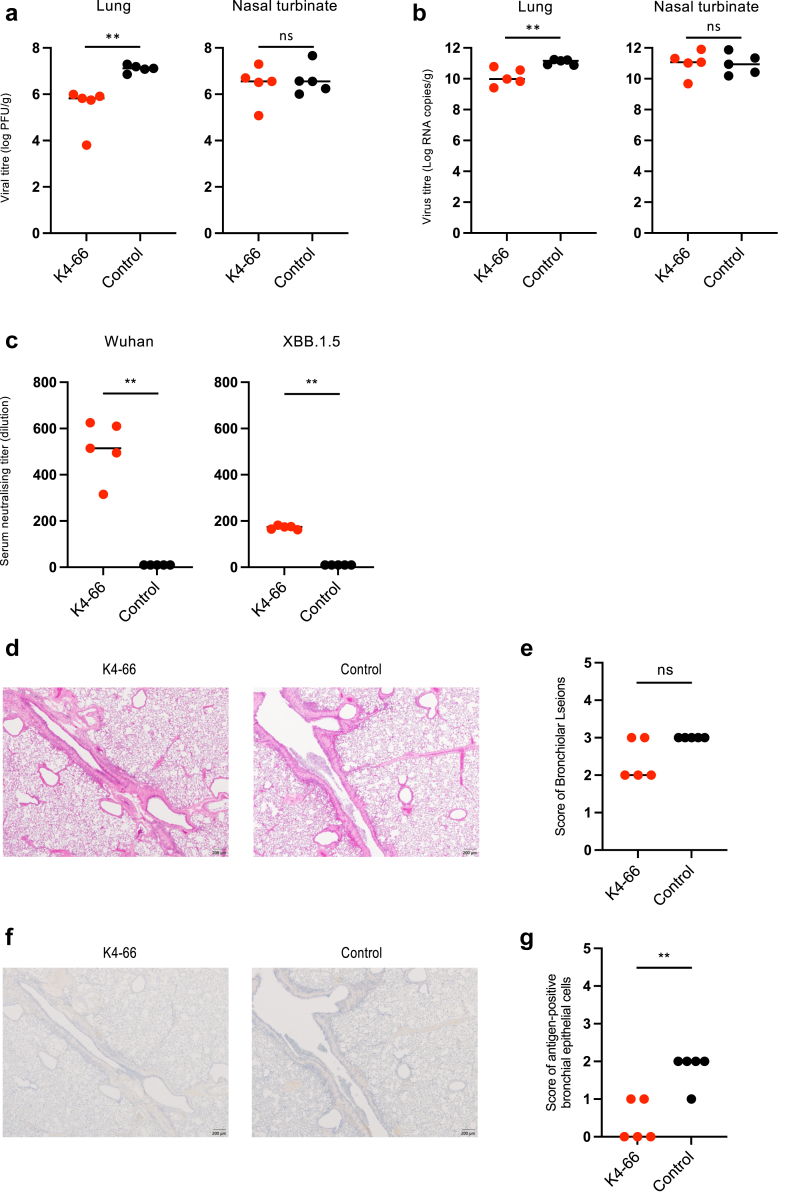
K4-66, which showed broad neutralising activity in vitro, was examined for its ability to suppress viral replication in vivo using a hamster model. Syrian hamsters inoculated with 103 PFU of Omicron subvariant XBB.1.5 (hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022) were intraperitoneally administered with 5 mg/kg K4-66 1 day post infection. Virus titres in the nasal turbinates and lungs and neutralising activities in plasma samples were determined at 4 days post infection. The viral loads of hamsters administered with K4-66 were significantly decreased in the lungs, but not in the nasal turbinates (Fig. 7a and b). Neutralising activities against both the Wuhan strain and Omicron subvariant XBB.1.5 were detected in plasma, although the neutralising titre against XBB.1.5 was lower than that against Wuhan (Fig. 7c). The histopathological analysis of lung revealed that the K4-66-treated groups showed slightly reduced scores for bronchiolar lesions by HE staining, although the differences did not reach statistical significance (Fig. 7d and e). We also found that K4-66 treatment significantly reduced the numbers of SARS-CoV-2 antigen-positive bronchial epithelial cells compared to the control antibody treatment, by immunohistochemistry staining of SARS-CoV-2 N protein (Fig. 7f and g). These results demonstrated that K4-66 neutralised subvariant XBB.1.5 in vivo and suppressed viral replication in the lungs.
Fig. 7.
Effect of mAb K4-66 on infection with Omicron subvariant XBB.1.5 in hamsters. Syrian hamsters were inoculated with 103 PFU of XBB.1.5 and intraperitoneally administered with 5 mg/kg K4-66 (n = 5) or normal human IgG (n = 5, control) 1 day later. Viral load titre was determined by PFU (a) and quantitative PCR (b) at 4 days post infection in the lungs (left) and nasal turbinates (right). (c) Serum neutralisation titres against Wuhan (left) and XBB.1.5 (right) in plasma samples at 4 days post infection. (d) Representative images of the lungs of infected hamsters stained by haematoxylin and eosin (H&E). (e) The extent of inflammatory cell infiltration in the lungs. (f) Representative images of the lungs stained by anti-SARS-CoV-2 nucleocapsid protein. (g) The extent of viral antigen-positive cells in the lung. The scores were determined as follows: 0, no change; 1, mild; 2, slight; 3, moderate; 4, severe. P value was calculated using the Mann–Whitney test. ∗P < 0.05, ∗∗P < 0.01, and ns: not significant.
Structure of K4-66 and SARS-CoV-2 S complex
To reveal the epitope on the SARS-CoV-2 S protein recognised by K4-66, we performed a cryo-EM analysis of the SARS-CoV-2 XBB.1.5 S ectodomain bound to Fab K4-66 and determined the cryo-EM map with C3 symmetry at a resolution of 3.8 Å (Supplementary Fig. S6 and Table S3). Fab K4-66 recognised a large part of the ACE2 receptor-binding motif (RBM) in the RBD-up form (Fig. 8a). Local refinement for the XBB.1.5 S RBD in complex with Fab K4-66 resulted in the cryo-EM map at a resolution of 5.3 Å (Fig. 8b). To better fit the Fab structure into the cryo-EM map of the RBD-Fab complex, we crystallized Fab K4-66 alone and determined the structure at 2.90 Å resolution (Fig. 8c and Supplementary Table S4). The fitting of crystal structures into the cryo-EM map revealed that K4-66 recognised the same epitope as the previously reported IGHV3-53/3-66 mAbs, such as CC12.1,12 but the bonding angle was approximately 30° more inclined in Fab K4-66 than in Fab CC12.1 (Fig. 8d).
Fig. 8.
Structural analysis of SARS-CoV-2 XBB.1.5 S and Fab K4-66 complex. (a) Overall cryo-EM map of XBB.1.5 S trimer in complex with Fab K4-66. Each protomer in the S trimer is colored by green, red, and blue, while the Fab is dark blue. (b) Cryo-EM map of the locally refined RBD-Fab complex with the rigidly fitted atomic models of XBB.1.5 S RBD (orange, PDB ID: 8JYP) and Fab K4-66 (PDB ID: 9II9). Structures are shown in ribbon representation. H- and L-chains are shown in sky blue and light blue, respectively. XBB.1.5 RBD is shown in orange. (c) Crystal structure of Fab K4-66. Same colors as in Fig. b. (d) Superimposed structures of Fab K4-66-RBD complex with Fab CC12.1-RBD complex (grey, PDB ID: 6XC2). (e) Electrostatic surface of the paratope and epitope. Surface is visualized using APBS plugin in PyMOL. (f) Structure of Fab K4-66 and close-up views of the residues substituted from the inferred germline sequence that are important for neutralising activity (Fig. 3).
To understand the driving force of antigen–antibody binding, we analysed the electrostatic potential molecular surface of the paratope and found that the antigen-binding surface of Fab K4-66 consisted of positive (R36, R106, and H110) and negative (D28, D86, and D108) patches on either side of the central concavity (Fig. 8e). Correspondingly, the epitopes on the RBDs of ancestral, BA.5, and XBB.1.5 variants had negative (D420) and positive (K444 and Q/R498) patches (Fig. 8e). This variant-independent electrostatic interaction would explain the broad reactivity of antibody K4-66. Interestingly, however, the electrostatic potential molecular surface of the paratope of antibody CC12.1, which exhibits a very similar binding mode with antibody K4-66, was quite different, indicating that the driving forces of the RBD binding between two antibodies are very different (Fig. 8e). The Q493, L455, and F456 residues of the RBD was close to the central concavity of the Fab K4-66 paratope, forming the antigen–antibody interaction core. Mutations at Q493, L455, and F456 decreased the neutralising potency of K4-66 (Supplementary Fig. S5b), suggesting the importance of the antigen–antibody interaction core in the interaction between K4-66 and RBD.
The F28I and A96G residues in the K4-66 heavy chain, which contributed significantly to the neutralising potency of K4-66 (Fig. 3c), were absent at the antigen–antibody binding interface (Fig. 8f). Accordingly, F28I and A96G would serve to adjust the paratope structure to facilitate a better antigen binding by slightly altering the structures of CDRL1 and framework of K4-66, respectively.
Discussion
Most public antibodies have not been effective against Omicron subvariants, whereas a fraction of IGHV3-53/3-66 public antibodies, as well as the mAb K4-66 identified in this study, showed broadly neutralising activity against a range of variants.4,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 These findings suggest that IGHV3-53/3-66 public antibodies induced by vaccination or infection with pre-Omicron SARS-CoV-2 variants may have the potential to neutralise a broad range of variants, including the recently circulating Omicron subvariants BQ.1.1, XBB.1.5, EG.5.1, BA.2.86, and JN.1.
BTI results in hybrid immunity, which is more effective against variants than infection-induced or vaccine-induced immunity.15, 16, 17 Broadly neutralising mAbs against SARS-CoV-2 variants have mainly been isolated from individuals with BTI,4,45,46,49, 50, 51 but were also induced in vaccinated4,47,52 or convalescent individuals.4,41, 42, 43,48 An analysis of several thousand mAbs isolated by Cao et al.4 revealed that many IGHV3-53/3-66 mAbs neutralised pseudoviruses carrying S proteins from D614G, BA.1, BA.2, BA.2.75, BA.5, BQ.1.1, and XBB. These IGHV3-53/3-66 mAbs were isolated from vaccinees and patients convalescing from infection with SARS-CoV, SARS-CoV-2 wild type, BA.1, BA.2, and BA.5. In contrast to the present study, the frequency of IGHV3-53/3-66 mAbs among the broadly neutralising mAbs in their study was not high, and was lower than that of IGHV1-69 mAbs. The acquisition of broadly neutralising activity only in the IGHV3-53/3-66 mAbs in the present study may be attributable to the difference in binding activity of mAbs to the Delta variant (Supplementary Fig. S3), although we only analysed one Delta BTI case. While the IGHV3-53/3-66 mAbs showed the same level of neutralising activity against both the prototype and Delta variant, the IGHV1-69 mAbs neutralised the prototype much better than the Delta variant, as previously reported.53 This raised the possibility that Delta BTI effectively activated antibodies using IGHV3-53/3-66, resulting in the maturation of antibodies with broadly neutralising activity. The low frequency of IGHV3-53/3-66 mAbs in the study by Cao et al.4 may be due to the lack of cases involving Delta BTI, which may activate IGHV3-53/3-66 mAbs better than IGHV1-69 mAbs. Conversely, broadly neutralising IGHV1-69 mAbs were frequently isolated from cases of Omicron BTI, which may activate IGHV1-69 antibodies.4 Further studies are required to clarify the mechanism of maturation of the IGHV3-53/3-66 antibodies, because we analysed memory B cells after Delta BTI from only one donor. Comparison of the IGHV3-53/3-66 antibodies before and after BTI in multiple donors will be useful to understand the effect of BTI on the breadth of neutralisation.
Increase in neutralising breadth in hybrid immunity may be due to diversification by SHM and masking of immunodominant epitopes.4,12,14,42, 43, 44,54,55 The critical importance of SHM for the acquisition of broadly neutralising activity in the K4-66 lineage antibodies was indicated by the lack of neutralisation of Omicron subvariants by the inferred germline precursor of K4-66 and the effect of F28I/L and A96 G/V mutations on neutralisation breadth. The Delta variant was neutralised by the K4-66 precursor better than 614G, suggesting that B cells expressing the K4-66 precursor were activated and expanded to K4-66 lineage antibodies after the Delta BTI. Mutations accumulated during evolution of the K4-66 lineage may not have significantly affected neutralisation of the 614G and Delta variants, but were important for neutralisation of Omicron subvariants. Consistently, IGHV3-53/3-66 public antibodies have been found to strongly neutralise prototype SARS-CoV-2 with minimal mutations, but most of the IGHV3-53/3-66 mAbs with broadly neutralising activity have more mutations than those without cross-reactivity.4,12,14,43,44 This implies that IGHV3-53/3-66 public antibodies induced by vaccination or infection with pre-Omicron variants may have potential as broadly neutralising antibodies with additional mutations. The importance of additional mutations for cross-neutralisation was also reported for IGHV1-58 mAbs, in which the mutations with minimal impact on neutralisation of prototype virus were critical for neutralisation of Omicron subvariants.56 Diversification of antibodies via SHM is an effective countermeasure against newly emerging SARS-CoV-2 variants.
While K4-66 neutralised all of the variants tested in this study, some Omicron subvariants were relatively resistant to this mAb. The gene usage observed in K4-66, IGHV3-53 and IGKV1-33, is the major combination identified in mAbs against the epitope A in the classification by Cao et al.4 that corresponds to part of class 1 in the classification by Barnes et al.57 Cryo-EM structure analysis of K4-66 and XBB.1.5 S protein confirmed that K4-66 targets the epitope A. This group of mAbs is encoded by the VH3-53 gene with a short H-chain CDR 3 (CDRH3) loop and binds to the RBD using germline-encoded motifs.57, 58, 59 K4-66 also has a short CDRH3 (11 amino acids) and efficiently blocked binding between angiotensin-converting enzyme 2 and RBD (data not shown), as class 1 mAbs do. Analysis of the K4-66 paratope suggests that the positive and negative patches on the binding surface enable the interaction with a range of RBDs from variants. The central concavity of K4-66, which is close to the cluster consisting of Q493, L455, and F456 of RBD, is critical for the interaction with RBD, because Q493R, F456L, and L455S mutations of RBD significantly decreased neutralisation potency. Neutralisation breadth of the K4-66 lineage mAbs was expanded by SHM, such as F28I/L and A96 G/V. Accumulation of mutations at specific positions of the VH3-53 gene14 in the class 1 VH3-53 antibodies, which are abundant in the human B-cell repertoire,60 is one strategy by which a vaccine can prevent infection with emerging variants. Furthermore, it becomes unnecessary to overcome the immune imprinting problem because this strategy involves maturation of antibodies that have already been induced by vaccine or infection. However, the frequency of occurrence of public antibodies with the potential to acquire broad neutralising activity is unclear. This will require identification of the potential broadly neutralising antibodies in vaccinated and infected individuals by comprehensive analysis, such as single cell RNA sequencing.
In summary, our study demonstrated that public antibodies have the potential to maturate to broadly neutralising antibodies. Public IGHV3-53/3-66 antibodies induced by vaccination are generally prototype-specific, but can acquire broad neutralising activity via accumulation of SHM, as observed in the Delta BTI case. Induction of broadly neutralising antibodies from public antibodies represents a potential strategy for the development of vaccines and therapeutic antibodies against newly emerging SARS-CoV-2 variants.
Contributors
Takeo Kuwata, and Shuzo Matsushita designed this study. Takeo Kuwata performed neutralisation assay and wrote the manuscript. Yu Kaku, Shashwata Biswas, Kaho Matsumoto, Mikiko Shimizu, and Yoko Kawanami isolated mAbs. Ryuta Uraki, Mutsumi Ito, Maki Kiso, Seiya Yamayoshi, Masaki Imai, and Yoshihiro Kawaoka performed a hamster experiment. Kyo Okazaki, and Hiroshi Morioka performed an SPR analysis. Rumi Minami, Yoji Nagasaki, Takako Inoue, and Yasuhito Tanaka collected samples. Mami Nagashima, Isao Yoshida, Kenji Sadamasu, Kazuhisa Yoshimura, Mako Toyoda, and Takamasa Ueno prepared authentic viruses. Takeo Kuwata, Mikiko Shimizu, Terumasa Ikeda, and Kei Sato prepared plasmids. Kanako Tarakado Kimura, Takao Hashiguchi, Yukihiko Sugita, and Takeshi Noda prepared RBD and S proteins and performed a structure analysis. G2P-Japan Consortium provide plasmids and information for experiments. All authors accessed and verified the underlying data, and read and approved the final manuscript.
Data sharing statement
All the data will be available upon reasonable request to the corresponding author with publication.
Declaration of interests
Rumi Minami declares payment or honoraria for lectures from ViiV Healthcare and Gilead Sciences, Inc. Kei Sato declares consulting fees from Moderna Japan Co., Ltd. and Takeda Pharmaceutical Co. Ltd., and payment or honoraria for lectures from Gilead Sciences, Inc., Moderna Japan Co., Ltd., and Shionogi & Co., Ltd. Yoshihiro Kawaoka declares funding supports, which are not related to the study, from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. Ltd., Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio.
Other authors declare no conflict of interests regarding any financial and personal relationships with other people or organisations that could inappropriately influence our work.
Acknowledgements
We thank all the clinical staff who provided care for the patients in Kyusyu Medical Center, Nagoya City University Hospital, and Kumamoto University. Casirivimab, imdevimab, bamlanivimab, and sotrovimab were kindly provided from Meiji Seika Pharma. We thank Michelle Kahmeyer-Gabbe, PhD, from Edanz for editing a draft of this manuscript. This study was supported by grants from the Japan Agency for Medical Research and Development (JP23ym0126048, JP22ym0126048, JP21ym0126048, JP23wm0125002, JP233fa627001, JP223fa627009, JP24jf0126002, JP22fk0108572), and the Japan Society for the Promotion of Science (JP21H02970, JK23K20041, and JPJSCCA20240006).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105439.
Contributor Information
Takeo Kuwata, Email: tkuwata@kumamoto-u.ac.jp.
Shuzo Matsushita, Email: shuzo@kumamoto-u.ac.jp.
The Genotype to Phenotype Japan (G2P-Japan) Consortium:
Jumpei Ito, Naoko Misawa, Arnon Plianchaisuk, Ziyi Guo, Alfredo Hina, Jr., Keiya Uriu, Kaoru Usui, Wilaiporn Saikruang, Spyridon Lytras, Ryo Yoshimura, Shusuke Kawakubo, Luca Nishimura, Yusuke Kosugi, Shigeru Fujita, Luo Chen, Jarel Elgin M. Tolentino, Lin Pan, Wenye Li, Maximilian Stanley Yo, Kio Horinaka, Mai Suganami, Adam P. Strange, Mika Chiba, Keiko Iida, Naomi Ohsumi, Kaho Okumura, Shiho Tanaka, Eiko Ogawa, Kyoko Yasuda, Tsuki Fukuda, Rina Osujo, Takasuke Fukuhara, Tomokazu Tamura, Rigel Suzuki, Saori Suzuki, Hayato Ito, Keita Matsuno, Hirofumi Sawa, Naganori Nao, Shinya Tanaka, Masumi Tsuda, Lei Wang, Yoshikata Oda, Zannatul Ferdous, Kenji Shishido, Keita Mizuma, Isshu Kojima, Jingshu Li, Tomoya Tsubo, Shuhei Tsujino, So Nakagawa, Kotaro Shirakawa, Akifumi Takaori-Kondo, Kayoko Nagata, Ryosuke Nomura, Yoshihito Horisawa, Yusuke Tashiro, Yugo Kawai, Kazuo Takayama, Rina Hashimoto, Sayaka Deguchi, Yukio Watanabe, Ayaka Sakamoto, Naoko Yasuhara, Tateki Suzuki, Kanako Kimura, Jiei Sasaki, Yukari Nakajima, Hisano Yajima, Yoshitaka Nakata, Hiroki Futatsusako, Takashi Irie, Ryoko Kawabata, Kaori Tabata, Hesham Nasser, Ryo Shimizu, MST Monira Begum, Michael Jonathan, Yuka Mugita, Otowa Takahashi, Kimiko Ichihara, Chihiro Motozono, Sharee Leong, Akatsuki Saito, Maya Shofa, Yuki Shibatani, Tomoko Nishiuchi, Hiroyuki Asakura, Jiri Zahradnik, Prokopios Andrikopoulos, Miguel Padilla-Blanco, and Aditi Konar
Appendix ASupplementary data
References
- 1.Mannar D., Saville J.W., Zhu X., et al. SARS-CoV-2 Omicron variant: antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science. 2022;375:760–764. doi: 10.1126/science.abn7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N., Stowe J., Kirsebom F., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/nejmoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uraki R., Ito M., Kiso M., et al. Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate. Lancet Infect Dis. 2023;23:402–403. doi: 10.1016/S1473-3099(23)00070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y., Jian F., Wang J., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2023;614:521–529. doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uriu K., Ito J., Kosugi Y., et al. Transmissibility, infectivity, and immune evasion of the SARS-CoV-2 BA.2.86 variant. Lancet Infect Dis. 2023;23:e460–e461. doi: 10.1016/s1473-3099(23)00575-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaku Y., Okumura K., Padilla-Blanco M., et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect Dis. 2024;24:e82. doi: 10.1016/S1473-3099(23)00813-7. [DOI] [PubMed] [Google Scholar]
- 7.Saito A., Tamura T., Zahradnik J., et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant. Cell Host Microbe. 2022;30:1540–1555.e15. doi: 10.1016/j.chom.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito J., Suzuki R., Uriu K., et al. Convergent evolution of SARS-CoV-2 Omicron subvariants leading to the emergence of BQ.1.1 variant. Nat Commun. 2023;14:1–20. doi: 10.1038/s41467-023-38188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura T., Ito J., Uriu K., et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat Commun. 2023;14:13–15. doi: 10.1038/s41467-023-38435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uriu K., Ito J., Zahradnik J., et al. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect Dis. 2023;23:280–281. doi: 10.1016/S1473-3099(23)00051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasoba D., Uriu K., Plianchaisuk A., et al. Virological characteristics of the SARS-CoV-2 omicron XBB.1.16 variant. Lancet Infect Dis. 2023;23:655–656. doi: 10.1016/S1473-3099(23)00278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan M., Liu H., Wu N.C., et al. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Yuan M., Lv H., Peng J., Wilson I.A., Wu N.C. A large-scale systematic survey reveals recurring molecular features of public antibody responses to SARS-CoV-2. Immunity. 2022;55:1105–1117.e4. doi: 10.1016/j.immuni.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Chen X., Wang Z., et al. Breakthrough infection elicits hypermutated IGHV3-53/3-66 public antibodies with broad and potent neutralizing activity against SARS-CoV-2 variants including BQ and XBB lineages. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds C.J., Pade C., Gibbons J.M., et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377 doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechmere T., Snell L.B., Graham C., et al. Broad neutralization of SARS-CoV-2 variants, including omicron, following breakthrough infection with Delta in COVID- 19-vaccinated individuals. mBio. 2022;13 doi: 10.1128/mbio.03798-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walls A.C., Sprouse K.R., Bowen J.E., et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185:872–880.e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuBridge R.B., Tang P., Hsia H.C., Leong P.M., Miller J.H., Calos M.P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto M., Kiso M., Sakai-Tagawa Y., et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaku Y., Kuwata T., Zahid H.M., et al. Resistance of SARS-CoV-2 variants to neutralization by antibodies induced in convalescent patients with COVID-19. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez Valdez KP., Kuwata T., Maruta Y., et al. Complementary and synergistic activities of anti-V3, CD4bs and CD4i antibodies derived from a single individual can cover a wide range of HIV-1 strains. Virology. 2015;475:187–203. doi: 10.1016/j.virol.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Hansen J., Baum A., Pascal K.E., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones B.E., Brown-Augsburger P.L., Corbett K.S., et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021;13:1–18. doi: 10.1126/scitranslmed.abf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto D., Park Y.J., Beltramello M., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 25.Westendorf K., Žentelis S., Wang L., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozono S., Zhang Y., Ode H., et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12:848. doi: 10.1038/s41467-021-21118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakurai Y., Ngwe Tun M.M., Kurosaki Y., et al. 5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro. Biochem Biophys Res Commun. 2021;545:203–207. doi: 10.1016/j.bbrc.2021.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amano M., Otsu S., Uemura Y., et al. Neutralization against Omicron sublineages (BA.2/BA.5/BQ.1.1/XBB/XBB.1.5) in bivalent BNT162b2-vaccinated HCWs with or without risk factors, or following BT infection with Omicron. Sci Rep. 2023;13:1–11. doi: 10.1038/s41598-023-44484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai M., Iwatsuki-Horimoto K., Hatta M., et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA. 2020;117:16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uraki R., Kiso M., Iida S., et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607:119–127. doi: 10.1038/s41586-022-04856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uraki R., Kiso M., Imai M., et al. Therapeutic efficacy of monoclonal antibodies and antivirals against SARS-CoV-2 Omicron BA.1 in Syrian hamsters. Nat Microbiol. 2022;7:1252–1258. doi: 10.1038/s41564-022-01170-4. [DOI] [PubMed] [Google Scholar]
- 32.Tamura T., Irie T., Deguchi S., et al. Virological characteristics of the SARS-CoV-2 Omicron XBB.1.5 variant. Nat Commun. 2024;15:1–17. doi: 10.1038/s41467-024-45274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheres S.H.W. A bayesian view on cryo-EM structure determination. J Mol Biol. 2012;415:406–418. doi: 10.1016/j.jmb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 35.Kabsch W. XDS. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agirre J., Atanasova M., Bagdonas H., et al. The CCP4 suite: integrative software for macromolecular crystallography. Acta Crystallogr Sect D Struct Biol. 2023;79:449–461. doi: 10.1107/S2059798323003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagin A., Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- 38.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 39.Murshudov G.N., Skubák P., Lebedev A.A., et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams P.D., Afonine P.V., Bunkóczi G., et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reincke S.M., Yuan M., Kornau H.C., et al. SARS-CoV-2 Beta variant infection elicits potent lineage-specific and cross-reactive antibodies. Science. 2022;375:782–787. doi: 10.1126/science.abm5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruhn M., Obara M., Chiyyeadu A., et al. Memory B cells anticipate SARS-CoV-2 variants through somatic hypermutation. J Infect. 2024;88:57–60. doi: 10.1016/j.jinf.2023.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Sheward D.J., Pushparaj P., Das H., et al. Structural basis of broad SARS-CoV-2 cross-neutralization by affinity-matured public antibodies. Cell Reports Med. 2024;5 doi: 10.1016/j.xcrm.2024.101577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruhn M., Obara M., Salam A., et al. Diversification of the VH3-53 immunoglobulin gene segment by somatic hypermutation results in neutralization of SARS-CoV-2 virus variants. Eur J Immunol. 2024;54:1–9. doi: 10.1002/eji.202451056. [DOI] [PubMed] [Google Scholar]
- 45.Kaku C.I., Bergeron A.J., Ahlm C., et al. Recall of pre-existing cross-reactive B cell memory following Omicron BA.1 breakthrough infection. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abq3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z., Zhou P., Muecksch F., et al. Memory B cell responses to Omicron subvariants after SARS-CoV-2 mRNA breakthrough infection in humans. J Exp Med. 2022;219 doi: 10.1084/jem.20221006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ju B., Fan Q., Liu C., et al. Omicron BQ.1.1 and XBB.1 unprecedentedly escape broadly neutralizing antibodies elicited by prototype vaccination. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ju B., Zhang Q., Wang Z., et al. Infection with wild-type SARS-CoV-2 elicits broadly neutralizing and protective antibodies against omicron subvariants. Nat Immunol. 2023;24:690–699. doi: 10.1038/s41590-023-01449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yisimayi A., Song W., Wang J., et al. Repeated Omicron exposures override ancestral SARS-CoV-2 immune imprinting. Nature. 2024;625:148–156. doi: 10.1038/s41586-023-06753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo M., Zhou B., Reddem E.R., et al. Structural insights into broadly neutralizing antibodies elicited by hybrid immunity against SARS-CoV-2. Emerg Microbes Infect. 2023;12 doi: 10.1080/22221751.2022.2146538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muecksch F., Wang Z., Cho A., et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022;607:128–134. doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreano E., Paciello I., Piccini G., et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600:530–535. doi: 10.1038/s41586-021-04117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer-Hermann M. Injection of antibodies against immunodominant epitopes tunes germinal centers to generate broadly neutralizing antibodies. Cell Rep. 2019;29:1066–1073.e5. doi: 10.1016/j.celrep.2019.09.058. [DOI] [PubMed] [Google Scholar]
- 55.Inoue T., Kurosaki T. Memory B cells. Nat Rev Immunol. 2024;24:5–17. doi: 10.1038/s41577-023-00897-3. [DOI] [PubMed] [Google Scholar]
- 56.Korenkov M., Zehner M., Cohen-Dvashi H., et al. Somatic hypermutation introduces bystander mutations that prepare SARS-CoV-2 antibodies for emerging variants. Immunity. 2023;56:2803–2815.e6. doi: 10.1016/j.immuni.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Barnes C.O., Jette C.A., Abernathy M.E., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hastie K.M., Li H., Bedinger D., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shrock E.L., Timms R.T., Kula T., et al. Germline-encoded amino acid–binding motifs drive immunodominant public antibody responses. Science. 2023;380 doi: 10.1126/science.adc9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banach B.B., Cerutti G., Fahad A.S., et al. Paired heavy- and light-chain signatures contribute to potent SARS-CoV-2 neutralization in public antibody responses. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.