Abstract
Immune responses from prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and COVID-19 vaccination mitigate disease severity, but they do not fully prevent subsequent infections, especially from genetically divergent strains. We examined the incidence of and immune differences against human endemic coronaviruses (eCoVs) as a proxy for response against future genetically heterologous coronaviruses (CoVs). We assessed differences in symptomatic eCoV and non-CoV respiratory disease incidence among those with known prior SARS-CoV-2 infection or previous COVID-19 vaccination but no documented SARS-CoV-2 infection or neither exposure. Retrospective cohort analyses suggest that prior SARS-CoV-2 infection, but not previous COVID-19 vaccination alone, associates with a lower incidence of subsequent symptomatic eCoV infection. There was no difference in non-CoV incidence, implying that the observed difference was eCoV specific. In a second cohort where both cellular and humoral immunity were measured, those with prior SARS-CoV-2 spike protein exposure had lower eCoV-directed neutralizing antibodies, suggesting that neutralization is not responsible for the observed decreased eCoV disease. The three groups had similar cellular responses against the eCoV spike protein and nucleocapsid antigens. However, CD8+ T cell responses to the nonstructural eCoV proteins nsp12 and nsp13 were higher in individuals with previous SARS-CoV-2 infection as compared with the other groups. This association between prior SARS-CoV-2 infection and decreased incidence of eCoV disease may therefore be due to a boost in CD8+ T cell responses against eCoV nsp12 and nsp13, suggesting that incorporation of nonstructural viral antigens in a future pan-CoV vaccine may improve vaccine efficacy.
INTRODUCTION
There are seven known human coronaviruses (HCoVs), with consequences ranging from asymptomatic infection or mild respiratory symptoms to severe respiratory distress or death (1). Four (HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1) of these, termed endemic CoVs (eCoVs), mostly cause only mild symptomatic illness and are responsible for 15 to 30% of the “common cold” in human adults (2). However, in the past 20 years, three HCoVs that are highly pathogenic and much more deadly have emerged: severe acute respiratory syndrome–related coronavirus (SARS-CoV); Middle East respiratory syndrome–related coronavirus (MERS-CoV); and SARS-CoV-2, responsible for the COVID-19 pandemic (3). Betacoronavirus, one of four coronavirus (CoV) genera, includes several of the most clinically relevant HCoVs, including HCoV-OC43, HCoV-HKU1, SARS-CoV, SARS-CoV-2, and MERS-CoV (4).
Numerous SARS-CoV-2 vaccines, primarily targeting the spike protein, are highly effective in reducing the incidence of hospitalization and progression to severe disease after infection (5). The SARS-CoV-2 vaccines or prior SARS-CoV-2 infection, however, are less likely to prevent breakthrough or reinfection especially with the most recent lineages, such as the Omicron variants and subvariants (6, 7). Numerous studies have demonstrated that antibodies generated from previous vaccination, prior SARS-CoV-2 infection, or both have lower neutralization potency against Omicron subvariants (8, 9). The less potent neutralizing antibodies (nAbs) potentially account for the limited protection against subsequent infections among those with prior SARS-CoV-2 exposure by vaccination or infection.
Besides nAbs, cellular responses are also likely important in reducing the incidence of symptomatic infection and onset of severe disease (10). nAbs generally target the SARS-CoV-2 spike protein, whereas T cells respond to various peptides present across the entire SARS-CoV-2 coding genome (11, 12). In general, spike protein–encoding sequences are more variable than other parts of the genome, both when comparing different SARS-CoV-2 variants and when comparing with other CoVs (13). In addition, nonstructural proteins, such as the viral RNA-dependent RNA polymerase (nsp12) and viral helicase (nsp13), show greater genetic conservation among the different CoVs (13). Furthermore, in contrast with nAbs, adaptive T cell responses show slower decay over time (14, 15). Thus, cellular responses generated by vaccination and infection may be especially important in protecting against the development of symptomatic disease by the SARS-CoV-2 variants and the other circulating CoVs.
Previous studies have demonstrated that individuals without prior SARS-CoV-2 exposure have preexisting antibodies and cellular responses against SARS-CoV-2 spike, nucleocapsid, nsp12, nsp13, and other proteins (16, 17). It has been speculated that these immune reactions may arise from prior eCoV infections. Studies from our group and others suggest that prior infection with eCoVs and the subsequent immune response can attenuate COVID-19 severity after SARS-CoV-2 infection (18, 19). These studies imply that an eCoV infection may generate heterotypic immunity against other CoV viral family members. Deciphering the immune basis for this potential heterologous immunity is important because of the ongoing threat of yet another CoV as the etiologic agent for a future pandemic.
In this study, we examined the effect of heterotypic immunity generated from previous SARS-CoV-2 antigen exposure, either by infection or COVID-19 vaccination. We find that prior SARS-CoV-2 infection, but not COVID-19 vaccination alone, associates with protection against subsequent symptomatic disease from the eCoVs. Non-CoV incidence was similar among those with different prior SARS-CoV-2 antigen exposure histories, implying that the heterotypic immunity is specific to CoVs. This heterologous immunity may be mediated by CD8+ T cell responses targeting nonstructural proteins, such as nsp12 and nsp13, and not nAbs. Our observations have important implications for future pan-CoV vaccines and other disease prevention strategies.
RESULTS
Individuals with prior SARS-CoV-2 infection have lower incidence of subsequent symptomatic eCoVs
We examined the incidence symptomatic eCoV and non-CoV infections in a retrospective cohort (Fig. 1A), and we analyzed ex vivo immune responses in a separate prospective cohort (Fig. 1B). We collected all eCoV and non-CoV infection instances documented on comprehensive respiratory panel (CRP) polymerase chain reaction (PCR) tests among individuals who presented for clinical evaluation during the study period of 30 November 2020 to 8 October 2021 at Boston Medical Center (BMC) (Fig. 1A). The CRP-PCR test detects the four eCoVs, SARS-CoV-2, and 16 other common non-CoV respiratory pathogens. The follow-up time was a median of 170 days with an interquartile range (IQR) of 156 days. All individuals were categorized into three groups on the basis of their pre-CRP-PCR test history: (i) prior documented SARS-CoV-2 infection; (ii) antecedent COVID-19 vaccination but no known SARS-CoV-2 infection; and (iii) no previous SARS-CoV-2 antigen exposure. There were 5713 CRP-PCR results from 4935 (median, one test per person; range, 1 to 8) different individuals. Most individuals in all three groups had one CRP-PCR test with an IQR from 1 to 1. Early in the pandemic, testing and vaccination were limited, and SARS-CoV-2 infection was documented more often in and COVID-19 vaccines were preferentially given to older individuals with preexisting comorbidities. Thus, as expected, the individuals with no prior SAR-CoV-2 antigen exposure were younger and healthier compared with the people in the other two groups (table S1). In general, the differences in age, gender, and the proportion with preexisting diagnoses was smaller among those with previous documented SARS-CoV-2 infection and those with prior COVID-19 vaccination but no known SARS-CoV-2 infection.
Fig. 1. Overall study design used in the investigation.
(A) Black arrows detail the grouping and analysis for the incidence of respiratory infections at BMC. (B) Red arrows detail the recruitment and grouping of individuals for the ex vivo assessment of CoV-specific immunity.
Among the 4935 individuals, 617 had a non–SARS-CoV-2 respiratory pathogen detected on the CRP-PCR test at some time during the study period (Table 1). There were 103 eCoVs, and rhinovirus/enterovirus (n = 409) was the most common non-CoV infection detected on the CRP-PCR tests (Table 1). Of the eCoV infections, 79 were HCoV-OC43, 19 were HCoV-229E, and 5 were HCoV-NL63. Individuals with prior SARS-CoV-2 infection had the lowest incidence of subsequent symptomatic eCoV as compared with the two other groups as judged by outcome alone (Table 1). Those with prior documented SARS-CoV-2 infection had lower incidence of Alpha but not Beta eCoVs. In contrast, non-CoV and enterovirus/rhinovirus detection was not different in the three groups. Time to event analysis also showed that eCoV (P < 0.0001, log-rank test; Fig. 2A) but not non-CoV (P = 0.2823; Fig. 2B) infections occurred less frequently among those with prior documented SARS-CoV-2 infections as compared with the individuals with COVID-19 vaccination or no known antigen exposure.
Table 1.
Infections detected on CRP-PCR tests. N/A, not applicable.
| Prior SARS-CoV-2 infection (n = 501) | Prior COVID-19 vaccine/No SARS-CoV-2 infection (n = 1565) | No prior SARS-CoV-2 exposure (n = 2869) | P value* | |
|---|---|---|---|---|
| Positive CRP-PCR † | 51 (10.2) | 226 (14.4) | 340 (11.9) | 0.0113 |
| eCoV | 5 (1.0) | 45 (2.9) | 53 (1.8) | 0.0145 |
| Alpha-eCoV ‡ | 0 (0.0) | 13 (0.8) | 11 (0.4) | 0.0316 |
| Beta-eCoV § | 5 (1.0) | 32 (2.0) | 42 (1.5) | 0.1777 |
| eCoV–full vaccine ¶ | 3 of 209 (1.4)# | 44 of 1463 (3.0) | N/A | 0.2638†† |
| eCoV–partial vaccine ** | 0 of 17 (0.0) | 1 of 102 (1.0) | N/A | 0.9999†† |
| eCoV–no vaccine | 2 of 275 (0.7) | N/A | 53 of 2869 (1.8) | 0.2301†† |
| Non-CoV | 46 (9.2) | 181 (11.6) | 287 (10.0) | 0.1688 |
| Rhinovirus/enterovirus | 31 (6.2) | 127 (8.1) | 251 (8.7) | 0.1518 |
Chi-square test unless noted otherwise.
For 1 of the 20 non–SARS-CoV-2 respiratory pathogens detected.
Includes HCoV-229E and HCoV-NL63.
Includes HCoV-OC43 and HCoV-HKU-1.
Received at least two doses of the Pfizer BioNTech BNT162b2 or Moderna mRNA-1273 or one dose of the Janssen/Johnson & Johnson Ad26.COV2.S COVID-19 vaccine a minimum of 14 days before the CRP-PCR test.
Two of 124 (1.6%),Moderna mRNA-1273; 1 of 66 (1.5%), Pfizer BioNTech BNT162b2; and 0 of 19 (0%), Janssen/Johnson & Johnson Ad26.COV2.S.
Received only one dose of the Pfizer BioNTech BNT162b2 (n = 43) or Moderna mRNA-1273 COVID-19 (n = 76) vaccine.
Fisher’s exact test.
Fig. 2. Previous SARS-CoV-2 infection is associated with lower incidence of symptomatic eCoV infections.
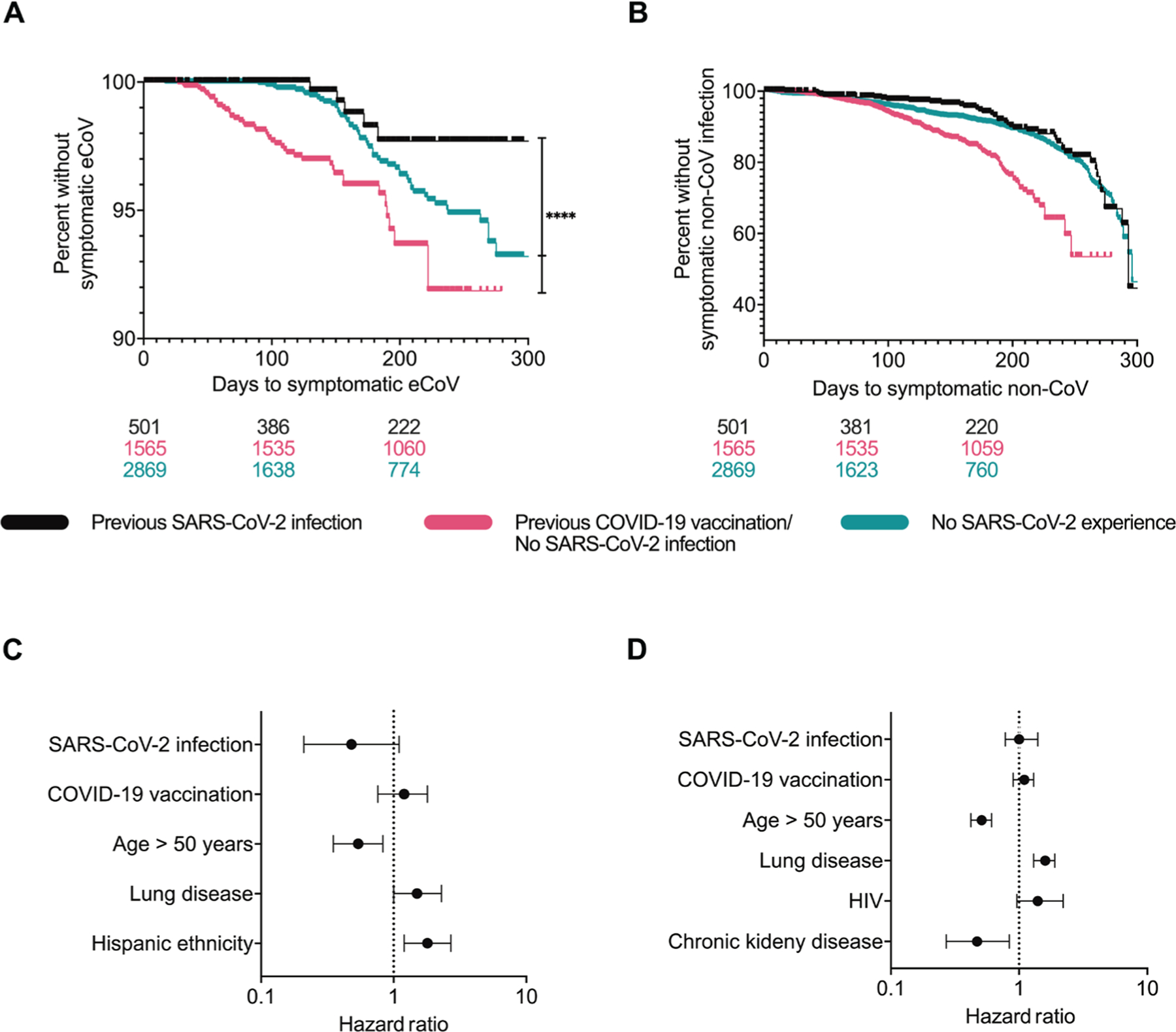
(A and B) Shown are Kaplan Meier plots for eCoV (A) and non-CoV (B) in those with no prior documented SARS-CoV-2 antigen exposure (teal), prior COVID-19 vaccination but no known SARS-CoV-2 infection (pink), and prior SARS-CoV-2 infection (black). Plots show unadjusted analyses with number of individuals at risk in each group at the bottom. Time to event analysis was performed using a log-rank test. (C and D) Shown are HRs of eCoV (C) and non-CoV (D) incidence in time-varying adjusted models. The figures show the covariates that had a P value ≤0.1 in the multivariable Cox-proportional hazard model. No prior documented SARS-CoV-2 antigen exposure group is the reference category. The horizontal lines indicate the HR for each variable along with the 95% confidence interval, and the vertical dotted line indicates an HR of 1.0. **** represent P values <0.0001.
Among those with prior COVID-19 vaccination but no documented SARS-CoV-2 infection, 1463 of the 1565 (93%) were deemed fully vaccinated because they had received the last dose of the recommended primary vaccine series at least 14 days before the CRP-PCR result (Table 1). During the study period, individuals who had received two doses of the Pfizer BioNTech BNT162b2 or Moderna mRNA-1273 COVID-19 vaccine or one dose of the Janssen / Johnson & Johnson Ad26.COV2.S COVID-19 vaccine were considered to have completed the recommended primary vaccination series according to national guidelines. In these 1463 individuals, there was no difference in eCoV incidence among those who got two doses of the Moderna mRNA-1273 (29 of 831, 3.5%), Pfizer BioNTech BNT162b2 (12 of 483, 2.5%), or one dose of the Janssen/Johnson & Johnson Ad26.COV2.S (3 of 149, 2.0%) COVID-19 vaccine (P = 0.4448, chi-square test). Among the 501 individuals with prior SARS-CoV-2 infection, 226 (45%) had at least one COVID-19 vaccine dose antecedent to the subsequent CRP-PCR test (Table 1). Thus, these individuals possibly had hybrid immunity associated with enhanced responses (20). Incidence of symptomatic eCoV infection was significantly lower in those with prior SARS-CoV-2 infection and no vaccination (2 of 275, 0.7%) as compared with the individuals who had been deemed fully vaccinated but had no known prior SARS-CoV-2 infection (44 of 1463, 3.0%, P = 0.0245, Fisher’s exact test, Table 1). One dose of the Janssen/Johnson & Johnson Ad26.COV2.S was often deemed to induce an inadequate immune response (21). Even after excluding individuals with Janssen/Johnson & Johnson Ad26.COV2.S immunization, those with prior documented SARS-CoV-2 infection (5 of 482, 1.0%) had lower subsequent symptomatic eCoV disease as compared with those with prior mRNA-based COVID vaccine (42 of 1416, 3.0%, P = 0.0169, Fisher’s exact test).
The prior results may lead to an incorrect conclusion that COVID-19 vaccination enhances disease likelihood from respiratory pathogens. The previous analyses did not account for the various differences among the three groups (table S1). Thus, we conducted time-varying covariate analysis to account for the baseline demographics and because some individuals had SARS-CoV-2 infection or COVID-19 vaccination during and not before the study period. In these instances, the CRP-PCR test of interest also occurred after the exposure. Previous COVID-19 infection [hazard ratio (HR) 0.48, 95% confidence interval (CI) 0.21, 1.1, P = 0.0810] associated with around 50% reduced risk of a future symptomatic eCoV (Fig. 2C), but it did not affect non-CoV incidence (Fig. 2D). On the other hand, COVID-19 vaccination showed no effect on the risk for eCoV or non-CoV infections. There was no statistically significant interaction between prior SARS-CoV-2 infection and antecedent COVID-19 vaccination in these models. Age greater than 50 years had significantly lower eCoV (HR 0.54, 95% CI 0.35, 0.83, P = 0.0121) and non-CoV (HR 0.51, 95% CI 0.42, 0.61, P < 0.0001) incidence. The lower hazard for both eCoV and non-CoV suggests decreased overall exposure or lower likelihood of developing symptomatic disease in this older age group. Those with preexisting lung disease, however, had higher risk for both eCoV (HR 1.5, 95% CI 1.0, 2.3, P = 0.0419) and non-CoV (HR 1.6, 95% CI 1.3, 1.9, P < 0.0001) infection. This may suggest that individuals with preexisting lung disease have greater physiologic risk for developing symptomatic disease after exposure to respiratory pathogens.
Ex vivo immunological differences to SARS-CoV-2 antigens define SARS-CoV-2 exposure groups
We collected blood samples from November 2020 to December 2022 to examine immune differences associated with the observed protection against symptomatic eCoVs. In contrast to the retrospective analysis, individuals in this independent cohort were grouped into three categories with stricter definitions (Fig. 1B). First, none of those with prior PCR-documented SARS-CoV-2 infection had COVID-19 vaccination (n = 20). Second, all the individuals in the COVID-19 vaccine group had received the second dose of either the Pfizer BioNTech BNT162b2 or Moderna mRNA-1273 COVID-19 vaccine at least 14 days before blood collection (n = 25). Last, individuals with no known SARS-CoV-2 antigen exposure (n = 28) and COVID-19 vaccination alone were interviewed regarding prior suspected infection during the consent process before blood collection. We specifically did not choose prepandemic samples for the no antigen exposure group because eCoV circulation was substantially higher in the preceding years as compared with the year when COVID-19 was declared a public health emergency in the city of Boston (22). Thus, samples collected before as compared with after pandemic onset may have higher eCoV immune responses from greater community virus circulation. The three groups had relatively similar demographics other than age distribution (table S2). In addition, the duration between the last documented exposures to SARS-CoV-2 spike protein was shorter among the individuals in the COVID-19 vaccination as compared with the SARS-CoV-2 infection group.
Individuals classified as not having a previous SARS-CoV-2 infection may have had prior undiagnosed or asymptomatic COVID-19 (23). Thus, there is a possibility of misclassification. We used comprehensive antibody- and T cell–based assessments to evaluate this possibility. First, we measured anti–SARS-CoV-2 [receptor binding domain (RBD) and nucleocapsid] plasma immunoglobulin G (IgG) titers by the single molecule array (Simoa) SARS-CoV-2 IgG anti-body test (24). RBD antibody response has been demonstrated as highly indicative of SARS-CoV-2 spike protein exposure through either infection or vaccination (25). As expected, individuals with prior SARS-CoV-2 spike protein exposure by infection or vaccination had higher RBD antibody titers compared with those with no known antigen exposure (Fig. 3A). Nucleocapsid IgG reactivity was used to further classify some COVID-19 vaccine recipients who may have had prior SARS-CoV-2 infection (26). Again, as expected, nucleocapsid IgG titers were higher in individuals with documented SARS-CoV-2 infection as compared with the two other groups (Fig. 3B). We also examined the number of activated T cells (both CD4+ and CD8+) after exposure to various SARS-CoV-2 peptide pools using the activation-induced marker (AIM) assay (fig. S1) (27, 28). As expected, higher percentages of activated CD4+ (CD134+ CD137+; Fig. 3C) and CD8+ (CD69+ CD137+; Fig. 3D). T cells were observed after SARS-CoV-2 spike peptide pool stimulation in individuals with either a previous SARS-CoV-2 infection or COVID-19 vaccination compared with those individuals without a known history of SARS-CoV-2 exposure. The activated SARS-CoV-2 spike protein–specific CD4+ and CD8+ T cell frequencies were similar between individuals with either a previous SARS-CoV-2 infection or COVID-19 vaccination. Also as expected, individuals with a previous documented SARS-CoV-2 infection had elevated SARS-CoV-2 nucleocapsid responsive CD4+ (Fig. 3E) and CD8+ (Fig. 3F) T cells compared with individuals with COVID-19 vaccination only or no history of SARS-CoV-2 antigen experience.
Fig. 3. Ex vivo immune responses to SARS-CoV-2 antigens differentiate individuals with different SARS-CoV-2 immune histories.
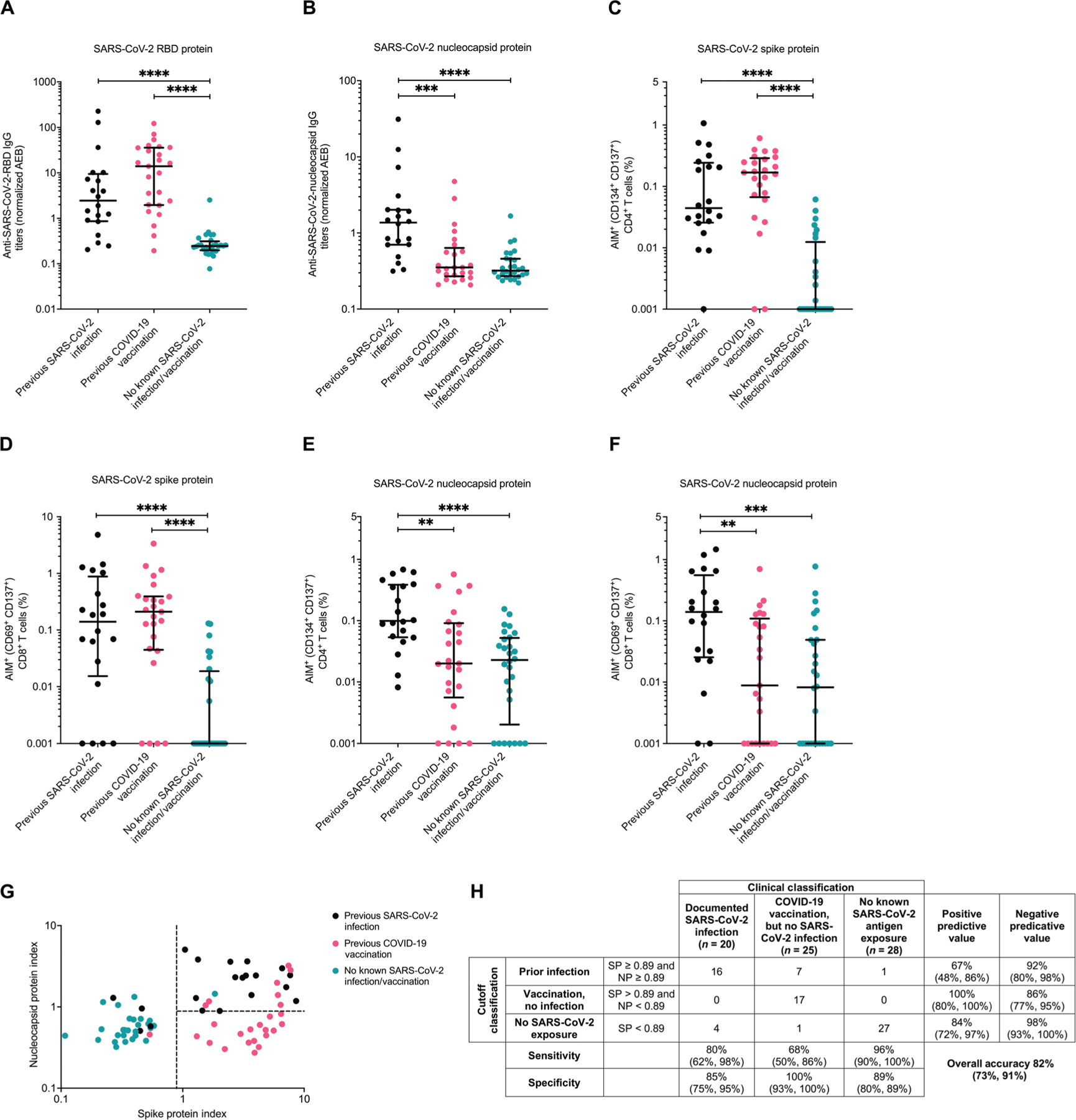
(A to F) Shown are antibody (A and B) and cellular responses (C to F) against SARS-CoV-2 spike protein (A, D, and E) and SARS-CoV-2 nucleocapsid (B, D, and E) in those with no prior SARS-CoV-2 exposure (teal), prior COVID-19 vaccination but no SARS-CoV-2 infection (pink), and prior documented SARS-CoV-2 infection (black). (A and B) SARS-CoV-2 IgG was calculated on the basis of the average enzyme per bead (AEB) against RBD (A) or nucleocapsid (B) measured using Simoa detection system. Statistical analyses were performed using a Kruskal-Wallis test with Dunn multiple comparison test. (C to F) Shown are results of AIM assays with SARS-CoV-2 spike protein (C and D) or SARS-CoV-2 nucleocapsid (E and F) peptide pools. T cell activation was measured on CD4+ (CD134+ CD137+) (C and E) and CD8+ (CD69+ CD137+) (D and F) T cells by flow cytometry. Data were background subtracted against the negative control (DMSO only). The dark horizontal lines in each scatter dot plot denote the median and interquartile range. Statistical analyses were performed using a Kruskal-Wallis test with Dunn multiple comparison test. (G) The antibody and T cell responses against SARS-CoV-2 (A to F) were log transformed and combined to create a spike protein and nucleocapsid protein index. The dotted lines represent the cutoff values to best differentiate the groups. (H) Accuracy and 95% confidence intervals of clinical classifications based on SARS-CoV-2 spike protein (SP) and nucleocapsid protein (NP) immunity index values from (H). **, ***, and **** represent P values <0.01, <0.001, and <0.0001, respectively.
Log-transformed values from the three nucleocapsid (IgG, AIM CD4+, and AIM CD8+) and three spike protein (RBD, AIM CD4+, and AIM CD8+) measurements were combined to generate a nucleocapsid and spike protein index. Spike protein and nucleocapsid cutoffs that best separated the three groups were chosen (Fig. 3G). These cutoffs yielded relatively similar test characteristics and overall accuracy (82%, 95% CI 73%, 91%; Fig. 3H) compared to another method (accuracy range 84 to 90%) that was based on examining SARS-CoV-2 cellular responses only (29). One and seven in the no antigen exposure and COVID-19 vaccine only group, respectively, clustered with most of the individuals with prior documented SARS-CoV-2 infection based on the established cutoffs. Thus, these eight individuals may have had prior undocumented or asymptomatic SARS-CoV-2 infection. These potentially “misclassified” individuals are highlighted in the ensuing figures, and the subsequent analyses were conducted both including and excluding these people. Furthermore, four individuals with documented SARS-CoV-2–positive PCR results had either a low spike protein or nucleocapsid index. Two of these four had severe COVID-19 with admission to the intensive care unit (ICU), whereas the other two were not hospitalized during their primary infection. Seven and nine of the remaining 16 individuals with documented SARS-CoV-2 infection were not hospitalized and not admitted to an ICU floor during their disease course, respectively.
Humoral immune responses are unlikely to account for the decreased symptomatic eCoV incidence
We first investigated humoral immune responses because nAbs have been described as the correlate of protection for SARS-CoV-2 (14, 30). We measured the ability of patient plasma to neutralize pseudoviruses that express the spike protein of one of three CoVs (SARS-CoV-2-Wuhan, HCoV-OC43, and HCoV-229E) (Fig. 4). Although spike protein exposure was more recent in those with vaccination (table S2), neutralization of the SARS-CoV-2 spike protein–based pseudovirus was similar in the individuals with a previous SARS-CoV-2 infection as compared to COVID-19 vaccination, even when seven vaccinated individuals with possible misclassification were excluded (Fig. 4A). Individuals with SARS-CoV-2 spike protein exposure either by infection or vaccination had higher SARS-CoV-2 nAbs as compared with the no SARS-CoV-2 antigen exposure group (P = 0.0439; Fig. 4B), although this was not the case when the one misclassified person from the no antigen group was excluded (P = 0.0566). Similar to previous reports, multiple individuals categorized into the no known SARS-CoV-2 spike protein exposure group had SARS-CoV-2 nAbs (16, 31, 32). These humoral responses may occur from a previous unknown SARS-CoV-2 infection or heterotypic responses from prior eCoV infections. SARS-CoV-2 RBD IgG–binding titers weakly correlated with pseudovirus neutralization (Spearman ρ = 0.1810, P = 0.03). Weak association may have been observed because we only quantified SARS-CoV-2 RBD IgG–binding antibodies. On the other hand, plasma contains both nAbs that target other spike protein domains, besides RBD, and non-IgG isotypes, which can also affect neutralization (33, 34).
Fig. 4. Prior SARS-CoV-2 spike protein exposure associates with more HCoV-OC43 nonneutralizing antibodies.
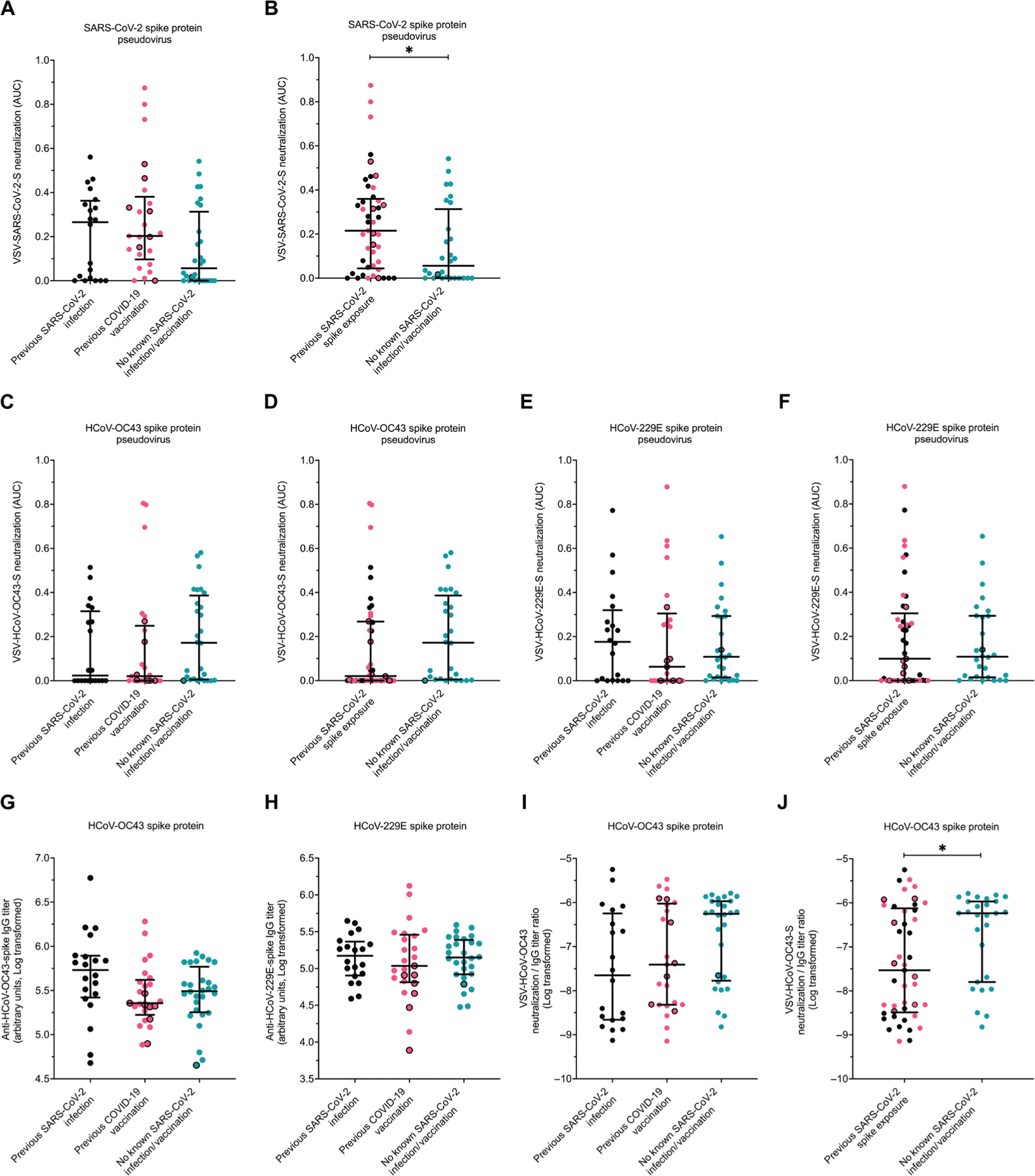
AUC (A to F) and spike protein–binding (G and H) antibody responses to various CoV spike proteins in those with no prior SARS-CoV-2 exposure (teal), prior COVID-19 vaccination but no SARS-CoV-2 infection (pink), and prior documented SARS-CoV-2 infection but no COVID-19 vaccination (black). (A and B) Neutralization responses against pseudoviruses expressing SARS-CoV-2 spike protein are shown for groups categorized by type of exposure (infection versus vaccination) (A) or with any exposure combined (B). (C and D) Neutralization responses against pseudoviruses expressing HCoV-OC43 spike protein are shown for groups categorized by type of exposure (infection versus vaccination) (C) or with any exposure combined (D). (E and F) Neutralization responses against pseudoviruses expressing HCoV-229E spike protein are shown for groups categorized by type of exposure (infection versus vaccination) (E) or with any exposure combined (F). (G and H) HCoV-OC43 (G) and HCoV-229E (H) spike protein–binding IgG antibody titers were measured by ELISA. (I and J) Ratios of HCoV-OC43 spike protein neutralization to binding antibodies are shown for groups categorized by type of exposure (infection versus vaccination) (I) or with any exposure combined (J). Black borders represent the eight individuals identified as potentially having prior asymptomatic SARS-CoV-2 infection. The dark horizontal lines denote the median and interquartile range. Statistical analyses were performed using either Kruskal-Wallis test with Dunn multiple comparison test (A, C, E, G, H, and I) or Mann-Whitney U test (B, D, F, and J). * represents P values <0.05.
No differences in HCoV-OC43 neutralization were observed between the prior SARS-CoV-2 infection group compared to the COVID-19–vaccinated group with or without excluding the seven people with possible prior occult infection (Fig. 4C). Individuals with any SARS-CoV-2 spike protein exposure either by SARS-CoV-2 infection or COVID-19 vaccination had lower HCoV-OC43 neutralization responses as compared with the no SARS-CoV-2 antigen experience individuals but only when excluding the one misclassified person from the no antigen group (P = 0.0407 when excluding the one misclassified person from the no antigen group; otherwise, P = 0.0645; Fig. 4D). On the other hand, there were no differences in the ability to neutralize HCoV-229E spike protein–containing pseudoviruses between any of the three groups (Fig. 4E) or between those with as compared to without previous spike protein exposure (Fig. 4F).
A previous investigation demonstrated that SARS-CoV-2 infection enhances antibodies against the Beta, but not Alpha eCoVs (35). We observed that binding antibodies against HCoV-OC43 (Fig. 4G) and HCoV-229E (Fig. 4H) spike proteins as measured by enzyme-linked immunosorbent assay (ELISA) were not different between the three groups. However, in multivariate linear regression analysis that accounted for age, gender, and comorbidities, prior SARS-CoV-2 infection associated with higher HCoV-OC43 binding antibody titers (table S3). Individuals with no known prior SARS-CoV-2 antigen experience had the highest ratio of HCoV-OC43 neutralization relative to total binding antibodies (Fig. 4I). The ratio of neutralization relative to total binding antibodies was lower in those with any previous SARS-CoV-2 spike protein experience as compared with those with no spike protein antigen exposure (P = 0.0473, P = 0.0412 after excluding the one person with possible prior asymptomatic infection, Fig. 4J). These results possibly suggest that any SARS-CoV-2 spike protein exposure, either by infection or vaccination, enhances nonneutralizing binding antibodies more than nAbs against HCoV-OC43.
HCoV-OC43 nonstructural protein–directed cellular immune responses are higher among those with prior SARS-CoV-2 infection
We next examined differences in cellular immune responses among the different groups using the AIM assay. We focused our testing on HCoV-OC43 peptides because HCoV-OC43 was the primary eCoV circulating in Boston during the study period, and peripheral blood mononuclear cell (PBMC) quantity limitations prevented testing against a large number of peptide pools. We also focused our study on spike protein, nucleocapsid, and nsp12/nsp13 T cell activity, which allowed us to compare spike protein, nonspike protein, structural protein, and nonstructural protein responses. Similar HCoV-OC43–reactive CD4+ and CD8+ T cells were observed between the three groups when PBMCs were stimulated with HCoV-OC43 spike protein (Fig. 5, A and B) and HCoV-OC43 nucleocapsid (Fig. 5, C and D) peptide pools, even when the individuals with possible prior asymptomatic infection were excluded from the analysis. We next examined cellular responses to nonstructural proteins nsp12 and nsp13 because preexisting responses against these viral targets have been associated with lack of detectable SARS-CoV-2 infection after presumed exposure (36). We generated an HCoV-OC43 nsp12/nsp13 peptide pool on the basis of previously described SARS-CoV-2 nsp12- and nsp13-reactive T cell epitopes (12, 37–42). On the basis of the Immune Epitope Database and Analysis Resource population coverage analysis tool, this combined set of epitopes with their known human leukocyte antigen (HLA) interactions potentially covers 98.05% (HLA class I) and 99.3% (HLA class II) of the world population and averages 7.82 (HLA class I) and 5.61 (HLA class II) epitopes per HLA combination (43). The defined SARS-CoV-2 epitopes were mapped to the HCoV-OC43 protein sequence, and HCoV-OC43 nsp12/nsp13 peptides were synthesized and pooled together (table S4). Although no difference was observed between CD4+ T cells (Fig. 5E), HCoV-OC43 nsp12/nsp13-reactive CD8+ T cells (Fig. 5F) were more frequent in individuals with a previous SARS-CoV-2 infection compared with individuals without a known history of SARS-CoV-2 antigen exposure (P = 0.0018, P = 0.0009 after excluding the one with possible “misclassification”) or with a prior COVID-19 vaccination only (P = 0.0693, P = 0.0279 without the seven with possible prior occult infection). Individuals with prior SARS-CoV-2 infection had significantly higher CD8+ T cell responses to HCoV-OC43 nsp12/nsp13 peptide pools as compared with a combined group containing those with COVID-19 vaccination and no antigen exposure (Fig. 5G, P = 0.0009, P = 0.0002 when excluding the eight with possible prior infection). Previous SARS-CoV-2 infection associated with a 0.0714 higher percentage of nsp12/nsp13-responsive CD8+ T cells in multivariable linear regression analysis that accounted for baseline demographics and comorbidities (table S5). In this multivariate model, age greater than 50 years was not a predictor for HCoV-OC43 nsp12/nsp13 CD8+ T cell responses. This suggests that the lower incidence of both eCoVs and non-CoVs among those greater than 50 years (Fig. 2, C and D) is unlikely to be due to higher preexisting cellular responses. No differences were observed between the groups in the CD4+ or CD8+ T cell responses to control peptide pools, human cytomegalovirus (CMV) pp65, or CMV, Epstein-Barr virus, and influenza (CEF) (fig. S2). Similar results were observed when stimulation index was used to calculate the relative T cell activation compared with the negative control condition (fig. S3). This suggests that the individuals in the groups did not have baseline differences in cellular reactivity.
Fig. 5. Prior SARS-CoV-2 infection associates with elevated CD8+ T cell responses to HCoV-OC43 nonstructural proteins.
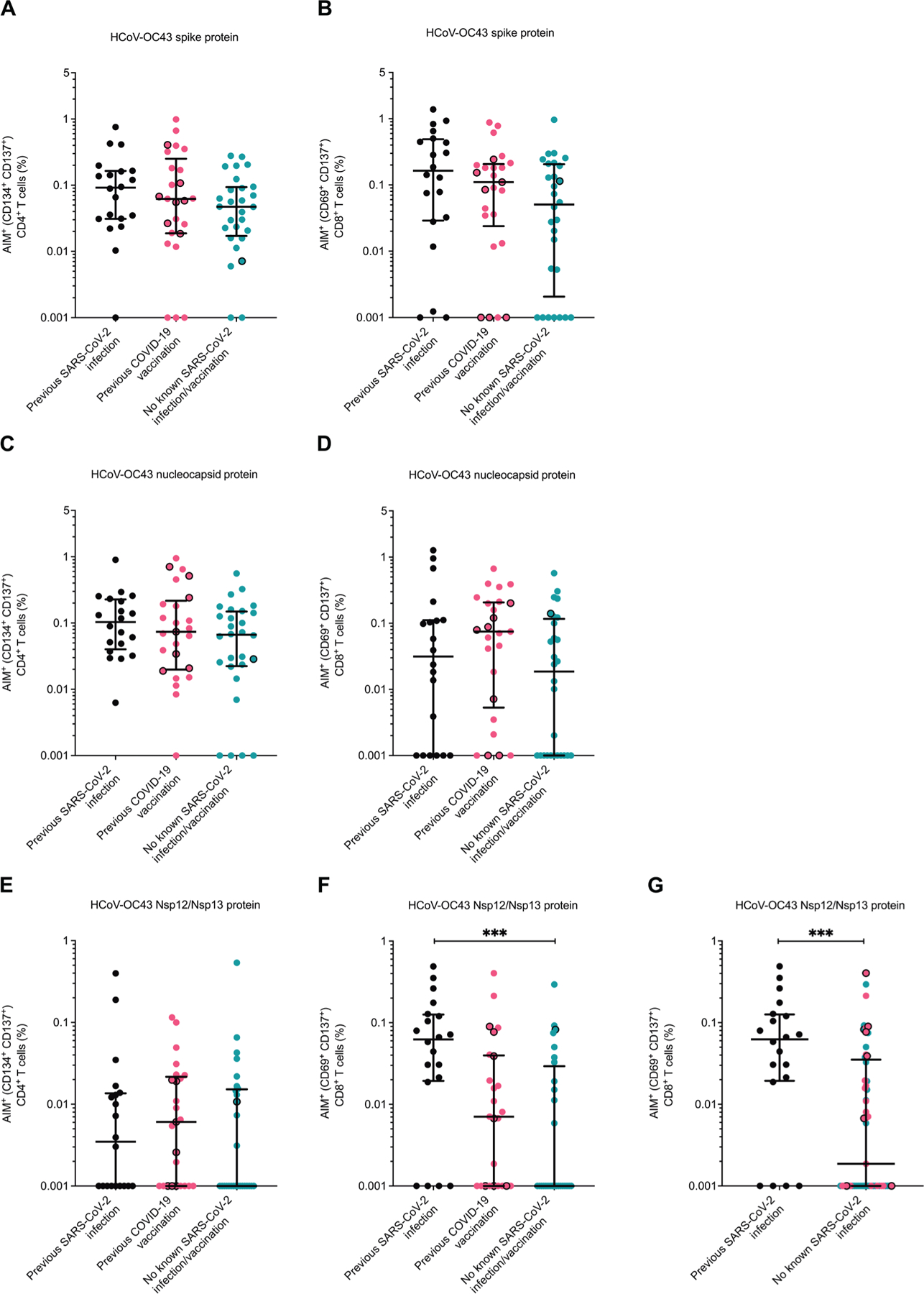
(A to G) The percentage of activated CD4+ and CD8+ T cells was measured by flow cytometry in response to various HCoV-OC43 proteins in those with no prior SARS-CoV-2 exposure (teal), prior COVID-19 vaccination but no SARS-CoV-2 infection (pink), and prior documented SARS-CoV-2 infection (black). (A and B) PBMCs were stimulated with peptides from HCoV-OC43 spike protein, and CD4+ (A) or CD8+ (B) T cell responses were measured. (C and D) PBMCs were stimulated with peptides from HCoV-OC43 nucleocapsid protein, and CD4+ (C) or CD8+ (D) T cell responses were measured. (E and F) PBMCs were stimulated with peptides from HCoV-OC43 nsp12 and nsp13 proteins, and CD4+ (E) or CD8+ (F) T cell responses were measured. (G) As in (F), but groups are categorized according to those with and without documented SARS-CoV-2 infection. Black borders represent the eight individuals identified as potentially having prior undocumented or asymptomatic SARS-CoV-2 infection. Data were background subtracted against the negative control (DMSO only). The dark horizontal lines in each scatter dot plot denote the median and interquartile range. Note, the y axis varies among the different panels. Statistical analyses were performed using either Kruskal-Wallis test with Dunn multiple comparison test (A to F) or Mann-Whitney U test (G). *** represents P values <0.001.
DISCUSSION
Some, but not all, prior studies have implied that previous eCoV immunity can provide protection against SARS-CoV-2 infection and COVID-19 morbidity (19, 44, 45). At BMC, which was the focus for the epidemiological examination in this study, we have previously shown that recent documented eCoV infection was associated with less COVID-19 disease severity and morbidity. We previously reasoned that nAbs are unlikely to mediate this effect because there was no difference in SARS-CoV-2 incidence (18). The biological mechanism for this observation remains uncertain, although numerous groups have suggested that prior eCoV infection can generate heterotypic humoral and cellular responses against SARS-CoV-2 (17, 46). Examining whether SARS-CoV-2 infection or COVID-19 vaccination protects against a subsequent eCoV disease can provide further insights into the nature of heterotypic immune responses among CoVs. Although nearly all adults have preexisting eCoV immune responses, these common cold CoVs share less genetic similarity with SARS-CoV-2 as compared with the relatedness among different SARS-CoV-2 lineages. Thus, even with the prior complex immunological history, eCoVs as compared with the SARS-CoV-2 variants are a better, although not a perfect, surrogate for a possible future genetically distant novel CoV. The incidence of eCoVs after SARS-CoV-2 infection and COVID-19 vaccination provides insights into the prospects for preventing disease from another CoV outbreak. Furthermore, the immunological characterization can inform future pan-CoV vaccine design.
We found that individuals with a prior SARS-CoV-2 infection, but not COVID-19 vaccination alone, had a lower likelihood of having subsequent symptomatic eCoV disease, although statistically significant differences were only observed for the more distantly related Alpha-CoVs. This suggests that SARS-CoV-2 infection, as compared with COVID-19 vaccination, may provide greater protection against genetically disparate CoVs. Similar to our results, SARS-CoV-2 spike protein immunization alone provided minimal protection against subsequent HCoV-OC43 infection in mice (47). In some respects, these animal results support our observations because COVID-19 vaccines predominantly only contain the SARS-CoV-2 spike antigen and no other viral protein. Along with spike protein–directed immunity, individuals with prior SARS-CoV-2 infection harbor additional immune responses against nonspike viral proteins, such as nucleocapsid, nsp12, and nsp13. The nonspike proteins are more conserved among the different CoVs, and, thus, the immune responses are likely cross-reactive (48). The SARS-CoV-2 infection– and COVID-19 vaccine–generated heterotypic immunity, especially nAbs, may not be able to fully prevent a heterologous CoV infection. After initial infection, however, SARS-CoV-2 heterotypic nonspike protein–specific cellular responses could lower virus load and reduce the onset of symptomatic disease (49).
There are several strengths with our observational retrospective results. The data incorporate a relatively large number of individuals, although they were acquired from a single medical center. The large population size allowed us to adjust for various demographics that are likely important for symptomatic eCoVs, such as age, gender, and preexisting morbidities (50). Furthermore, we also analyzed non-CoV incidence as a control among the groups. Prior SARS-CoV-2 infection associated with lower subsequent eCoV incidence with all the statistical methods used to analyze the data. In the time-varying multivariable models that accounted for baseline demographics and preexisting comorbidities, non-CoV incidence was not different among those with prior SARS-CoV-2 infection or COVID-19 vaccination as compared to the no SARS-CoV-2 antigen experience group. This finding is important because it suggests that behaviors and unmeasured confounders, such as physician’s reasons for testing and patient’s health-seeking behaviors, were likely similar in these groups. On the other hand, older age associated with lower eCoV infections. This likely reflects differences in exposure to respiratory pathogens because non-CoV incidence was also lower in the older age group. Conversely, the higher incidence of both eCoV and non-CoV incidence among those with preexisting lung disease likely reflects physiological predisposition to developing symptomatic disease after exposure to a respiratory pathogen. The impact of SARS-CoV-2 infection as compared with COVID-19 vaccination on eCoV incidence should also be examined in other cohorts, although the widespread and often undocumented infection with the SARS-CoV-2 Omicron variant will make a prospective analysis extremely challenging now (51).
There are also limitations with these retrospective observational data. First, the sample size was based on a specific time period, not statistical power, so lack of statistical significance may not imply lack of clinical relevance. There is a risk of misclassification because some individuals may have had asymptomatic COVID-19. In our ex vivo analysis, 8 of the 53 (15%) individuals classified as not having prior SARS-CoV-2 infection may have had prior occult disease (table S2). Prior SARS-CoV-2 infection, however, would still be associated with lower eCoV incidence even with the worst-case assumption of nondifferential misclassification of up to 10% of the vaccinated and no previous antigen exposure group (table S6). Analyses may also be subject to residual confounding, and formal tests for statistical interaction may be underpowered. In addition, HRs may be subject to selection bias. Our observational data include symptomatic individuals that present for clinical care; it does not record possible asymptomatic infections. It remains uncertain whether prior SARS-CoV-2 infection potentially also associates with lower incidence of asymptomatic eCoV infections.
We conducted ex vivo analyses to identify immune mechanisms for the observed protection against symptomatic eCoV infections. These ex vivo studies were restricted to individuals with more strict definitions for the different groups. Individuals in the three prespecified groups were sampled during the same time period because this assured that there was similar community eCoV exposure across the entire population. This, along with the emergence of both the highly infectious Omicron variant and widespread home testing, limited sample sizes for these ex vivo analyses because we aimed to avoid individuals who may have had an undocumented SARS-CoV-2 infection. We conducted numerous tests to identify misclassified individuals because it can be difficult to decipher previous asymptomatic and undocumented SARS-CoV-2 infections using single tests alone. We used six different independent measurements to identify misclassified individuals. In general, our methodology revealed similar results as other T cell–based methods to differentiate routes of SARS-CoV-2 spike protein exposure (29). However, no assay or methodology can identify prior undocumented SARS-CoV-2 infection with complete certainty (52).
Numerous previous studies have reported on the importance of nAbs in preventing CoV infections and ameliorating disease severity (30, 53). nAbs are deemed as a correlate of protection against SARS-CoV-2 and as a corollary against CoVs in general (54). Animal models also suggest that cross-reactive nAbs can protect against heterologous CoV challenge if the two CoVs have high degrees of genetic conservation (47). In our analysis, we found that nAbs against SARS-CoV-2, HCoV-OC43, and HCoV-229E were not different among those with prior SARS-CoV-2 infection or COVID-19 vaccination alone. Previous reports have suggested that SARS-CoV-2 spike protein exposure boosts antibody titers against the Beta-CoVs, such as HCoV-OC43, and not necessarily against the Alpha-CoVs, such as HCoV-229E (55). We also found that binding antibodies against HCoV-OC43 spike protein were increased after SARS-CoV-2 infection, although we did not observe a boost in HCoV-OC43 antibodies in our COVID-19 vaccinated group. In contrast, we observed that HCoV-OC43 neutralization was generally lower in those with prior documented SARS-CoV-2 infection or COVID-19 vaccination as compared with those without any known SARS-CoV-2 antigen exposure. The lower HCoV-OC43 neutralizing to total antibody binding ratio in those with prior SARS-CoV-2 spike protein exposure suggests that SARS-CoV-2 spike protein preferentially induces antibodies that are not critical for HCoV-OC43 neutralization (56). We did not assess mucosal antibodies or Fc-mediated antibody functions against HCoV-OC43 or HCoV-229E among the groups, and differences in these parameters may be important for protection against symptomatic eCoVs (57). Collectively, our studies suggest that preexisting or de novo–generated plasma nAbs titers do not associate with the lower incidence of symptomatic eCoVs.
In contrast to nAbs, our ex vivo investigations imply that cellular responses are important in lowering the incidence of symptomatic eCoVs. Spike protein–based T cell immunity was similar between those individuals with either a previous SARS-CoV-2 infection and a previous COVID-19 vaccination, which agrees with previous work in the field (58). In general, the T cell responses against HCoV-OC43 structural proteins were relatively common in all individuals, highlighting the ubiquity of prior eCoV infections (11). The primary difference between the individuals with a previous SARS-CoV-2 infection and those with a COVID-19 vaccination were in the elevated CD8+ T cell responses to the nonstructural antigens nsp12 and nsp13. Memory T cell responses to these nonstructural antigens have been implicated in the protection against SARS-CoV-2 infections (19, 36). Animal models have also implied that cellular responses against nonstructural proteins are important for protection against infection and disease severity (49, 59). The nonstructural regions of the different CoVs are generally more conserved, and, thus, cross-reactive responses are more likely preserved (48, 60). The heterotypic boost of these cross-reactive T cells likely contributes to the killing of CoV-infected cells and the clearance of the infection before the onset of symptomatic disease. In our ex vivo analysis, greater than 50 years of age did not associate with higher HCoV-OC43 nsp12- and nsp13-directed CD8+ T cell responses (table S5). This further argues that the protection against symptomatic eCoV observed in those greater than 50 years of age in the retrospective analysis potentially reflects lower exposure to respiratory pathogens rather than enhanced immunity from a greater number of prior HCoV-OC43 infections with increasing age (61).
There are limitations to our conclusions from the ex vivo analyses. Our results demonstrate associations and do not prove causation. We chose to examine spike protein, nucleocapsid, and nsp12/nsp13 cellular responses only. Cellular responses against other structural and nonstructural proteins are also likely important. Cell quantities limited the breadth of the cellular responses that we could examine, and we focused on those that have been deemed most important for disease prevention (36, 62). In addition, we only tested cellular responses against HCoV-OC43. Although there is a relatively high degree of similarity between Alpha- and Beta-CoV non-spike proteins, cellular responses against an Alpha-CoV, such as HCoV-229E, should be tested to make our results more generalizable. We also did not incorporate COVID-19 disease parameters for the individuals with prior SARS-CoV-2 infection in our analyses. The magnitude of the immune responses differs on the basis of COVID-19 disease severity, and associations between eCoV incidence and mild versus severe COVID-19 disease may further highlight the potentially protective immune responses (63). Documenting lower eCoV incidence among those with as compared with those without preexisting cellular responses against nonstructural CoV proteins generated by vaccination or infection will be required to conclude biological mechanisms. These types of human studies will be exceedingly difficult because of the relatively low incidence of symptomatic, clinically recognized eCoV infections. Animal studies may also be challenging because there are not great nonhuman models to examine symptomatic eCoV disease after SARS-CoV-2 infection. Interferon-γ receptor–deleted transgenic mice expressing specific HLAs were recently used to document that cellular responses from prior HCoV-OC43 infection could mitigate SARS-CoV-2–induced disease (64). In these highly immunocompromised mice, however, HCoV-OC43 exposure generated immune responses but minimal to no disease. Future studies that examine cellular reactivity against SARS-CoV-2 nsp12/nsp13 among those with no previous SARS-CoV-2 infection and prior known eCoV infection history will also provide valuable insights into the heterotypic immunity observed in our study. Furthermore, examining nAbs, as has been done previously (65, 66), and cellular responses against CoVs that currently only exist in nonhuman species, such as bats, could also further highlight the extent of heterotypic immunity from SARS-CoV-2 infection and COVID-19 vaccination.
Prior SARS-CoV-2 infection associates with better protection against the heterologous eCoVs compared with spike protein–based COVID-19 vaccination alone. Although acute COVID-19 severity has been greatly reduced with the widespread institution of COVID-19 vaccination and immunity from prior SARS-CoV-2 infections, our results should not be used to promote SARS-CoV-2 infections as a means to limit future heterologous CoV disease (67). SARS-CoV-2 infections continue to pose a substantial risk for developing long-term symptoms, termed long COVID or post-acute sequelae of SARS-CoV-2 infection (68). Most efforts aimed at developing universal CoV vaccines are focused on eliciting broadly nAbs and inducing T cell responses that target conserved domains in structural proteins shared among diverse CoVs (69). Although these efforts should continue, we propose that the addition of nonstructural antigens to future universal CoV vaccines may induce a more diverse T cell repertoire and recapitulate the heterotypic immune benefit of a natural infection.
MATERIALS AND METHODS
Study design
The objective of this study was to determine whether prior SARS-CoV-2–elicited immunity associates with symptomatic eCoV incidence and to identify an immune correlate. We performed a retrospective analysis of the incidence of eCoV and non-CoV infections among individuals classified into three groups: (i) previous documented SARS-CoV-2 infection; (ii) COVID-19 vaccination and no documented or known SARS-CoV-2 infection; and (iii) neither exposure. Immune correlates were identified by examining ex vivo immune responses. All ex vivo assays were performed in duplicate or triplicate at independent times. The study was not blinded. This study was approved by the BMC Institutional Review Board.
Participants and data collection
The retrospective analysis of the incidence of eCoV and non-CoV infections included all available CRP-PCR (BioFire Diagnostics) results in the BMC electronic medical records (EMRs) performed from 30 November 2020 to 08 October 2021. In general, CRP-PCRs are used to evaluate patients that present with acute respiratory symptoms, but we did not confirm the medical reasons for the testing in all cases. The CRP-PCR was done at the discretion of the treating physician. All individuals less than 18 years of age were excluded from analyses. The start date was chosen because the first eCoV infection at BMC for the 2020 fall/winter season was documented on 30 November 2020. The end date of 08 October 2021 was chosen because this was before the first documented SARS-CoV-2 Omicron infection at BMC. The less-symptomatic and widely spread Omicron infections made it difficult to reliably differentiate individuals into the three prespecified groups (51). Individuals with a positive test for HCoV-229E, HCoV-HKU1, HCoV-NL63, or HCoV-OC43 were classified as having an eCoV infection. Each individual only contributed a single data point, although they may have had multiple CRP-PCR results during the study period. The date of the positive eCoV infection was used in the analysis for an individual even if they had other CRP-PCR results during the study period. Incidence of non-CoVs served as a control to assess for differences in exposure and susceptibility to respiratory pathogens among the three groups. For those individuals with positive non-CoV test results, the date of the first positive non-CoV result was used in the analysis, although the individual may have had other pathogens, besides eCoVs, in subsequent CRP-PCRs. Among individuals with multiple CRP-PCRs that did not detect eCoVs or non-CoVs, the date of the last test was used in the analyses. SARS-CoV-2 detected on the CRP-PCR tests was not classified as either eCoV or non-CoV. If an individual had SARS-CoV-2 on a CRP-PCR test and a subsequent CRP-PCR test, that person was placed in the prior SARS-CoV-2 infection group. On the other hand, an individual with SARS-CoV-2 detected on last CRP-PCR test within the study time period was categorized on the basis of prior history. Demographic and clinical information, including prior COVID-19 vaccination and a previous documented positive SARS-CoV-2 test result, were obtained for these individuals from the EMR.
Blood samples were obtained from BMC patients during a non-COVID-19–related medical visit. Before sample collection, all individuals’ status regarding COVID-19 vaccination and prior SARS-CoV-2 infection was confirmed during the consent process. Included individuals were greater than 18 years of age, and medical visits were for non–COVID-19 diagnosis. Exclusion criteria included a SARS-CoV-2–positive nasal swab within the past 14 days or use of an immunosuppressive drug. Plasma was obtained after centrifugation, and PBMCs were isolated using Ficoll Hypaque density centrifugation methods.
SARS-CoV-2–specific IgG detection
Anti–SARS-CoV-2 IgG antibody titers were assessed using a previously described ultrasensitive Simoa multiplexed assay that simultaneously measures anti–SARS-CoV-2 IgG antibodies against the RBD and nucleocapsid protein (24). Plasma samples were centrifuged at 4°C for 10 min at 2000g. The supernatant was then diluted 1000-fold in Homebrew Sample Diluent (Quanterix) before running the assay.
Pseudovirus production
CoV spike protein–expressing pseudoviruses were prepared as previously described (70). Briefly, HCoV-229E-spike protein (SinoBiological, VG40605-CF), HCoV-OC43-spike protein (SinoBiological, VG40607-CF), or SARS-CoV-2-spike protein (BEI Resources, NR52310) expression plasmids were transfected into human embryonic kidney (HEK)–293 T cells (American Type Culture Collection (ATCC), CRL-11268). After 24 hours, the transfected cells were infected with vesicular stomatitis virus–glycoprotein (VSV-G)–pseudotyped virus (G*ΔG-VSV) containing the firefly luciferase expression cassette. The pseudovirus-containing cell supernatant was collected after an additional 24 hours, filtered, concentrated, and stored at −80°C. Virus supernatants from HEK-293 T cells infected with VSV-G–pseudotyped virus (G*ΔG-VSV) only and not transfected with a CoV spike protein–encoding plasmid were used as a control to assess for the amount of infection in the absence of a CoV spike protein.
Neutralization assay
Pseudovirus neutralization assays were performed as previously described (71). Briefly, plasma was heat-inactivated at 56°C for 1 hour, and pseudovirus was incubated in five twofold serial dilutions, starting with a 1:40 highest dilution, at 37°C. After 1 hour, approximately 1.25×104 Vero E6 cells (ATCC, CRL-1586) were added to each well. After 48 hours, luciferase expression was measured using the Promega Bright-Glo Luciferase Assay System (Thermo Fisher Scientific) on a SpectraMax190 Microplate Reader (Molecular Devices). Percent neutralization was calculated in comparison with luciferase expression in infected wells without patient plasma. Area under the curve (AUC) values were calculated from the curve generated from the neutralizations across the serially diluted plasma (72). All neutralizations were tested in triplicate, a minimum of two independent times. Neutralization against VSV-G–pseudotyped virus (G*ΔG-VSV) was used as a control to assess activity against VSV-G protein in the absence of a CoV spike protein.
ECoV spike–specific IgG detection
Relative HCoV-OC43 and HCoV-229E spike protein–specific IgG titers were detected by ELISA as previously described (73). Briefly, a 96-well plate was coated with 2 μg/ml of either HCoV-OC43-spike protein (SinoBiological, 40607-V08B) or HCoV-229E-spike protein (SinoBiological, 40605-V08B) antigen for 1 hour at room temperature. Wells were washed thrice with phosphate-buffered saline (PBS) and blocked with casein blocking buffer (Thermo Fisher Scientific, 37528) for 90 min. The wells were then washed thrice with PBS before the wells were incubated with threefold dilutions of plasma for 1 hour. Wells were subsequently washed thrice with PBS containing 0.05% Tween 20 (PBST), followed by the addition of antihuman horseradish peroxidase–conjugated secondary antibodies for IgG detection (diluted 1:50000, Sigma-Aldrich, A0170) to each well for 30 min. The wells were washed with PBST four times, and then 3,3′, 5,5′ tetramethylbenzidine-ELISA substrate solution (Thermo Fisher Scientific, 34029) was added and incubated in the dark for 15 to 20 min. The reaction was stopped by the addition of 2 M sulfuric acid. Absorbance was measured on a SpectraMax190 Microplate Reader (Molecular Devices) at 450 nm, and the optical density (OD) from the no-antigen negative control wells was subtracted from all readings. A positive control standard (CR3022 IgG, Abcam, 273073) was serially diluted and measured against SARS-CoV-2 RBD (SinoBiological 40592-V08H) to create a standard curve on each plate. The CR3022 standard curve was used to calculate titers (relative units) for each sample by interpolating a four-parameter logistic curve.
Peptide pools
All peptides for a given antigen were reconstituted in dimethyl sulfoxide (DMSO), pooled at equal concentrations, and diluted in PBS. Aliquots of peptide pools were stored at −80°C. The following peptides pools consisted of overlapping peptides ranging from 13 to 18 amino acids with 10 to 12 amino acids of overlap covering the entire protein region of interest: SARS-CoV-2 spike protein (n = 181, BEI Resources, NR-52402); SARS-CoV-2 nucleocapsid (n = 59, BEI Resources, NR-52404); HCoV-OC43 spike protein (n = 226, BEI Resources, NR-53728); HCoV-OC43 nucleocapsid (n = 110, JPT, PM-OC43-NCAP-1); and human CMV pp65 (n = 138, NIH HIV Reagent Program, ARP-11549). The peptide pool containing CEF peptides consisted of 32 peptides of 8 to 11 amino acids covering immunodominant CD8+ T cell epitopes (NIH HIV Reagent Program, ARP-9808).
The HCoV-OC43 nsp12/nsp13 peptide pool consisted of 29 peptides of 14 to 16 amino acids in length (table S4). The defined SARS-CoV-2 epitopes were mapped to the HCoV-OC43 protein sequence (strain ATCC VR-759, NC_006213). Additional amino acids, corresponding to the HCoV-OC43 sequence, were added to the ends of each epitope, so each peptide was 14 to 16 amino acids in length. The HCoV-OC43 nsp12/nsp13 peptides were synthesized at greater than 95% purity (GenScript).
AIM assay
The AIM assay was performed as described previously (27, 28). Briefly, PBMCs were thawed, washed in RPMI 1640 medium, resuspended in complete RPMI 1640 medium with 5% human serum, and rested overnight. The cells were plated at concentration of 1 × 106 cells per well in a 96-well round bottom plate. For each stimulation, peptides were added to the well at a final concentration of 1 μg/ml, containing less than 0.1% DMSO. Peptide pools containing CEF or CMV pp65 were used as positive controls, and medium with 0.1% DMSO was used as a negative control. After 24 hours, the cell supernatant was removed, and the cells were collected for additional analysis. Stimulation experiments were performed twice at independent times.
Cell staining and flow cytometry
Cells from the stimulation experiment were washed twice in PBS and then stained for live/dead cell marker (1:200 dilution, Thermo Fisher Scientific, L23105) on ice for 20 min. Cells were washed in fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% fetal bovine serum and 2 mM EDTA) and then incubated with human Fc receptor block (1:100 dilution, BioLegend, 422302) for 15 min on ice. Cells were then stained with the following antibodies on ice for 30 min: Alexa Fluor 647 antihuman CD3 (1:100 dilution, BioLegend, clone HIT3a, 300321), Alexa Fluor 488 antihuman CD4 (1:200 dilution, BioLegend, clone SK3, 344618), allophycocyanin/Fire 750 antihuman CD8a (1:50 dilution, BioLegend, clone HIT8a, 300931), phycoerythrin (PE)/cyanine7 antihuman CD69 (1:50 dilution, BioLegend, clone FN50, 310911), Brilliant Violet 421 antihuman CD134 (OX40) (1:25 dilution, BioLegend, clone Ber-ACT35 (ACT-35), 350013), and PE antihuman CD137 (4–1BB) (1:100 dilution, BioLegend, clone 4B4–1, 309803). After staining, the cells were washed twice in FACS buffer, and samples were analyzed on a BD LSR II Flow Cytometer (BD Biosciences). The resulting flow cytometry data were analyzed using FlowJo software.
Statistical analysis
Individuals were initially classified into three groups: (i) those with a prior documented SARS-CoV-2 infection; (ii) those with no prior documented SARS-CoV-2 infection and with at least one prior COVID-19 vaccine dose; and (iii) those with no prior SARS-CoV-2 infection or COVID-19 vaccination. Vaccinated individuals were deemed fully vaccinated if they had received at least two doses of the Pfizer BioNTech BNT162b2 or Moderna mRNA-1273 COVID-19 vaccine or one dose of the Janssen / Johnson & Johnson Ad26.COV2.S COVID-19 vaccine a minimum of 14 days before the CRP-PCR test. No individual had received a COVID-19 vaccine booster because this practice was instituted after the end of the study period. Comparisons were done using chi-square or Fisher’s exact test for categorical variables and the Kruskal-Wallis or Mann-Whitney U test for continuous variables, as appropriate. A Kaplan Meier (KM) curve was used to visually display time to CoV or non-CoV for the three groups. Cox proportional hazards models were developed examining time to either eCoV or non-CoV infection using SARS-CoV-2 infection and COVID-19 vaccination as time-varying covariates. In the KM and Cox proportional hazards models, 30 November 2020 was used as the index or “start” date if SARS-CoV-2 infection or COVID-19 vaccination occurred before 30 November 2020. The start date was the day of exposure if SARS-CoV-2 infection or first COVID-19 vaccination was after 30 November 2020. The event day was the positive CRP-PCR test of interest or the date with the last negative CRP-PCR test. There was no loss to follow up.
Spike protein and nucleocapsid indices were generated by summing log-normalized [log2 (result +1)] spike protein and nucleocapsid SIMOA IgG, AIM-CD4+, and AIM-CD8+ measurements, respectively. The accuracy of clinical classifications based on SARS-CoV-2 adaptive immune responses, including sensitivity, specificity, positive predictive value, and negative predictive value, was examined along with asymptotic 95% CIs. In the case of perfect prediction, exact binomial CIs are presented.
In the multivariable linear regression models, antibody or T cell responses were the dependent variables. The independent predictors varied in the models but included the group categorization or a combined classification on the basis of SARS-CoV-2 spike protein exposure (SARS-CoV-2 infection and COVID-19 vaccination group) or no known SARS-CoV-2 infection (COVID-19 vaccination and no SARS-CoV-2 antigen experience group). In the multivariable models, covariates included the demographic variables and presence or absence of various comorbidities (tables S1 and S2). Age was dichotomized into above and below 50 years of age. Inclusion of age as a continuous variable did not yield any significant findings. All covariates in univariate models with P values less than or equal to 0.15 were initially included in multivariable models. Covariates with P values greater than 0.10 in multivariable models were then removed. A multiplicative interaction term between SARS-CoV-2 infection and COVID-19 vaccination was examined in Cox proportional hazards models but then removed because of lack of statistical significance. All model assumptions were found to be reasonable. Statistical analyses were performed using GraphPad Prism 8.2.1 and SAS v9.4. A two-sided P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments:
We thank the study participants for providing information and donating specimens. We thank the Boston University Flow Cytometry Core and phlebotomy team for help. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
This work was supported by National Institutes of Health grant K24-AI145661 (to M.S.), National Institutes of Health grant P30-AI042853 (to M.S.), National Institutes of Health training grant T32–5T32AI00730928 (to D.J.B.), and Massachusetts Consortium on Pathogen Readiness funds (to D.W. and M.S.).
Footnotes
Other Supplementary Material for this manuscript includes the following:
MDAR Reproducibility Checklist
Competing interests: D.W. has a financial interest in Quanterix, a company developing an ultrasensitive digital immunoassay platform and is an inventor of the Simoa technology, a founder of the company, and a member of its board of directors. The other authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials. All raw data will be made available from the corresponding author upon request and signature of a data transfer agreement. All materials, including the HCoV-OC43 nsp12/nsp13 peptide pools, will be made available from the corresponding author upon request and signature of a materials transfer agreement.
REFERENCES AND NOTES
- 1.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V, Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol 19, 155–170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE, Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol 48, 2940–2947 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung TS, Liu DX, Similarities and dissimilarities of COVID-19 and other coronavirus diseases. Annu. Rev. Microbiol 75, 19–47 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Hu B, Guo H, Zhou P, Shi Z-L, Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol 19, 141–154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Thelwall S, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ladhani SN, Ramsay M, Lopez Bernal J, Duration of protection against mild and severe disease by Covid-19 vaccines. N. Engl. J. Med 386, 340–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean DJ, Monroe J, Turcinovic J, Moreau Y, Connor JH, Sagar M, Severe acute respiratory syndrome coronavirus 2 reinfection associates with unstable housing and occurs in the presence of antibodies. Clin. Infect. Dis 75, e208–e215 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, Simons D, Blomquist PB, Zaidi A, Nash S, Aziz NIBA, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J, Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med 386, 1532–1546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J, SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N. Engl. J. Med 386, 698–700 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller J, Hachmann NP, Collier AY, Lasrado N, Mazurek CR, Patio RC, Powers O, Surve N, Theiler J, Korber B, Barouch DH, Substantial neutralization escape by SARS-CoV-2 omicron variants BQ.1.1 and XBB.1. N. Engl. J. Med 388, 662–664 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, Zhuang Y, Tham CYL, Chia A, Smith GJD, Young B, Kalimuddin S, Low JGH, Lye D, Wang L-F, Bertoletti A, Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 34, 108728 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A, Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, Mateus J, da Silva Antunes R, Moore E, Rubiro P, Methot N, Phillips E, Mallal S, Frazier A, Rawlings SA, Greenbaum JA, Peters B, Smith DM, Crotty S, Weiskopf D, Grifoni A, Sette A, Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med 2, 100204 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jungreis I, Sealfon R, Kellis M, SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat. Commun 12, 2642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng C, Shi J, Fan Q, Wang Y, Huang H, Chen F, Tang G, Li Y, Li P, Li J, Cui J, Guo L, Chen S, Jiang M, Feng L, Chen L, Lei C, Ke C, Deng X, Hu F, Tang X, Li F, Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery. Nat. Commun 12, 4984 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilich T, Nelde A, Heitmann JS, Maringer Y, Roerden M, Bauer J, Rieth J, Wacker M, Peter A, Hörber S, Rachfalski D, Märklin M, Stevanović S, Rammensee H-G, Salih HR, Walz JS, T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med 13, eabf7517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, Ulferts R, Earl C, Wrobel AG, Benton DJ, Roustan C, Bolland W, Thompson R, Agua-Doce A, Hobson P, Heaney J, Rickman H, Paraskevopoulou S, Houlihan CF, Thomson K, Sanchez E, Shin GY, Spyer MJ, Joshi D, O’Reilly N, Walker PA, Kjaer S, Riddell A, Moore C, Jebson BR, Wilkinson M, Marshall LR, Rosser EC, Radziszewska A, Peckham H, Ciurtin C, Wedderburn LR, Beale R, Swanton C, Gandhi S, Stockinger B, McCauley J, Gamblin SJ, McCoy LE, Cherepanov P, Nastouli E, Kassiotis G, Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370, 1339–1343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M, Chia WN, Chen MI-C, Wang L-F, Ooi EE, Kalimuddin S, Tambyah PA, Low JG-H, Tan Y-J, Bertoletti A, SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Sagar M, Reifler K, Rossi M, Miller NS, Sinha P, White LF, Mizgerd JP, Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Invest 131, e143380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu R, Narean JS, Wang L, Fenn J, Pillay T, Fernandez ND, Conibear E, Koycheva A, Davies M, Tolosa-Wright M, Hakki S, Varro R, McDermott E, Hammett S, Cutajar J, Thwaites RS, Parker E, Rosadas C, McClure M, Tedder R, Taylor GP, Dunning J, Lalvani A, Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun 13, 80 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds CJ, Pade C, Gibbons JM, Butler DK, Otter AD, Menacho K, Fontana M, Smit A, Sackville-West JE, Cutino-Moguel T, Maini MK, Chain B, Noursadeghi M, UK COVIDsortium Immune Correlates Network, Brooks T, Semper A, Manisty C, Treibel TA, Moon JC, UK COVIDsortium Investigators, Valdes AM, McKnight Á, Altmann DM, Boyton R, Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 372, 1418–1423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, Cortes FH, Grifoni A, Tarke A, Chang J, Escarrega EA, Kim C, Goodwin B, Bloom NI, Frazier A, Weiskopf D, Sette A, Crotty S, Humoral and cellular immune memory to four COVID-19 vaccines. Cell 185, 2434–2451.e17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha P, Reifler K, Rossi M, Sagar M, Coronavirus disease 2019 mitigation strategies were associated with decreases in other respiratory virus infections. Open Forum Infect. Dis 8, ofab105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalish H, Klumpp-Thomas C, Hunsberger S, Baus HA, Fay MP, Siripong N, Wang J, Hicks J, Mehalko J, Travers J, Drew M, Pauly K, Spathies J, Ngo T, Adusei KM, Karkanitsa M, Croker JA, Li Y, Graubard BI, Czajkowski L, Belliveau O, Chairez C, Snead KR, Frank P, Shunmugavel A, Han A, Giurgea LT, Rosas LA, Bean R, Athota R, Cervantes-Medina A, Gouzoulis M, Heffelfinger B, Valenti S, Caldararo R, Kolberg MM, Kelly A, Simon R, Shafiq S, Wall V, Reed S, Ford EW, Lokwani R, Denson J-P, Messing S, Michael SG, Gillette W, Kimberly RP, Reis SE, Hall MD, Esposito D, Memoli MJ, Sadtler K, Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci. Transl. Med 13, eabh3826 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman M, Gilboa T, Ogata AF, Maley AM, Cohen L, Busch EL, Lazarovits R, Mao C-P, Cai Y, Zhang J, Feldman JE, Hauser BM, Caradonna TM, Chen B, Schmidt AG, Alter G, Charles RC, Ryan ET, Walt DR, Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat. Biomed. Eng 4, 1180–1187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indenbaum V, Koren R, Katz-Likvornik S, Yitzchaki M, Halpern O, Regev-Yochay G, Cohen C, Biber A, Feferman T, Saban NC, Dhan R, Levin T, Gozlan Y, Weil M, Mor O, Mandelboim M, Sofer D, Mendelson E, Lustig Y, Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLOS ONE 15, e0241164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burbelo PD, Riedo FX, Morishima C, Rawlings S, Smith D, Das S, Strich JR, Chertow DS, Davey RT Jr., Cohen JI, Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J. Infect. Dis 222, 206–213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A, Crotty S, A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J. Immunol 197, 983–993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss S, Baxter AE, Cirelli KM, Dan JM, Morou A, Daigneault A, Brassard N, Silvestri G, Routy J-P, Havenar-Daughton C, Crotty S, Kaufmann DE, Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLOS ONE 12, e0186998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu ED, Wang E, Garrigan E, Goodwin B, Sutherland A, Tarke A, Chang J, Gálvez RI, Mateus J, Ramirez SI, Rawlings SA, Smith DM, Filaci G, Frazier A, Weiskopf D, Dan JM, Crotty S, Grifoni A, Sette A, da Silva Antunes R, Development of a T cell-based immunodiagnostic system to effectively distinguish SARS-CoV-2 infection and COVID-19 vaccination status. Cell Host Microbe 30, 388–399.e3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP, Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med 27, 1205–1211 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Galipeau Y, Siragam V, Laroche G, Marion E, Greig M, McGuinty M, Booth RA, Durocher Y, Cuperlovic-Culf M, Bennett SAL, Crawley AM, Giguère PM, Cooper C, Langlois M-A, Relative ratios of human seasonal coronavirus antibodies predict the efficiency of cross-neutralization of SARS-CoV-2 spike binding to ACE2. EBioMedicine 74, 103700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrwani K, Sharma R, Krishnan M, Jones T, Mayora-Neto M, Cantoni D, Temperton NJ, Dobson SL, Subramaniam K, McNamara PS, Cunliffe NA, Turtle L, Zhang Q, Detection of serum cross-reactive antibodies and memory response to SARS-CoV-2 in prepandemic and post-COVID-19 convalescent samples. J. Infect. Dis 224, 1305–1315 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voss WN, Hou YJ, Johnson NV, Delidakis G, Kim JE, Javanmardi K, Horton AP, Bartzoka F, Paresi CJ, Tanno Y, Chou C-W, Abbasi SA, Pickens W, George K, Boutz DR, Towers DM, McDaniel JR, Billick D, Goike J, Rowe L, Batra D, Pohl J, Lee J, Gangappa S, Sambhara S, Gadush M, Wang N, Person MD, Iverson BL, Gollihar JD, Dye JM, Herbert AS, Finkelstein IJ, Baric RS, McLellan JS, Georgiou G, Lavinder JJ, Ippolito GC, Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science 372, 1108–1112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt C-E, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte J-M, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, Gorochov G, IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med 13, eabd2223 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, Norwood C, Nyhoff LE, Edara VV, Floyd K, De Rosa SC, Ahmed H, Whaley R, Patel SN, Prigmore B, Lemos MP, Davis CW, Furth S, O’Keefe JB, Gharpure MP, Gunisetty S, Stephens K, Antia R, Zarnitsyna VI, Stephens DS, Edupuganti S, Rouphael N, Anderson EJ, Mehta AK, Wrammert J, Suthar MS, Ahmed R, McElrath MJ, Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med 2, 100354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swadling L, Diniz MO, Schmidt NM, Amin OE, Chandran A, Shaw E, Pade C, Gibbons JM, Le Bert N, Tan AT, Jeffery-Smith A, Tan CCS, Tham CYL, Kucykowicz S, Aidoo-Micah G, Rosenheim J, Davies J, Johnson M, Jensen MP, Joy G, McCoy LE, Valdes AM, Chain BM, Goldblatt D, Altmann DM, Boyton RJ, Manisty C, Treibel TA, Moon JC, van Dorp L, Balloux F, McKnight Á, Noursadeghi M, Bertoletti A, Maini MK, Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 601, 110–117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferretti AP, Kula T, Wang Y, Nguyen DMV, Weinheimer A, Dunlap GS, Xu Q, Nabilsi N, Perullo CR, Cristofaro AW, Whitton HJ, Virbasius A, Olivier KJ, Buckner LR, Alistar AT, Whitman ED, Bertino SA, Chattopadhyay S, MacBeath G, Unbiased screens show CD8+ T cells of COVID-19 patients recognize shared epitopes in SARS-CoV-2 that largely reside outside the spike protein. Immunity 53, 1095–1107.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saini SK, Hersby DS, Tamhane T, Povlsen HR, Amaya Hernandez SP, Nielsen M, Gang AO, Hadrup SR, SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8+ T cell activation in COVID-19 patients. Sci. Immunol 6, eabf7550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kared H, Redd AD, Bloch EM, Bonny TS, Sumatoh H, Kairi F, Carbajo D, Abel B, Newell EW, Bettinotti MP, Benner SE, Patel EU, Littlefield K, Laeyendecker O, Shoham S, Sullivan D, Casadevall A, Pekosz A, Nardin A, Fehlings M, Tobian AA, Quinn TC, SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J. Clin. Invest 131, e145476 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, Burger ZC, Rawlings SA, Smith DM, Phillips E, Mallal S, Lammers M, Rubiro P, Quiambao L, Sutherland A, Yu ED, da Silva Antunes R, Greenbaum J, Frazier A, Markmann AJ, Premkumar L, de Silva A, Peters B, Crotty S, Sette A, Weiskopf D, Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, Sagar FD, Lago MS, Decker A, Luxenburger H, Binder B, Bettinger D, Sogukpinar O, Rieg S, Panning M, Huzly D, Schwemmle M, Kochs G, Waller CF, Nieters A, Duerschmied D, Emmerich F, Mei HE, Schulz AR, Llewellyn-Lacey S, Price DA, Boettler T, Bengsch B, Thimme R, Hofmann M, Neumann-Haefelin C, Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med 27, 78–85 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Nelde A, Bilich T, Heitmann JS, Maringer Y, Salih HR, Roerden M, Lübke M, Bauer J, Rieth J, Wacker M, Peter A, Hörber S, Traenkle B, Kaiser PD, Rothbauer U, Becker M, Junker D, Krause G, Strengert M, Schneiderhan-Marra N, Templin MF, Joos TO, Kowalewski DJ, Stos-Zweifel V, Fehr M, Rabsteyn A, Mirakaj V, Karbach J, Jäger E, Graf M, Gruber L-C, Rachfalski D, Preuß B, Hagelstein I, Märklin M, Bakchoul T, Gouttefangeas C, Kohlbacher O, Klein R, Stevanović S, Rammensee H-G, Walz JS, SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol 22, 74–85 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Bui H-H, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A, Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics 7, 153 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dugas M, Grote-Westrick T, Merle U, Fontenay M, Kremer AE, Hanses F, Vollenberg R, Lorentzen E, Tiwari-Heckler S, Duchemin J, Ellouze S, Vetter M, Fürst J, Schuster P, Brix T, Denkinger CM, Müller-Tidow C, Schmidt H, Tepasse P-R, Kühn J, Lack of antibodies against seasonal coronavirus OC43 nucleocapsid protein identifies patients at risk of critical COVID-19. J. Clin. Virol 139, 104847 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abela IA, Pasin C, Schwarzmüller M, Epp S, Sickmann ME, Schanz MM, Rusert P, Weber J, Schmutz S, Audigé A, Maliqi L, Hunziker A, Hesselman MC, Niklaus CR, Gottschalk J, Schindler E, Wepf A, Karrer U, Wolfensberger A, Rampini SK, Meyer Sauteur PM, Berger C, Huber M, Böni J, Braun DL, Marconato M, Manz MG, Frey BM, Günthard HF, Kouyos RD, Trkola A, Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat. Commun 12, 6703 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song G, He W, Callaghan S, Anzanello F, Huang D, Ricketts J, Torres JL, Beutler N, Peng L, Vargas S, Cassell J, Parren M, Yang L, Ignacio C, Smith DM, Voss JE, Nemazee D, Ward AB, Rogers T, Burton DR, Andrabi R, Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat. Commun 12, 2938 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dangi T, Palacio N, Sanchez S, Park M, Class J, Visvabharathy L, Ciucci T, Koralnik IJ, Richner JM, Penaloza-MacMaster P, Cross-protective immunity following coronavirus vaccination and coronavirus infection. J. Clin. Invest 131, e151969 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphal T, Mader M, Karsten H, Cords L, Knapp M, Schulte S, Hermanussen L, Peine S, Ditt V, Grifoni A, Addo MM, Huber S, Sette A, Lütgehetmann M, Pischke S, Kwok WW, Sidney J, Wiesch JSZ, Evidence for broad cross-reactivity of the SARS-CoV-2 NSP12-directed CD4+ T-cell response with pre-primed responses directed against common cold coronaviruses. Front. Immunol 14, 1182504 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dagotto G, Ventura JD, Martinez DR, Anioke T, Chung BS, Siamatu M, Barrett J, Miller J, Schäfer A, Yu J, Tostanoski LH, Wagh K, Baric RS, Korber B, Barouch DH, Immunogenicity and protective efficacy of a rhesus adenoviral vaccine targeting conserved COVID-19 replication transcription complex. NPJ Vaccines 7, 125 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cummings DAT, Radonovich LJ, Gorse GJ, Gaydos CA, Bessesen MT, Brown AC, Gibert CL, Hitchings MDT, Lessler J, Nyquist A-C, Rattigan SM, Rodriguez-Barradas MC, Price CS, Reich NG, Simberkoff MS, Perl TM, Risk factors for healthcare personnel infection with endemic coronaviruses (HKU1, OC43, NL63, 229E): Results from the Respiratory Protection Effectiveness Clinical Trial (ResPECT). Clin. Infect. Dis 73, e4428–e4432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, Gundlapalli AV, Hall AJ, MacNeil A, Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb. Mortal. Wkly Rep 71, 606–608 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loesche M, Karlson EW, Talabi O, Zhou G, Boutin N, Atchley R, Loevinsohn G, Chang JBP, Hasdianda MA, Okenla A, Sampson E, Schram H, Magsipoc K, Goodman K, Donahue L, MacGowan M, Novack LA, Jarolim P, Baden LR, Nilles EJ, Longitudinal SARS-CoV-2 nucleocapsid antibody kinetics, seroreversion, and implications for seroepidemiologic studies. Emerg. Infect. Dis 28, 1859–1862 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, Humphries HE, Jepson B, Kelly EJ, Plested E, Shoemaker K, Thomas KM, Vekemans J, Villafana TL, Lambe T, Pollard AJ, Voysey M, Oxford COVID Vaccine Trial Group, Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med 27, 2032–2040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, Borgert BA, Moreno CA, Solomon BD, Trimmer-Smith L, Etienne V, Rodriguez-Barraquer I, Lessler J, Salje H, Burke DS, Wesolowski A, Cummings DAT, A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun 11, 4704 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson EM, Goodwin EC, Verma A, Arevalo CP, Bolton MJ, Weirick ME, Gouma S, McAllister CM, Christensen SR, Weaver J, Hicks P, Manzoni TB, Oniyide O, Ramage H, Mathew D, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, D’Andrea K, Kuthuru O, Dougherty J, Pattekar A, Kim J, Han N, Apostolidis SA, Huang AC, Vella LA, Kuri-Cervantes L, Pampena MB, Upenn COVID Processing Unit, Betts MR, Wherry EJ, Meyer NJ, Cherry S, Bates P, Rader DJ, Hensley SE, Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 184, 1858–1864.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowley AR, Natarajan H, Hederman AP, Bobak CA, Weiner JA, Wieland-Alter W, Lee J, Bloch EM, Tobian AAR, Redd AD, Blankson JN, Wolf D, Goetghebuer T, Marchant A, Connor RI, Wright PF, Ackerman ME, Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike. eLife 11, e75228 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, Wu Y, Behl S, Taylor JJ, Chakaraborty R, Johnson AJ, Shiavo DN, Utz JP, Reisenauer JS, Midthun DE, Mullon JJ, Edell ES, Alameh MG, Borish L, Teague WG, Kaplan MH, Weissman D, Kern R, Hu H, Vassallo R, Liu S-L, Sun J, Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol 7, eadd4853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurme A, Jalkanen P, Heroum J, Liedes O, Vara S, Melin M, Teräsjärvi J, He Q, Pöysti S, Hänninen A, Oksi J, Vuorinen T, Kantele A, Tähtinen PA, Ivaska L, Kakkola L, Lempainen J, Julkunen I, Long-lasting T cell responses in BNT162b2 COVID-19 mRNA vaccinees and COVID-19 convalescent patients. Front. Immunol 13, 869990 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai W, Feng S, Chai B, Lu S, Zhao G, Chen D, Yu W, Ren L, Shi H, Lu J, Cai Z, Pang M, Tan X, Wang P, Lin J, Sun Q, Peng X, Cheng G, An mRNA-based T-cell-inducing antigen strengthens COVID-19 vaccine against SARS-CoV-2 variants. Nat. Commun 14, 2962 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nesterenko PA, McLaughlin J, Tsai BL, Burton Sojo G, Cheng D, Zhao D, Mao Z, Bangayan NJ, Obusan MB, Su Y, Ng RH, Chour W, Xie J, Li Y-R, Lee D, Noguchi M, Carmona C, Phillips JW, Kim JT, Yang L, Heath JR, Boutros PC, Witte ON, HLA-A∗02:01 restricted T cell receptors against the highly conserved SARS-CoV-2 polymerase cross-react with human coronaviruses. Cell Rep 37, 110167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chow EJ, Uyeki TM, Chu HY, The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol 21, 195–210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hajnik RL, Plante JA, Liang Y, Alameh M-G, Tang J, Bonam SR, Zhong C, Adam A, Scharton D, Rafael GH, Liu Y, Hazell NC, Sun J, Soong L, Shi P-Y, Wang T, Walker DH, Sun J, Weissman D, Weaver SC, Plante KS, Hu H, Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci. Transl. Med 14, eabq1945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S, Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371, eabf4063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dos Santos Alves RP, Timis J, Miller R, Valentine K, Pinto PBA, Gonzalez A, Regla-Nava JA, Maule E, Nguyen MN, Shafee N, Landeras-Bueno S, Olmedillas E, Laffey B, Dobaczewska K, Mikulski Z, McArdle S, Leist SR, Kim K, Baric RS, Ollmann Saphire E, Elong Ngono A, Shresta S, Human coronavirus OC43-elicited CD4+ T cells protect against SARS-CoV-2 in HLA transgenic mice. Nat. Commun 15, 787 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB, COVID-19-neutralizing antibodies predict disease severity and survival. Cell 184, 476–488.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan C-W, Chia W-N, Young BE, Zhu F, Lim B-L, Sia W-R, Thein T-L, Chen MI-C, Leo Y-S, Lye DC, Wang L-F, Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N. Engl. J. Med 385, 1401–1406 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Exline MC, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Olson SM, Kobayashi M, Verani JR, Patel MM, Influenza and Other Viruses in the Acutely Ill (IVY) Network, Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 326, 2043–2054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM, Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw. Open 4, e2128568 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su S, Li W, Jiang S, Developing pan-β-coronavirus vaccines against emerging SARS-CoV-2 variants of concern. Trends Immunol 43, 170–172 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitt MA, Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 169, 365–374 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, Lu Q, Li X, Sun Q, Liu J, Fan C, Huang W, Xu M, Wang Y, Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc 15, 3699–3715 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Yu X, Gilbert PB, Hioe CE, Zolla-Pazner S, Self SG, Statistical approaches to analyzing HIV-1 neutralizing antibody assay data. Stat. Biopharm. Res 4, 1–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuen RR, Steiner D, Pihl RMF, Chavez E, Olson A, Smith EL, Baird LA, Korkmaz F, Urick P, Sagar M, Berrigan JL, Gummuluru S, Corley RB, Quillen K, Belkina AC, Mostoslavsky G, Rifkin IR, Kataria Y, Cappione AJ, Gao W, Lin NH, Bhadelia N, Snyder-Cappione JE, Novel ELISA protocol links pre-existing SARS-CoV-2 reactive antibodies with endemic coronavirus immunity and age and reveals improved serologic identification of acute COVID-19 via multi-parameter detection. Front. Immunol 12, 614676 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. All raw data will be made available from the corresponding author upon request and signature of a data transfer agreement. All materials, including the HCoV-OC43 nsp12/nsp13 peptide pools, will be made available from the corresponding author upon request and signature of a materials transfer agreement.


