Highlights
-
•
A large real-world data on prostate cancer detection in Ghanaian men using standard diagnostic tests - PSA, DRE, and biopsy.
-
•
The cancer detection rate was high at 48.5%, indicating a significant disease burden in this population.
-
•
Elevated PSA strongly correlated with a higher likelihood of a cancer diagnosis, though 25% with "normal" PSA (<4 ng/mL) still had cancer.
-
•
Abnormal DRE findings also predicted increased cancer risk, especially when combined with PSA for levels <10 ng/mL.
-
•
77.8% of cancers detected were clinically significant (Gleason ≥7), suggesting potential benefits of early screening.
Keywords: Prostate cancer, PSA, DRE, Africa, TRUS biopsy
Abstract
Purpose of the study
This study aims to determine the role of serum prostate-specific antigen (PSA) levels and digital rectal examination (DRE) in predicting the histological outcomes of prostate biopsies by analyzing a database of over 7000 patients who underwent transrectal ultrasound (TRUS)-guided prostate biopsies.
Methods
We conducted a retrospective analysis of men who underwent TRUS-guided prostate biopsies at Korle Bu Teaching Hospital, a tertiary referral center in Accra, Ghana, from July 2005 to December 2022. The biopsies, which included 10 to 12 core samples, were prompted by PSA levels greater than 4.0 ng/mL, abnormal DRE findings, or both. We then correlated histopathology results with PSA and DRE findings.
Results
Out of 7,338 patients who presented for biopsy, 76.3% were between the ages of 60 and 79. Histology reports were available for 5,289 patients, of whom 2,564 (48.5%) were diagnosed with prostate cancer. Cancer detection rates based on PSA levels were as follows: 21.6% for PSA <4 ng/mL, 21.7% for PSA 4-10 ng/mL, 32.7% for PSA 10-20 ng/mL, 53.0% for PSA 20-50 ng/mL, 71.5% for PSA 50-100 ng/mL, and 92.0% for PSA >100 ng/mL. When DRE findings were classified according to the 2016 TNM System (AJCC 8th Edition) as T1, T2, T3, and T4, cancer detection rates were 26.8%, 51.8%, 87.6%, and 95.7%, respectively. The overall cancer detection rate was significantly higher with abnormal DRE findings (64.6% vs. 26.7%, p < 0.001). Additionally, 78.2% of the detected cancers were high-grade (Gleason score of 7 or more).
Conclusion
This extensive study of Ghanaian men undergoing TRUS biopsies reveals a high prostate cancer detection rate, with nearly 80% of the detected cancers being high-grade. These findings underscore the importance of PSA and DRE in the early detection of prostate cancer and should be considered in patient counseling and discussions regarding the implementation of prostate cancer screening programs in this population.
Introduction
Prostate cancer is the most frequently diagnosed male cancer in men and the second-leading cause of male cancer death in Ghana [1]. In African populations, prostate cancer remains one of the most prevalent and lethal malignancies, with incidence and mortality rates ranking among the highest globally [2]. A recent increase in incidence in Africa has been reported, which can be attributed to such factors such as heightened awareness, better detection methods, an aging population, and shifts in diagnostic practice [3]. Early detection and accurate diagnosis are pivotal for effective management and treatment. Prostate-specific antigen (PSA) testing, digital rectal examination (DRE), transrectal ultrasonography (TRUS), and biopsy have been shown to enhance detection rates but are not without limitations. PSA testing's diagnostic accuracy is hampered by high sensitivity but low specificity, with factors like prostate volume, infection, trauma, and ethnicity reducing specificity, leading to potential overdiagnosis and unnecessary biopsies. This inaccuracy is especially noted in higher and more variable age-stratified PSA levels in black men compared to white men [4], raising concerns about the applicability of reference ranges primarily established for white populations to those of African ancestry [5]. Yet, the relationship between standard diagnostic tools like PSA, DRE, and histopathology reports following TRUS-guided biopsy in African contexts is not fully understood, owing to a lack of data [3,4]. The intricate interplay among age, PSA levels, prostate volume, and DRE findings requires in-depth analysis of large databases to create individualized screening and treatment strategies.
This study examines the rate of diagnosing prostate cancer by TRUS biopsy in men with a PSA level ≥4.0 ng/mL, a suspicious DRE irrespective of PSA, or both. Through real-world data analysis, we aim to provide insight into the accuracy of these diagnostic tools in the unique context of Ghanaian men's characteristics and healthcare landscape. As diagnosis and management of prostate cancer continue to evolve, this investigation will inform more effective counselling approaches and diagnostic strategies designed to improve patient outcomes in Ghana and other regions with similar challenges.
Patients and methods
Between July 2005 and December 2022, men with suspected prostate carcinoma due to an abnormal DRE or a PSA ≥4.0 ng/mL underwent transrectal ultrasound–guided prostate biopsy at the Korle Bu Teaching Hospital. The unit transitioned from digitally guided biopsies to TRUS-guided biopsies in 2005. Since then, a prospective record of all patients who presented for biopsy has been maintained. The recorded data included information such as age, PSA level, findings of DRE performed by a urologist, prostate volume, number of biopsy cores obtained, and any complications encountered during the biopsy procedure.
Biopsy technique
Patients scheduled for procedures were given an information sheet that outlined all possible complications, and informed consent was obtained. Each patient was prescribed 5 tablets of oral levofloxacin 500 mg (Tavanic; Sanofi). The first tablet was to be taken the evening before the procedure, the second at 30 min before the procedure, the third on the evening of the procedure, and the remaining two at 24 h and 48 h after the procedure. Patients on aspirin, warfarin, or herbal preparations were instructed to discontinue these 7 days before the procedure. Additionally, a starting dose of gentamicin 160 mg, given just before insertion of the rectal biopsy probe, was later added to the periprocedural regimen.
Biopsies were performed on an outpatient basis by either a urologist or an experienced senior urology resident. Patients were positioned in the left decubitus position. Local anaesthesia was provided with a periprostatic nerve block using 5 mL of 2% plain xylocaine. Prostate imaging was conducted in transverse and sagittal planes using a 7.5-MHz transrectal end-firing probe (type 8538; B&K Medical AS). Prostate dimensions were recorded, and volume was calculated. Between 10 and 12 prostatic biopsy cores were collected using an 18G needle and a Bard biopsy gun.
After biopsy, patients were prescribed oral paracetamol for 4 days, as needed. They also were given an information sheet that listed expected complications. In the event of profuse haemorrhage, symptoms of septicaemia (pyrexia >38 degrees), or difficulty with micturition, patients were advised to call the urology ward or return to the hospital.
To ensure efficient communication and follow-up, contact information, including telephone numbers of the patients or their relatives, was recorded in the database. Additionally, details of the laboratory responsible for histopathologic examination, along with the subsequent report, were diligently documented in the database.
Initially, there were some challenges in obtaining copies of the histology report for the database, because the biopsy specimens were given to the patients to be sent to the pathology lab for examination. The patients were responsible for returning the reports to their attending urologist. Unfortunately, some patients failed to do so, leading to some data loss; however, improvements were made to the process, and the current system was implemented. Currently, biopsy specimens are collected at the biopsy room and sent to the histopathology lab via courier. The reports are delivered to the biopsy room for collection by the patients.
Data analysis
Descriptive analysis was done using frequency tables. The χ2 test was adopted to test for association between categorical variables. Analysis of variance was used to determine the difference in prostate cancer outcomes by age (continuous). The primary outcome in this study was prostate cancer detection (cancer, no cancer). Independent variables were age, DRE, PSA, and cancer grade.
Ethics
Before study commencement, the hospital's Ethics Committee approved establishing and using the prostate cancer database.
Results
The descriptive variables in the database are displayed in Table 1. A total of 7338 patients presented for biopsy, with the majority falling within the age range of 60 to 79 years (76.3%). The distribution was as follows: <40 years, 0.2%; 40 to 49 years, 1.5%; 50 to 59 years, 16.8%; 60 to 69 years, 41.3%; 70 to 79 years, 35.0%; 80 to 89 years, 5.1%; and 90+ years, 0.1%.
Table 1.
Descriptive variables.
| Variable | Values | Frequency | Percent (%) |
|---|---|---|---|
| Age group | <40 | 15 | 0.2 |
| 40-49 | 108 | 1.5 | |
| 50-59 | 1230 | 16.8 | |
| 60-69 | 3033 | 41.3 | |
| 70-79 | 2565 | 35.0 | |
| 80-89 | 377 | 5.1 | |
| 90+ | 10 | 0.1 | |
| Total | 7338 | 100.0 | |
| PSA groups | <4 | 135 | 2.0 |
| 4-10 | 1584 | 23.4 | |
| 10-20 | 1633 | 24.1 | |
| 20-50 | 1497 | 22.1 | |
| 50-100 | 746 | 11.0 | |
| 100+ | 1181 | 17.4 | |
| Total | 6776 | 100.0 | |
| Cancer outcome | No cancer | 2725 | 51.5 |
| Had cancer | 2564 | 48.5 | |
| Total | 5289 | 100.0 | |
| DRE class | Abnormal | 2613 | 59.5 |
| Normal | 1778 | 40.5 | |
| Total | 4391 | 100.0 | |
| Cancer grade | Low (ISUP grade 1) | 538 | 22.2 |
| High (ISUP grade >1) | 1882 | 77.8 | |
| Total | 2420 | 100.0 |
The PSA levels among 6,776 patients were categorised into six groups: <4 ng/mL (2.0%), 4 to 10 ng/mL (23.4%), 10 to 20 ng/mL (24.1%), 20 to 50 ng/mL (22.1%), 50 to 100 ng/mL (11.0%), and 100+ ng/mL (17.4%). Of 5,289 patients, 2,564 (48.5%) were diagnosed with prostate cancer, while 2,725 (51.5%) had no evidence of cancer based on histology reports. A total of 4,391 patients had DRE documented in the database, with 2,613 (59.5%) declared abnormal and 1,778 (40.5%) deemed normal. Among the 2,420 patients diagnosed with cancer, 538 (22.2%) had low-grade cancer and 1,882 (77.8%) had high-grade cancer.
Age and cancer diagnosis
The age ranges of patients in the database are depicted in Table 2.
Table 2.
Cancer diagnosis by age.
| Variables |
Prostate Cancer Outcome |
Total | χ2 (df) | p-value | ||
|---|---|---|---|---|---|---|
| No Cancer | Had Cancer | |||||
| Age groups (yrs.) | <40 | 5 (45.5) | 6 (54.5) | 11 (0.2) | 81.2 [6] | <0.001 |
| 40-49 | 41 (57.7) | 30 (42.3) | 71 (1.3) | |||
| 50-59 | 500 (60.4) | 328 (39.6) | 828 (15.7) | |||
| 60-69 | 1192 (54.3) | 1002 (45.7) | 2194 (41.7) | |||
| 70-79 | 869 (46.5) | 998 (53.5) | 1867 (35.5) | |||
| 80-89 | 104 (36.4) | 182 (63.6) | 286 (5.4) | |||
| 90+ | 2 (22.2) | 7 (77.8) | 9 (0.2) | |||
| Total | 2713 (51.5) | 2553 (48.5) | 5266 (100) | |||
| No. of Patients | 2725 (51.5) | 2564 (48.5) | 5289 (100.0) | |||
| Mean Age (±SD) yrs. | 67 (±8.2) | 66.29 (±8.0) | 68.23 (±8.2) | <0.001a | ||
χ2 =Chi-square df= degrees of freedom n=Sample Sig. = P value
The mean age of the total sample was 67 years, with a standard deviation of ±8.2. A significant difference in mean age was found between patients without cancer and those with cancer (66.29 ± 8.0 vs. 68.23 ± 8.2 years; p < 0.001). The relationship between age group and cancer outcome also was statistically significant (χ2 [6] = 81.2; p < 0.0001). Among 5,266 men with an available histology report, 51.5% were without cancer and 48.5% had a cancer diagnosis. The prevalence of cancer decreased with age, from 54.5% in the <40 years group to 42.3% in the 40 to 49 group and 39.6% in the 50 to 59 group, then increased to 45.7% in the 60 to 69 group, 53.5% in the 70 to 79 group, 63.6% in the 80 to 89 group, and 77.8% in the 90+ group (Table 2).
PSA and cancer detection
The association between PSA level and prostate cancer detection is illustrated in Table 3.
Table 3.
PSA and cancer detection.
| Variables |
Prostate Cancer Outcome |
Total | χ2 (df) | p-value | ||
|---|---|---|---|---|---|---|
| No Cancer | Had Cancer | |||||
| PSA group | <4 | 76 (78.4) | 21 (21.6) | 97 (2.0) | 1240.2 [5] | <0.001 |
| (ng/ml) | 4-10 | 850 (78.3) | 235 (21.7) | 1085 (22.5) | ||
| 10-20 | 769 (67.3) | 374 (32.7) | 1143 (23.7) | |||
| 20-50 | 502 (47.0) | 565 (53.0) | 1067 (22.1) | |||
| 50-100 | 157 (28.5) | 394 (71.5) | 551 (11.4) | |||
| 100+ | 70 (8.0) | 806 (92.0) | 876 (18.2) | |||
| Total | 2424 (50.3) | 2395 (49.7) | 4819 (100.0) | |||
χ2 =Chi-square df= degrees of freedom n=Sample Sig. = P value
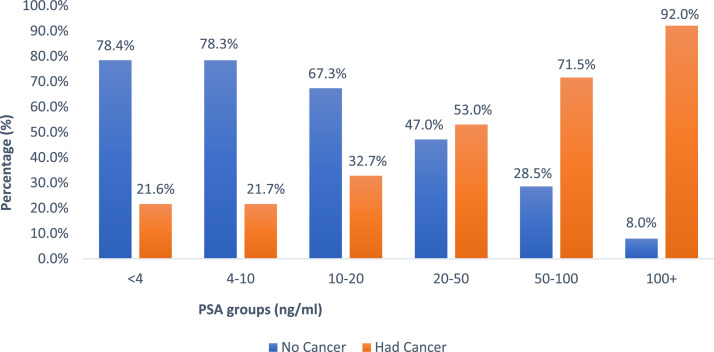
The results indicate a strong association between PSA level and the likelihood of a cancer diagnosis (χ2 [5] = 1240.2; p < 0.0001). The prevalence of cancer was lowest in the PSA <4 ng/mL group at 21.6% and highest in the PSA 100+ ng/mL group at 92.0%. The cancer detection rates in intermediate PSA ranges were 21.7% in the 4 to 10 ng/mL group, 32.7% in the 10 to 20 ng/mL group, 53.0% in the 20 to 50 ng/mL group, and 71.5% in the 50 to 100 ng/mL group (Fig. 1).
Fig. 1.
(PSA and Cancer Detection Rate). The figure shows the percentage of patients with benign histology (no cancer) and those with cancer diagnoses based on serum PSA level.
Digital rectal examination and cancer diagnosis
Table 4 presents the DRE findings classified according to the 2016 TNM system (AJCC 8th Edition) [6] and cancer detection rate.
Table 4.
DRE and prostate cancer detection rate.
| Variables | Total | No Cancer | Had cancer | χ2 (df) | p-value |
|---|---|---|---|---|---|
| DRE findings | |||||
| T1, n (%) | 1267 (23.9) | 927 (73.2) | 340 (26.8) | 705 [4] | <0.001 |
| T2, n (%) | 1321 (24.9) | 637 (48.2) | 684 (51.8) | ||
| T3, n (%) | 458 (8.6) | 57 (12.4) | 401 (87.6) | ||
| T4, n (%) | 208 (3.9) | 9 (4.3) | 199 (95.7) | ||
| Total | 3254 (100.0) | 1630 (50.1) | 1624 (49.9) |
T1: Clinically inapparent (identified by needle biopsy (e.g., because of elevated PSA level)
T2: Tumour palpable and confined within the prostate
T3: Tumour extends through prostatic capsule but is not fixed and does not invade adjacent structures, that is, rectal mucosa is mobile over prostate
T4: Tumour is fixed or invades adjacent structures other than seminal vesicles such as rectum (rectal mucosa immobile over prostate)
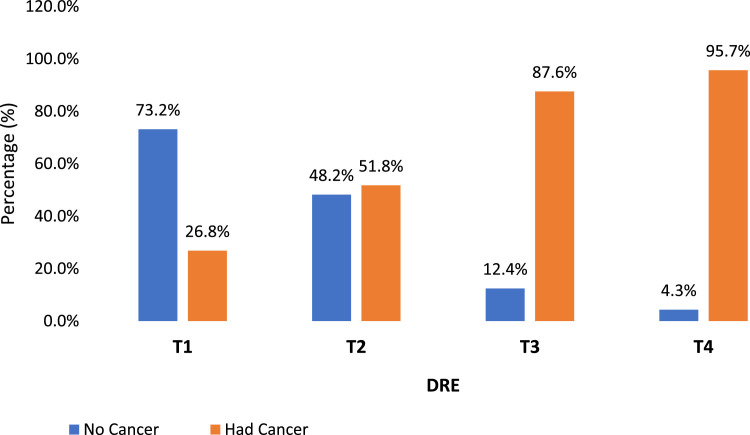
The DRE findings showed a statistically significant association (χ2 [4] = 713; p < 0.001) with cancer outcomes. The results in Table 3 demonstrate a robust association between DRE stages and the likelihood of a cancer diagnosis, with higher DRE stages corresponding to an increased probability of having cancer (Fig. 2).
Fig. 2.
DRE T stage and cancer detection. DRE classified according to the 2016 TNM system and percentage diagnosed with cancer.
Combination of DRE and PSA and cancer diagnosis
The data presented in Table 5 show the cancer detection rate categorised based on DRE findings as normal (T1) or abnormal (T2, T3, or T4) and further stratified by PSA level.
Table 5.
Overall prostate cancer detection rates based on PSA levels and DRE findings.
| DRE | PSA grp | Incidence | Cancer Outcome |
χ2 (df) | Sig. | |
|---|---|---|---|---|---|---|
| (ng/ml) | Total n | No Cancer | Cancer n (%) | |||
| Abnormal | <4 | 28 | 17 | 11 (39.3) | 492.13 [5] | <0.001 |
| (n=1956) | 4-10 | 244 | 171 | 73 (29.9) | ||
| 10-20 | 385 | 226 | 159 (41.3) | |||
| 20-50 | 427 | 184 | 243 (56.9) | |||
| 50-100 | 271 | 62 | 209 (77.1) | |||
| 100+ | 601 | 33 | 568 (94.5) | |||
| 1956 | 693 | 1263 (64.6) | ||||
| Normal | <4 | 15 | 13 | 2 (13.3) | 115.15 [5] | <0.001 |
| (n=1261) | 4-10 | 490 | 415 | 75 (15.3) | ||
| 10-20 | 378 | 286 | 92 (24.3) | |||
| 20-50 | 255 | 155 | 100 (39.2) | |||
| 50-100 | 81 | 43 | 38 (46.9) | |||
| 100+ | 42 | 12 | 30 (71.4) | |||
| 1261 | 924 | 337 (26.7) | ||||
χ2 =Chi-square df= degrees of freedom n=Sample Sig. = P value
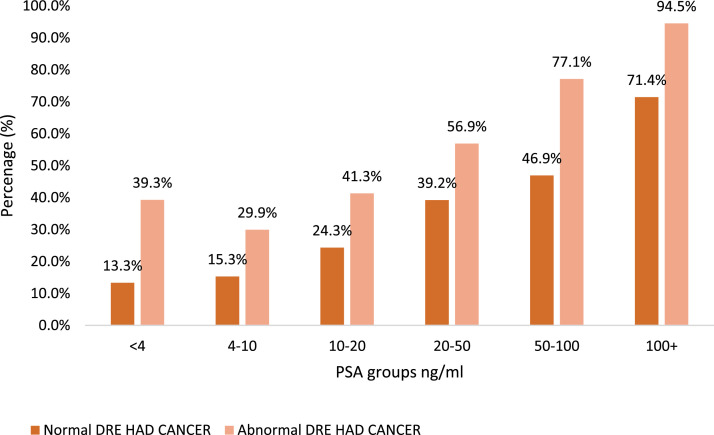
The cancer detection rate for patients with an abnormal DRE showed a progressive increase with increasing PSA level (Fig. 3). At the lowest range of PSA (<4 ng/mL), the detection rate was 39.3%. After a dip to 29.9% in the 4-10 ng/ml range, the detection rate rose across the PSA categories, reaching 41.3% in the 10-20 ng/ml range, 56.9% in the 20-50 ng/ml range, 77.1% in the 50-100 ng/ml range, and a substantial 94.5% in the highest category of 100+ ng/ml. The overall cancer detection rate for the abnormal DRE group was 64.6%. In contrast, the cancer detection rate for patients with a normal DRE was generally lower across the corresponding PSA ranges. For PSA <4 ng/ml, the rate was only 13.3%. It remained relatively low at 15.3% in the 4-10 ng/ml range and 24.3% in the 10-20 ng/ml range. A more pronounced increase was seen at the higher PSA levels, with a detection rate of 39.2% in the 20-50 ng/ml range, 46.9% in the 50-100 ng/ml range, and 71.4% in the 100+ ng/ml range. The overall detection rate for the normal DRE group was 26.7%.
Fig. 3.
Prostate cancer detection rates based on PSA and DRE.
Cancer grade and PSA
The data in Table 6 demonstrate a statistically significant association between PSA level and prostate cancer grade, as evidenced by a chi-square test result of χ2 [5] = 307.37 (p < 0.001). The table categorises prostate cancer into low-grade (Gleason 6) and high-grade (Gleason 7 and above) and further stratifies it according to different PSA levels. Out of the 2292 cases diagnosed with cancer, 508 (22.8%) were classified as low-grade and 1784 (77.2%) were classified as high-grade. There was a clear relationship between PSA level and cancer grade. As PSA level increased, the proportion of low-grade cases decreased and the prevalence of high-grade cancers increased, indicating that higher PSA levels may be more indicative of high-grade malignancies. However, the proportion of high-grade cancers was higher in patients with PSA <4 ng/ml compared to those with PSA between 4 and 20 ng/ml.
Table 6.
PSA association with high-grade cancer.
| PSA ng/ml |
Cancer Grading |
Total | χ2 (df) | Sig. | ||
|---|---|---|---|---|---|---|
| Low Grade | High Grade | |||||
| PSA groups | <4 | 6 (33.3) | 12 (66.7) | 18 (0.8) | 307.37 [5] | <0.001 |
| 4-10 | 112 (51.4) | 106 (48.6) | 218 (9.5) | |||
| 10-20 | 131 (37.0) | 223 (63.0) | 354 (15.4) | |||
| 20-50 | 159 (29.4) | 381 (70.6) | 540 (23.6) | |||
| 50-100 | 56 (14.9) | 321 (85.1) | 377 (16.4) | |||
| 100+ | 44 (5.6) | 741 (94.4) | 785 (34.2) | |||
| Total | 508 (22.8) | 1784 (77.8) | 2292 (100.0) | |||
Discussion
The incidence of prostate cancer increases with age, consistent with our data, which found that the mean age of patients who presented for biopsy was 67 years, with 77% of patients between 60 and 80 years old. Although diagnosis before age 40 years is rare, the incidence is not zero. Sakr and colleagues, in their autopsy studies, have shown that microscopic foci of high-grade prostatic intraepithelial neoplasia (HGPIN), a precursor of invasive prostate cancer, can be found in males in the 3rd and 4th decades, although it is more extensive in older men [7]. They also discovered that HGPIN is more prevalent and appears earlier in African Americans. It is rare to have patients coming for prostate biopsy in their 30s because no guideline recommends screening for the disease before age 40. Despite this, 11 patients in this series had a biopsy before age 40, and 6 of them were diagnosed with cancer. Such young patients tend to have aggressive disease and present for evaluation due to a strong family history, which motivated them to undergo PSA testing or seek urologic consultation earlier. Therefore, patients in their late 30s who are worried about prostate cancer should not be denied a thorough evaluation, especially if they have a strong family history of cancer. The autopsy studies also showed a very high incidence in patients in their 80s and 90s. Therefore, unsurprisingly, the cancer diagnosis rate was very high (78%) in these elderly patients. However, the biopsy rate was low (5.6%). An explanation for this low biopsy rate in patients age >80 years is that unless they present with symptoms suggestive of advanced disease, biopsy will not be performed, even if the PSA is elevated or the DRE is abnormal. An 80-year-old with localised asymptomatic disease is more likely to die of another comorbidity than from prostate cancer [8,9], which explains the low percentage of patients above 80 and the high rate of cancer in these patients because they presented with symptomatic disease. However, in a recent study, 15% of the patients who died from prostate cancer were older than 80 years [10]. Therefore, a fit patient in his late 70s who is also worried about prostate cancer should be carefully evaluated, because he can harbour a potentially life-threatening localised disease and might benefit from early diagnosis and treatment despite his advanced age.
Approximately 75% of the patients in our series presented with a PSA level >10 ng/ml, which is generally higher than rates reported from the Asia, Europe, and the United States [[11], [12], [13]]. The reason for this could be multifactorial. It could be related to a lack of awareness of screening services, resulting in patients presenting for medical attention when symptomatic with higher PSA from advanced disease. Another reason could be the higher incidence of nonmalignant causes such as prostatitis, which occurs more frequently in men of African descent [4]. The traditional 4 ng/mL PSA threshold for prostate cancer screening has been increasingly called into question due to emerging evidence showing that a significant number of cases go undetected at this cutoff level [14]. In our investigation, a concerning 25% of individuals with a PSA level considered “normal” were diagnosed with cancer. These patients underwent biopsy primarily because of abnormal DRE findings or elevated PSA velocity rates. This is particularly relevant for patients at high risk for developing prostate cancer, such as those with a substantial family history of the disease. Detecting aggressive forms of prostate cancer early, when localised to the prostate gland, is crucial for reducing mortality rates. Relying solely on a PSA level >4 ng/mL as the trigger for further investigation may be insufficient and potentially could result in delayed diagnoses. For high-risk populations, a comprehensive approach that includes not only standard PSA testing, but also meticulous DRE and monitoring of PSA velocity could offer a more effective early detection strategy, even when PSA levels remain within what is traditionally considered the “normal” range. Conversely, 78% of patients with a PSA of 4 to 10 ng/mL, 68% of those with a PSA of 10 to 20 ng/mL, and 47% of those with a PSA of 20 to 50 mg/mL had benign histology. These patients underwent unnecessary biopsies with attendant complications, including sepsis and bleeding. [15,16]. The use of multiparametric magnetic resonance imaging (MRI) has been shown to limit these negative biopsies without compromising cancer diagnosis [17,18].
Another approach to reducing unnecessary biopsies is to administer a trial of antibiotic therapy [19]. This is rational because of the high incidence of prostatitis in this group. A drop in the PSA on antibiotic therapy suggests prostatitis, and biopsy can be deferred. The downside of this approach may be the development of fluoroquinolone-resistant bacteria [20] and the cost and side effects of fluoroquinolone antibiotics, including spontaneous rupture of the Achilles tendon.
DRE is a cornerstone of prostate cancer diagnosis and management, offering such advantages such as accessibility, cost-effectiveness, and complementary insights over PSA testing. [21]. DRE is particularly useful in resource-limited settings [22], can detect abnormalities even in asymptomatic patients [23], and provides valuable prognostic information about the tumour, with abnormal DRE independently associated with clinically significant prostate cancer and prostate cancer-specific mortality [24]. DRE is not without limitations, however. Its effectiveness can vary depending on the examiner's skill and experience, leading to inconsistencies in diagnosis [25,26]. Moreover, DRE has limited sensitivity and specificity, often missing small or anteriorly located tumours [27,28]. The procedure can be uncomfortable for patients, causing anxiety and reluctance to undergo the examination, particularly among Africans and Caribbeans [27,29]. Therefore, although DRE remains a valuable tool, its role should be considered in conjunction with other diagnostic methods for a more comprehensive approach to prostate cancer care.
The database revealed that a carefully performed DRE is highly diagnostic of prostate cancer ((χ2 [4] = 713; p < 0.001). This is important because DRE has come under scrutiny and criticism from various publications as being invasive and not offering important information that will influence prostate cancer management [30]. In a resource-challenged environment, DRE is a critical physical examination skill that should not be neglected. The study found that an abnormal DRE T3 and T4 are virtually diagnostic of prostate cancer, with detection rates of 87.6% and 95.7%, respectively. Combining PSA and DRE is associated with an increased prostate cancer detection rate compared to either PSA or DRE alone, especially in the PSA “grey zone” between 4 and 10 ng/mL. In patients presenting with a PSA level of >100 ng/mL and T3 or T4 on DRE, the cancer detection rate is nearly 100%, making a biopsy potentially unnecessary and even risky, especially for those who are frail due to advanced disease [10,31].
The data compellingly support that the combination of PSA and DRE has a significantly greater predictive capability for diagnosing prostate cancer than either test alone [32,33]. However, a nuanced observation from the data is that the influence of an abnormal DRE is especially pronounced when PSA level is below 10 ng/ml. In this specific range, an abnormal DRE nearly doubles the cancer detection rate, serving as a critical adjunct to PSA testing for early and accurate diagnosis. For example, in patients with an abnormal DRE and a PSA level <4 ng/ml, the incidence of cancer was 39.3%, whereas in those with a PSA level between 4 and 10 ng/ml, the incidence was 29.9%, which is in stark contrast to patients with a normal DRE, in whom the incidence of cancer was significantly lower in both PSA groups, at 13.35% and 15.3%, respectively.
Prostate biopsy is essential for detecting cancer cells inside the prostate gland. While surgical removal and examination of the entire gland would be the most accurate method, it is clinically inapplicable. Therefore, TRUS-guided biopsy is accepted as the best diagnostic technique [34]. One of the inherent challenges of TRUS-guided biopsy is the limited amount of tissue sampled, leading to the possibility of missing cancer cells [35,36]. Therefore, the reliability of the 12-core prostate biopsy procedure has been called into question because of the potential for false-negative results. A study of 90 prostate cancer patients (mean age, 64 years; range, 49-77 years) diagnosed with TRUS-guided 12-core prostate biopsy revealed a detection rate of 67.8% with repeated ex vivo biopsies using the same mapping postoperatively [37]. This finding underscores the reality that repeat biopsies can still be negative despite the presence of prostate cancer. The false-negative rate also depends on the size of the prostate, with more samples from larger prostates recommended. Another cause of false-negative biopsies is anteriorly located tumours that are not accessible transrectally [38]. Anteriorly located tumours have been shown to be more prevalent in Africans and might be one of the reasons for delayed diagnosis and subsequent poor outcomes in Africans [39]. Therefore, declaring a patient cancer-free after a single TRUS biopsy may be premature. A repeat biopsy may be necessary in patients with high PSA and negative histology, especially if the DRE is abnormal. Despite multiple TRUS biopsies, both patients (especially those at high risk) and their urologists may still have lingering concerns about the accuracy of the biopsy results. Studies show that prostate cancer–specific mortality for men with an initially benign biopsy can still be significant, ranging from 3.6% in men with a PSA between 10 and 20 ng/mL to as high as 17.6% in those with a PSA >20 ng/mL [35]. The use of multiparametric MRI and targeted biopsy has been shown to reduce the false-negative rate, especially in large prostates [40]. A transperineal template biopsy with more than 50 cores (saturation biopsy) is sometimes required to convince a young, high-risk patient that he does not have prostate cancer [41]. The high cost and lack of equipment and radiologists skilled in MRI interpretation may limit the widespread adoption of MRI-targeted biopsies; however, it may be cost-effective if reserved for patients with a PSA <10 ng/ml and a normal DRE or in patients coming for a repeat biopsy. For patients with an abnormal DRE and a PSA >20 ng/ml, a pre-biopsy MRI will not be cost-effective, given the >50% yield from TRUS biopsy in this study.
The cancer detection rate of 48.5 % is higher than the 31% and 36% reported from similar studies in Caucasians [12] and Asians [11], respectively, but consistent with reports from other sub-Saharan African countries, with more than 77.2% having high-grade features making them clinically significant [42]. This shows that prostate cancer is more aggressive in Africans, and that early detection and active treatment will offer survival benefits. A noteworthy observation is the significant correlation between PSA level and cancer grade; however, this relationship is most evident when the PSA level exceeds 10 ng/ml. Interestingly, the proportion of high-grade cancers was higher in patients with a PSA level within the normal range (<4 ng/ml) compared to patients with a PSA of 4 to 20 ng/ml, which is in agreement with studies showing that highly aggressive tumours are often so poorly differentiated that they produce less PSA [14]. Moreover, certain non-adenocarcinoma prostate cancers, such as neuroendocrine and transitional cell cancers, do not produce PSA. These low-PSA–producing cancers might not respond well to androgen deprivation therapy and carry a high risk of prostate cancer death [14,43]. Early detection of these non–PSA-producing tumours largely depends on a thorough DRE.
Limitations
This study has some notable limitations. First, PSA assays were performed at different laboratories, which might have impacted the consistency of results due to variability across assays. Second, specimen interpretation by multiple pathologists without a central pathology review could have led to interobserver differences, particularly in Gleason grading. Third, we did not distinguish between initial and repeat biopsies, an important factor because detection rates decline with subsequent biopsies. Fourth, multiparametric MRI was not incorporated, even though it has become standard of care for prostate cancer detection in highly resourced settings. Nonetheless, our results reflect real-world prostate cancer detection patterns in sub-Saharan Africa given limited resources. These baseline data obtained using standard biopsy methods enables future comparisons if more advanced tools like MRI-fusion biopsy become accessible.
Conclusion
This study provides valuable real-world data on prostate cancer detection rates in Ghanaian men using standard diagnostic approaches of PSA, DRE, and TRUS-guided biopsy. The high cancer detection rate of 48.5% likely reflects later stages at presentation and the increased prostate cancer burden in this population. PSA levels correlated strongly with cancer detection, with higher levels conferring greater risk; however, cancer was identified in 25% of men with a PSA <4 ng/mL, highlighting potential limitations of rigid PSA cutoffs for this population. DRE also provided incremental diagnostic value, especially when combined with PSA. The predominance of aggressive, clinically significant cancers (ISUP grade 2-5: 77.8%) has critical implications for Ghanaian men and underscores the vital need for early detection to enable timely treatment of localised disease.
These findings should inform appropriate, evidence-based clinical decision making regarding diagnostic evaluation of Ghanaian men presenting for prostate cancer evaluation. More work is needed to optimize screening and detection strategies for this understudied population. Integration of emerging techniques such as MRI-fusion biopsy and molecular biomarkers could improve early diagnosis and risk stratification when resources allow. Reducing unnecessary biopsies while ensuring that cancer is not missed remains an area for improvement. Overall, these real-world data provide valuable insights into prostate cancer patterns in Ghanaian men that can guide clinical practice. The study also fills a research gap and lays groundwork for further exploration of tailored early detection approaches.
CRediT authorship contribution statement
James Edward Mensah: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Evans Akpakli: Writing – original draft, Formal analysis, Data curation, Conceptualization. Mathew Kyei: Writing – original draft, Formal analysis, Data curation, Conceptualization. Kenneth Klufio: Writing – original draft, Formal analysis, Data curation, Conceptualization. Isaac Asiedu: Writing – original draft, Formal analysis, Data curation, Conceptualization. Kweku Asante: Writing – original draft, Formal analysis, Data curation, Conceptualization. Bernard Toboh: Formal analysis, Data curation, Conceptualization. Micheal Darko Ashaley: Writing – original draft, Formal analysis, Data curation. Ben Molai Addo: Data curation, Conceptualization. Bernard Morton: Data curation. Erica Akoto Quist: Writing – original draft, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
We do not have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
References
- 1.Wiredu E.K., Armah H.B. Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality. BMC Public Health. 2006;6(1):159–165. doi: 10.1186/1471-2458-6-159. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong M.C.S., Goggins W.B., Wang H.H.X., Fung F.D.H., Leung C., Wong S.Y.S., et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur. Urol. 2016;70(5):862–874. doi: 10.1016/j.eururo.2016.05.043. Nov. [DOI] [PubMed] [Google Scholar]
- 3.Seraphin T.P., Joko-Fru W.Y., Kamaté B., Chokunonga E., Wabinga H., Somdyala N.I.M., et al. Rising prostate cancer incidence in Sub-Saharan Africa: a trend analysis of data from the african cancer registry network. Cancer Epidemiol. Biomark. Prev. 2021;30(1):158–165. doi: 10.1158/1055-9965.EPI-20-1005. Jan 1. [DOI] [PubMed] [Google Scholar]
- 4.Barlow M., Down L., Mounce L.T.A., Merriel S.W.D., Watson J., Martins T., et al. Ethnic differences in prostate-specific antigen levels in men without prostate cancer: a systematic review. Prostate Cancer Prostatic Dis. 2022;26(2):249–256. doi: 10.1038/s41391-022-00613-7. 2022 262Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nettey O.S., Walker A.J., Keeter M.K., Singal A., Nugooru A., Martin I.K., et al. Self-reported Black race predicts significant prostate cancer independent of clinical setting and clinical and socioeconomic risk factors. Urol. Oncol. Semin. Orig. Investig. 2018;36(11):501.e1–501.e8. doi: 10.1016/j.urolonc.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin M.B., Edge S., Greene F.E. 8th ed. Springer; New York, NY: 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 7.Sakr W.A., Grignon D.J., Haas G.P., Heilbrun L.K., Edson Pontes J., Crissman J.D. Age and racial distribution of prostatic intraepithelial neoplasia. Eur. Urol. 1996;30(2):138–144. doi: 10.1159/000474163. [DOI] [PubMed] [Google Scholar]
- 8.Welch H.G., Albertsen P.C. Reconsidering prostate cancer mortality–the future of PSA screening. N. Engl. J. Med. 2020;382(16):1557–1563. doi: 10.1056/NEJMms1914228. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury S., Robinson D., Cahill D., Rodriguez-Vida A., Holmberg L., Møller H. Causes of death in men with prostate cancer: an analysis of 50 000 men from the Thames Cancer Registry. BJU Int. 2013;112(2):182–189. doi: 10.1111/bju.12212. Jul 1. [DOI] [PubMed] [Google Scholar]
- 10.Mensah J., Amoah Y., Ofori E., Vanderpuye M.A.V. Determinants of mortality among patients managed for prostate cancer: experience from korle bu teaching hospital in Ghana. J. West Afr. Coll. Surg. 2023;13(3):65. doi: 10.4103/jwas.jwas_26_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei T., Lin T., Chang Y., Chen T., Lin A.T.L., Chen K. Transrectal ultrasound-guided prostate biopsy in Taiwan : a nationwide database study. J. Chin. Med. Assoc. 2015;78(11):662–665. doi: 10.1016/j.jcma.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Ng T.K., Vasilareas D., Mitterdorfer A.J., Maher P.O., Lalak A. Prostate cancer detection with digital rectal examination, prostate-specific antigen, transrectal ultrasonography and biopsy in clinical urological practice. BJU Int. 2005;95(4):545–548. doi: 10.1111/j.1464-410X.2005.05336.x. Mar. [DOI] [PubMed] [Google Scholar]
- 13.Jones D., Friend C., Dreher A., Allgar V., Macleod U. The diagnostic test accuracy of rectal examination for prostate cancer diagnosis in symptomatic patients: a systematic review. BMC Fam. Pract. 2018;19(1) doi: 10.1186/s12875-018-0765-y. Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahal B.A., Yang D.D., Wang N.Q., Alshalalfa M., Davicioni E., Choeurng V., et al. Clinical and genomic characterization of low-prostate-specific antigen, high-grade prostate cancer. Eur. Urol. 2018;74(2):146–154. doi: 10.1016/j.eururo.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labi A.K., Obeng-Nkrumah N., Dayie N., Addo B.M., Osei M.M., Fenny A., et al. Occurrence and significance of fluoroquinolone-resistant and ESBL-producing Escherichia coli and Klebsiella pneumoniae complex of the rectal flora in Ghanaian patients undergoing prostate biopsy. JAC-Antimicrob. Resist. 2022;4(6) doi: 10.1093/jacamr/dlac113. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundström K.J., Drevin L., Carlsson S., Garmo H., Loeb S., Stattin P., et al. Nationwide population-based study of infections after transrectal ultrasound guided prostate biopsy. J. Urol. 2014 doi: 10.1016/j.juro.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 17.Rai B.P., Mayerhofer C., Somani B.K., Kallidonis P., Nagele U., Tokas T. Magnetic resonance imaging/ultrasound fusion-guided transperineal versus magnetic resonance imaging/ultrasound fusion-guided transrectal prostate biopsy-a systematic review. Eur. Urol. Oncol. 2021;4(6):904–913. doi: 10.1016/j.euo.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 18.NCCN. Prostate cancer : early detection. 2012;1–19.
- 19.Serretta V., Catanese A., Daricello G., Liotta R., Allegro R., Martorana A., et al. PSA reduction (after antibiotics) permits to avoid or postpone prostate biopsy in selected patients. Prostate Cancer Prostatic Dis. 2008;11(2):148–152. doi: 10.1038/sj.pcan.4500996. Jan. [DOI] [PubMed] [Google Scholar]
- 20.Akduman B.B.B., Akduman D., Tokgöz H., Erol B., Trker T., Ayolu F., et al. Long-term fluoroquinolone use before the prostate biopsy may increase the risk of sepsis caused by resistant microorganisms. Urology. 2011;78(2):250–255. doi: 10.1016/j.urology.2011.02.065. Aug. [DOI] [PubMed] [Google Scholar]
- 21.Walsh A.L., Considine S.W., Thomas A.Z., Lynch T.H., Manecksha R.P. Digital rectal examination in primary care is important for early detection of prostate cancer: a retrospective cohort analysis study. Br. J. Gen. Pract. 2014;64(629):e783–e787. doi: 10.3399/bjgp14X682861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udeh E., Dakum N., Aderibigbe S., Edeh J. The utility of digital rectal examination in estimating prostate volume in a rural hospital setting. Niger. J. Surg. 2015;21(2):111. doi: 10.4103/1117-6806.162570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpern J.A., Oromendia C., Shoag J.E., Mittal S., Cosiano M.F., Ballman K.V., et al. Use of digital rectal examination as an adjunct to prostate specific antigen in the detection of clinically significant prostate cancer. J. Urol. 2018;199(4):947–953. doi: 10.1016/j.juro.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halpern J.A., Shoag J.E., Mittal S., Oromendia C., Ballman K.V., Hershman D.L., et al. Prognostic significance of digital rectal examination and prostate specific antigen in the prostate, lung, colorectal and ovarian (PLCO) cancer screening arm. J. Urol. 2017;197(2):363–368. doi: 10.1016/j.juro.2016.08.092. Feb. [DOI] [PubMed] [Google Scholar]
- 25.Walsh A.L., Considine S.W., Thomas A.Z., Lynch T.H., Manecksha R.P. Digital rectal examination in primary care is important for early detection of prostate cancer: a retrospective cohort analysis study. Br. J. Gen. Pract. 2014;64(629):e783–e787. doi: 10.3399/bjgp14X682861. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naji L., Randhawa H., Sohani Z., Dennis B., Lautenbach D., Kavanagh O., et al. Digital rectal examination for prostate cancer screening in primary care: a systematic review and meta-analysis. Ann. Fam. Med. 2018;16(2):149–154. doi: 10.1370/afm.2205. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones D., Friend C., Dreher A., Allgar V., Macleod U. The diagnostic test accuracy of rectal examination for prostate cancer diagnosis in symptomatic patients: a systematic review. BMC Fam. Pract. 2018;19(1) doi: 10.1186/s12875-018-0765-y. Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelsayed G.A., Danial T., Kaswick J.A., Finley D.S. Tumors of the anterior prostate: implications for diagnosis and treatment. Urology. 2015;85:1224–1228. doi: 10.1016/j.urology.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Alexis O., Worsley A. An integrative review exploring black men of African and Caribbean backgrounds, their fears of prostate cancer and their attitudes towards screening. Health Educ. Res. 2018;33(2):155–166. doi: 10.1093/her/cyy001. Apr 1. [DOI] [PubMed] [Google Scholar]
- 30.Cui T., Kovell R.C., Terlecki R.P. Is it time to abandon the digital rectal examination? Lessons from the PLCO cancer screening trial and peer-reviewed literature. Curr. Med. Res. Opin. 2016;7995(September):1–7. doi: 10.1080/03007995.2016.1198312. [DOI] [PubMed] [Google Scholar]
- 31.Lundström K.J., Drevin L., Carlsson S., Garmo H., Loeb S., Stattin P., et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J. Urol. 2014;192(4):1116–1122. doi: 10.1016/j.juro.2014.04.098. Oct. [DOI] [PubMed] [Google Scholar]
- 32.Journal T., Mcleod C.D., Grayhack J., Reed W. Commentary comparison of DRE and PSA in the detection of prostate cancer. 2017;197 (February):208–9.
- 33.Gosselaar C., Roobol M.J., Roemeling S., Schrö F.H. The role of the digital rectal examination in subsequent screening visits in the European randomized study of screening for prostate cancer (ERSPC), Rotterdam. [DOI] [PubMed]
- 34.Ismail M.T., Gomella L.G. Transrectal prostate biopsy. Urol. Clin. N. Am. 2013;40(4):457–472. doi: 10.1016/j.ucl.2013.07.012. Nov. [DOI] [PubMed] [Google Scholar]
- 35.Klemann N., Røder M.A., Helgstrand J.T., Brasso K., Toft B.G., Vainer B., et al. Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. Lancet Oncol. 2017;18(2):221–229. doi: 10.1016/S1470-2045(17)30025-6. Feb 1. [DOI] [PubMed] [Google Scholar]
- 36.Lewicki P., Shoag J., Golombos D.M., Oromendia C., Ballman K.V., Halpern J.A., et al. Prognostic significance of a negative prostate biopsy: an analysis of subjects enrolled in a prostate cancer screening trial. J. Urol. 2017;197(4):1014–1019. doi: 10.1016/j.juro.2016.11.002. Apr 1. [DOI] [PubMed] [Google Scholar]
- 37.Serefoglu E.C., Altinova S., Ugras N.S., Akincioglu E., Asil E., Balbay M.D. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can. Urol. Assoc. J. 2013;7(5–6):E293. doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesen L., Nørgaard N., Løgager V., Balslev I., Thomsen H.S. Where do transrectal ultrasound- and magnetic resonance imaging-guided biopsies miss significant prostate cancer? Urology. 2017;110:154–160. doi: 10.1016/j.urology.2017.08.028. Dec 1. [DOI] [PubMed] [Google Scholar]
- 39.Sundi D., Kryvenko O.N., Carter H.B., Ross A.E., Epstein J.I., Schaeffer E.M. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J. Urol. 2014;191(1):60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drost F.J.H., Osses D., Nieboer D., Bangma C.H., Steyerberg E.W., Roobol M.J., et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: a cochrane systematic review and meta-analysis. Eur. Urol. 2020;77(1):78–94. doi: 10.1016/j.eururo.2019.06.023. Jan 1. [DOI] [PubMed] [Google Scholar]
- 41.Schaeffer E.M., An Y., Barocas D., Bitting R., et al. NCCN guidelines version 1.2023 prostate cancer. 2022.
- 42.Ogbetere F., Irekpita E. Detection rate of prostate cancer following 12-core extended biopsy in a Semi-urban Nigerian Tertiary Hospital. Urol. Ann. 2021;13(2):150. doi: 10.4103/UA.UA_136_20. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahal B.A., Aizer A.A., Efstathiou J.A., Nguyen P.L. Association of very low prostate-specific antigen levels with increased cancer-specific death in men with high-grade prostate cancer. Cancer. 2016;122(1):78–83. doi: 10.1002/cncr.29691. Jan 1. [DOI] [PubMed] [Google Scholar]