Highlights
-
•
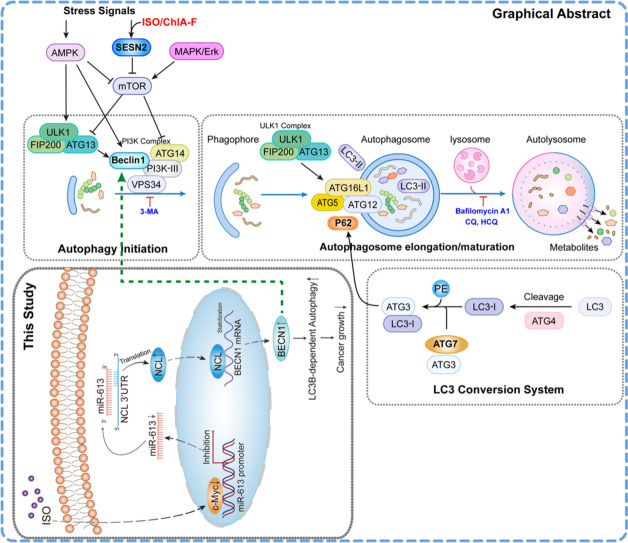
our results demonstrate that by activating c-Myc/miR-613/NCL axis, ISO treatment results in BECN1 posttranscriptional upregulation, which specific initiates LC3B-dependent autophagy and anti-cancer activity.
-
•
Our findings further strengths our application of ISO for therapy of high invasive BC patients.
Keywords: Isorhapontigenin (ISO), LC3B, autophagy, BECN1, NCL
Abstract
Isorhapontigenin (ISO), an active compound isolated from the Chinese herb Gnetum Cleistostachyum, exhibited strong preventive and therapeutic effects on bladder cancer (BC) both in vitro and in vivo. Our previous studies revealed that ISO-induced autophagy is crucial for its anti-cancer activity. However, the underlying mechanism remains unclear. Here, we showed that BECN1, an important autophagic protein, was induced by ISO treatment and played crucial roles in ISO-induced late phase of LC3B-dependent, and LC3A-independent autophagy, as well as anti-cancer activity. Downregulation of BECN1 was observed in human BCs and BBN-induced mouse invasive BC tissues, whereas co-treatment with ISO completely reversed BECN1 downregulation in BBN-induced mouse invasive BCs. Consistently, ISO treatment significantly increased BECN1 expression in vitro in a dose- and time-dependent manner. Depletion of BECN1 significantly impaired LC3B-dependent autophagy following ISO treatment, as well as abolished the inhibitory effect of ISO on anchorage-independent growth of human BC cells. Mechanistic studies revealed that BECN1 induction was mediated by ISO downregulation of c-Myc, which resulted in miR-613 reduction, in turn leading to increased NCL translation and further promoting NCL binding to BECN1 mRNA, subsequently stabilizing BECN1 mRNA. In conclusion, our results demonstrate that by activating c-Myc/miR-613/NCL axis, ISO treatment results in BECN1 posttranscriptional upregulation, which specifically initiates LC3B-dependent autophagy and anti-cancer activity. Our findings further strengths our application of ISO for therapy of high-grade invasive BC (HGIBC) patients.
Graphical abstract
Introduction
With approximately 80,000 new cases annually, bladder cancer (BC) ranks as the sixth most common cancer in the United States and the fourth most common in men [1]. Over 90% of BCs originate from the uroepithelium with median age of diagnosis being 65 years [1]. Given that HGIBC can develop to life-threatening metastases, and <20% of patients with lymph node-only have a 5-year overall survival [2], the identification of novel anti-HGIBC drugs is urgently needed.
Isorhapontigenin (ISO), a new derivative of stilbene compound isolated from the Chinese herb Gnetum Cleistostachyum [3], exhibits multiple anticancer activities in human muscle invasive BCs (MIBCs). Our previous studies show that ISO effectively induces cell cycle arrest at low dosages (5–10 µM) through inhibiting the key cell-cycle regulatory protein Cyclin D1 expression, and causes cancer cell apoptosis at high dosages (20–60 µM) by inhibiting the anti-apoptotic XIAP gene transcription [4,5]. Furthermore, at sub-lethal doses (10–20 µM), ISO induces autophagy, which is crucial for inhibiting anchorage-independent growth in human BC cells [6]. Moreover, oral administration of ISO effectively inhibits tumor growth in BC tumor xenograft model and HGIBC formation caused by N‑butyl‑N- (4-hydroxybutyl) nitrosamine (BBN) in mouse BC model [7]. Collectively, these studies strongly prove that ISO is a promising anti-cancer drug with notable inhibitory activity on BC growth and progression in vivo and in vitro.
Autophagy, a highly conserved catabolic process, involves the degradation of cytoplasmic proteins and organelles in lysosomes [8]. Originally identified as a response to nutrient scarcity, autophagy is now known to play key roles in various cellular processes such as cell proliferation and differentiation, embryo development, and diseases [[9], [10], [11], [12], [13]]. In human cancers, autophagy can either inhibit or promote cancer cell growth and tumor progression depending on the cell type and conditions [14]. LC3 proteins (MAP1-LC3A, B, and C) are structural components of autophagosome membranes and are widely recognized as biomarkers of autophagy. Notably, LC3A and LC3B exhibit distinct expression patterns in normal tissues, whereas LC3C is generally absent in most tissues. Among these, LC3B is the most extensively studied endogenous autophagy marker. The accumulation of autophagosomes can be identified as GFP-LC3 puncta under fluorescence microscopy, or as the conversion of LC3-I (soluble) to LC3-II (lipidated) on a western blot, both of which reflect autophagosome turnover [15,16]. The regulation of autophagy involves a complex network of signaling pathways, including the mTOR and AMPK pathways. Transcriptional regulation of autophagy is primarily achieved through the upregulation of autophagy-related genes (ATGs) and lysosome-related genes [16,17]. Additionally, post-translational modifications of autophagy-related proteins play a significant role in this regulation. For instance, the protein kinases mTOR and AMPK, as key upstream regulators of autophagy, modulate the activity of the ULK1 complex and VPS34 complex 1 by phosphorylating various autophagy proteins, thereby influencing the initiation of autophagy (also displayed in Graphical Abstract) [15,16].
BECN1, another key initiating player in autophagy [18], interacts with the catalytic unit PI3KC3 and regulatory unit PI3KR4 to promote the recruitment of other autophagy proteins and regulate various stages of autophagosome formation [18,19]. Conversely, the interaction between BECN1 and anti-apoptotic protein Bcl-2 family members prevents BECN1 from assembling the pre-autophagosome and inhibits autophagy [[19], [20], [21]]. Due to its important function in autophagy, Becn1 null mice die early in embryogenesis [22]. Interestingly, Becn1± mice have a high incidence of spontaneous tumors [22,23], which is consistent with the finding that mice with monoallelically loss of BECN1 develop 40–75% of prostate, breast, and ovarian cancers [24,25], suggesting BECN1 as a tumor suppressor. In human BCs, down-regulation of BECN1 is more frequently observed in tumors with higher histological grades [26]. Our recent studies have shown that ISO induces SESN2 and BECN1 protein levels, two important regulators of autophagy in human BC cells [6]. Depletion of SESN2 in UMUC3 cells not only impaired ISO-mediated early phase of autophagy but also reversed the inhibitory effect of ISO on anchorage-independent growth [6], indicating that ISO-induced autophagy is crucial for its anti-cancer activity. This study delves into the role and mechanisms of BECN1 in ISO-induced autophagy and cancer inhibition.
Materials and methods
For more details, please see the Supplement of “Materials and Methods”.
Cell lines and cell culture
Human BC cell lines UMUC3 and T24T as well as human embryonic kidney cell line HEK293T were cultured as described in the Supplement of “Materials and Methods”.
Chemicals and antibodies
ISO, Actinomycin D, and cycloheximide were commercialized. Antibodies specifically against LC3A, LC3B, BECN1, c-Myc, p-c-Jun Ser73, ELK1, c-Fos, JunD, GAPDH, NCL, AUF1, and HuR were purchased from Antibody companies.
Plasmids
ShRNA constructs targeting human BECN1 and Nucleolin, GFP-RFP-LC3B and mCherry-LC3B were purchased. We constructed BECN1 promoter luciferase plasmid, NCL 3′UTR luciferase plasmid, miR-613 overexpression plasmid, miR-613 or miR-219a binding site mutation of NCL 3′UTR luciferase plasmid, GFP-LC3A, and mCherry-LC3A.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR were performed using ThermoScriptTM RT-PCR system following the manufacturer's instructions as described in our previous studies [27].
Luciferase reporter assay
This assay was conducted as described in previous studies [28].
Anchorage-independent growth assay
Anchorage-independent growth ability of ISO-treated cells was evaluated in soft agar as described in our previous study [29].
RNA immunoprecipitation (RNA-IP) assay
RNA-IP assay was performed as previously described [30]. For more details, please see the Supplement of “Materials and Methods”.
Western blot (WB) assay
Whole cell lysates were collected and subjected to WB analysis as previously described [31].
Confocal laser scanning microscopy
This assay was conducted according to the protocols as described in the Supplement of “Materials and Methods”.
Live-cell imaging
Live-cell imaging was performed using the Lysosome Staining Kit (AAT Bioquest, Sunnyvale, CA) according to the manufacturer's instructions.
BC patients and BC tissue specimens
Twelve pairs of primary invasive BC specimens and adjacent normal bladder tissues were obtained from patients who underwent radical cystectomy at Department of Urology of the Union Hospital of Tongji Medical College (Wuhan, China) between 2012 and 2013.
Animal experiments and immunohistochemistry paraffin (IHC-P) of mouse bladder specimens
The C57BL/6 J mice (males, 5- 6 weeks old, n= 30) were randomly divided into three groups and then treated with BBN or BBN+ISO [32].
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software. The significance of the difference between the treated and untreated groups was determined with the Student T Test or ANOVA methods. The results are expressed as mean ± S.D. from at least triplicates. P value < 0.05 was considered as significance.
Results
BECN1 was downregulated in BCs and upregulation of BECN1 contributed to ISO anti-cancer activity
Although BECN1 has been reported as a tumor suppressor in human breast, ovarian, and prostate tumors [33,34], its functional role in human BCs remains unclear. To investigate this, we analyzed BECN1 protein levels in primary bladder tumor tissues from patients with HGIBC, and their paired adjacent normal bladder tissues. While BECN1 protein was expressed in most normal bladder tissues (11/12), 8 out of 12 tumor samples (75%) exhibited a markedly reduced levels of BECN1 protein, compared to the adjacent normal bladder tissues (Fig. 1A). Thus, our results indicated a reduced expression of BECN1 in human bladder tumor tissues.
Fig. 1.
ISO treatment elevated BECN1 expression and inhibited BC formation. A BECN1 protein levels were detected by WB analysis in freshly collected human BC tissues (T) and paired adjacent non-cancer tissues (N). The values below the protein bands are BECN1/GAPDH ratios. B Pooled normal bladder tissue (vehicle group) or BC tissues (BBN group) were analyzed for BECN1 protein by WB. C-D Representative images (C) and integrated optical densities (IOD) (D) of mouse bladder tissue from the indicated groups showing BECN1 protein expression by IHC staining. *Significant decrease from the vehicle control group, ♣Significant increase from the BBN treatment group (n = 3). E BECN1 expressions were analyzed by WB in UMUC3 and T24T cells treated with various doses of ISO for 24 h for. F Cells treated with ISO (10μM) for different durations were also analyzed for BECN1 expression. G UMUC3 and T24T cells were stably transfected with nonsense shRNA or two BECN1 shRNA constructs (shBECN1–1# and shBECN1–4#) to evaluate BECN1 knockdown efficiency. H, I The anchorage-independent growth of indicated cells was assessed using soft-agar assay, with the number of colonies containing >32 cells in UMUC3 (H) and T24T (I) cells counted and presented. *Significant decrease from vehicle control, ♣Significant increase from nonsense transfectant (n = 3).
BBN, a nitrosamine with butyl and hydroxybutyl substituents, induces single-stranded DNA breaks and sister chromatid exchanges, making it a widely utilized bladder cancer inducer [35,36]. Specifically, BBN-induced bladder cancer models in mice are favored for laboratory studies due to their reproducibility and their ability to generate muscle-invasive tumors that are histologically and genetically comparable to muscle-invasive bladder cancer (MIBC) in human patients [35]. Owing to the ease of use and rapid development of bladder tumors, BBN-induced tumors in mice are well-documented preclinical models of urothelial carcinoma. Therefore, we investigated whether downregulation of BECN1 can be reproduced in BBN-induced mouse bladder tumor. C57/BJ mice were treated with BBN for 20 weeks, by which BBN-treated mice developed the high-grade muscle-invasive BCs with 100% penetration. Like the results from human BCs, BBN-induced mouse BCs also exhibited a significant reduction in BECN1 protein levels compared to normal bladder tissues (Fig. 1B-D). Our previous study showed that co-treatment with ISO significantly inhibited BBN-induced development of mouse MIBCs [7]. Surprisingly, co-treatment of mice with BBN+ISO remarkably reversed BBN-caused downregulated BECN1 protein levels to those from vehicle control group in the bladder tissues (Fig. 1C-D). Thus, our results indicated that BECN1 proteins were downregulated in both human BCs and mouse BCs, and co-treatment with ISO not only inhibited BBN-induced BC formation but also reversed BECN1 downregulation, suggesting a potential involvement of BECN1 in ISO-mediated anti-cancer activity.
To investigate the potential role of BECN1 in ISO-induced cancer inhibition, we first evaluated the effect of ISO on BECN1 expression in UMUC3 and T24T cells. The results showed a profound increase in BECN1 protein levels following ISO treatment in both cell lines in a time- and dose-dependent manner (Fig. 1E-F). Subsequently, stable knockdown of BECN1 was generated in UMUC3 and T24T cells (Fig. 1G) to investigate the contribution of ISO-upregulated BECN1 to its anti-cancer activity. The analysis revealed that BECN1 knockdown markedly reversed the inhibitory effects of ISO on anchorage-independent growth of BC cells in both cells (Fig. 1H-I). These findings suggest that ISO-induced elevation of BECN1 expression in BC cells may contribute to its anti-cancer activity.
BECN1 played an important role in ISO-induced autophagy in human BC cells
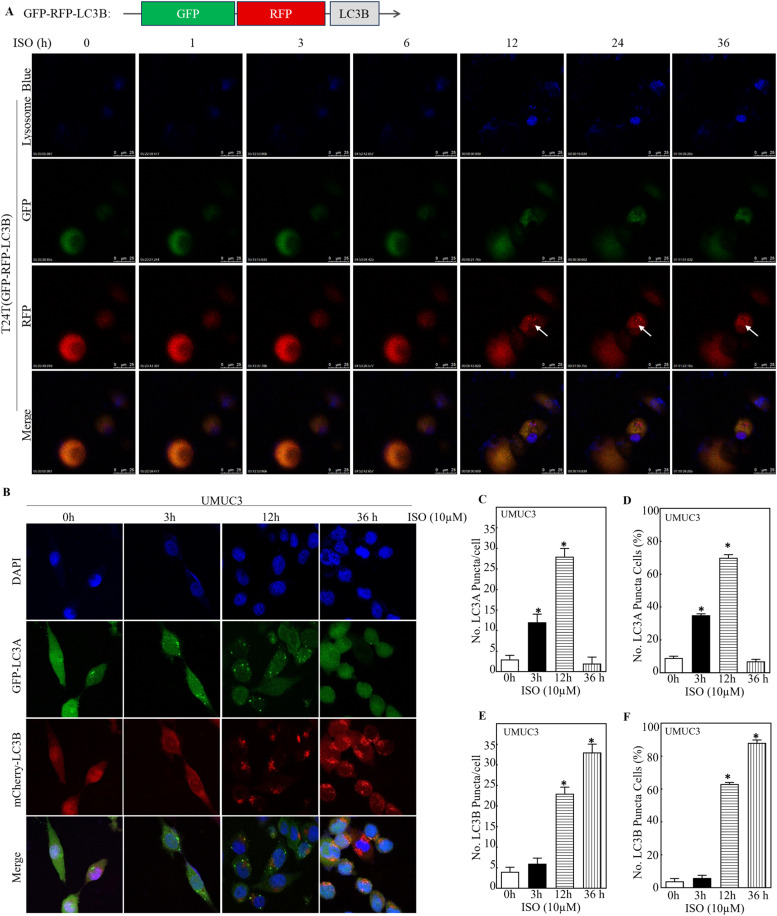
Our previous study demonstrates the importance of autophagic induction in ISO anti-cancer activity [6]. Since BECN1 is an important autophagy regulator [37], we then examined the role of BECN1 in ISO-induced cell autophagy. Using a tandem RFP-GFP-LC3B construct, which combines the acid-sensitive GFP with acid-stable RFP, we monitored ISO-induced autophagy flux in live cells. Punctate autophagosomes with both GFP and RFP signals were observed 12 h after ISO treatment, suggesting the initiation of autophagy. After 24 h, the number of GFP-LC3B puncta decreased while RFP puncta remained, indicating the fusion of autophagosomes with lysosomes (labeled with lysosome blue) (Fig. 2A). This confirmed increased autophagy flux in ISO-treated cells.
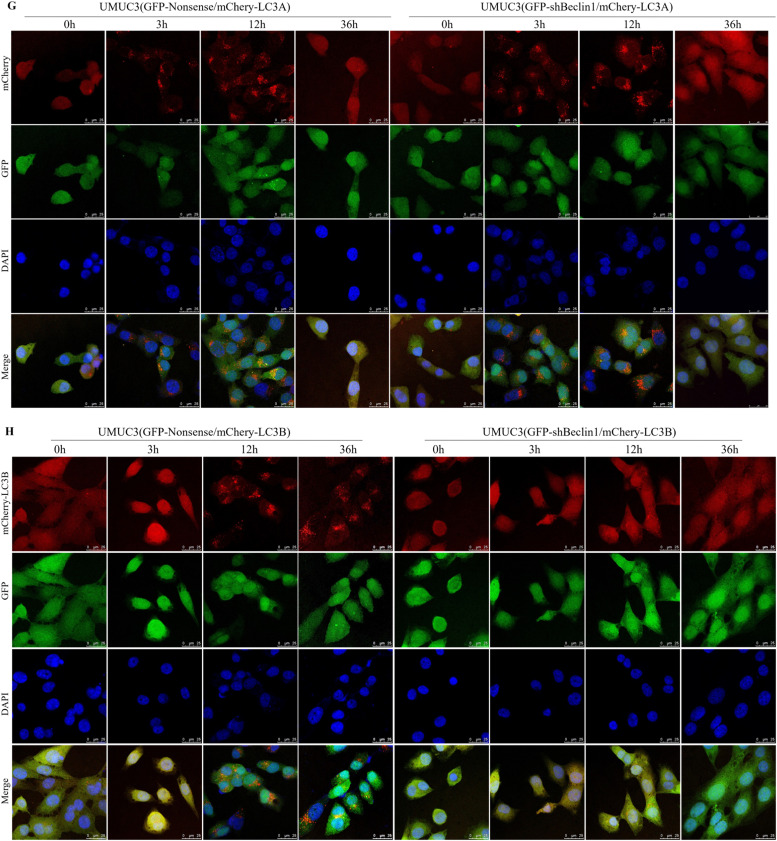
Fig. 2.
ISO induced autophagy through BECN1 in human BC cells. A T24T cells expressing GFP-RFP-LC3B were seeded into 35-mm dishes for live-cell imaging. Images were presented as the indicated time following ISO treatment. B-F UMUC3 cells expressing both GFP-LC3A and mCherry-LC3B were treated with 10μM of ISO for the indicated time periods. Confocal fluorescence microscopy was used to capture and count LC3A and LC3B puncta. The number of puncta per positive cell (C, E) and percentage of cells with LC3A or LC3B puncta (D, F) were calculated and presented. G-H UMUC3/shBECN1 or UMUC3/Nonsense cells expressing mCherry-LC3A (G) or mCherry-LC3B (H) were treated with 10μM of ISO for indicated times, and images of LC3A or LC3B puncta were captured and counted using confocal fluorescence microscopy. I-L The percentage of cells and number of puncta per positive cell with LC3A or LC3B puncta was calculated and presented. Cells with ≥5 intense LC3A or LC3B puncta were considered as positive autophagic cells. *Significant increase from vehicle group (n = 3). M, N Indicated transfectants in UMUC3 cells (M) and T24T cells (N) were treated with 0, 5 and 10μM of ISO for 36 h and the conversion of LC3B-I/II were assessed by WB. O Indicated cells were treated with ISO (10μM) for indicated times and the conversion of LC3B-I/II were assessed by WB using anti-LC3B and anti-LC3A antibodies. P UMUC3 cells treated with 10μM ISO, 2 nM BAF, 10μM CQ, or ISO+BAF, or ISO+CQ, for 24 h were analyzed by WB.
Subsequently, GFP-LC3A and mCherry-LC3B constructs were transfected into UMUC3 cells, punctate analysis was done at 3-, 12-, and 36-hours post-ISO treatment. Interestingly, punctate formation in UMUC3/GFP-LC3A cells occurred mainly at 3 and 12 h after ISO treatment, while in GFP-LC3B transfectants it occurred at 12 and 36 h under the same experimental conditions (Fig. 2B-F). This suggests different effects of ISO on LC3A- and LC3B-associated autophagic responses in human BC cells.
To further examined the impact of BECN1 knockdown on ISO-induced autophagy by monitoring the conversion of LC3-I to LC3-II, DNA construct containing mCherry-LC3A or mCherry-LC3B was transfected into UMUC3/shBECN1 or UMUC3/Nonsense cells, and LC3 punctate was analyzed at 3, 12, and 36 h after ISO treatment. Surprisingly, BECN1 knockdown did not affect early LC3A-I to LC3A-II conversion (Fig. 2G, I, J) but completely blocked late LC3B-I to LC3B-II conversion in human BC cells induced by ISO (Fig. 2H, K, L). This impaired conversion was confirmed by WB analysis (Fig. 2M-O). Increased autophagic flux from ISO treatment (36 h) was confirmed by co-treating cells with late-phase autophagy inhibitors BAF or CQ (Fig. 2P). This suggests that BECN1 is essential for ISO-induced late-phase autophagy, particularly in LC3B-I/II conversion, in human BC cells.
ISO treatment induced stabilization of BECN1 mRNA via NCL-dependent mechanism
To investigate the impact of ISO on BECN1 transcription, we examined BECN1 mRNA levels and promoter activity in UMUC3 cells following ISO treatment. Consistent with the increase in BECN1 protein, ISO treatment led to a time-dependent upregulation of BECN1 mRNA (Fig. 3A). Surprisingly, the luciferase reporter assay using the BECN1 promoter-driven sequence showed a significant decrease in promoter activity post-ISO treatment (Fig. 3B), suggesting a transcription-independent mechanism for ISO-induced BECN1 mRNA levels. Subsequent experiments focused on investigating the effect of ISO on BECN1 mRNA stability. Pre-treatment with ISO extended the half-life of BECN1 mRNA compared to treatment with the transcription inhibitor Act D alone (Fig. 3C), indicating that ISO upregulated BECN1 by increasing its mRNA stability.
Fig. 3.
ISO induced NCL expression and enhanced BECN1 mRNA stability in human BC cells. A UMUC3 cells were treated with ISO (10μM) for indicted times. BECN1 mRNA level was measured by RT-PCR. B UMUC3 cells expressing BECN1-promoter luciferase reporter were treated with various concentrations ISO for 12 h. The luciferase activity was measured and presented as relative promoter activity. *Significant decrease from vehicle control (n = 3). C UMUC3 cells were pretreated with ISO (10μM) for 6 h. The BECN1 mRNA stability were determined with or without Act D (20μM) for 12 and 24 h. *Significant decrease from vehicle control. ♣Significant increase from cells treated with Act D alone (n = 3). D UMUC3 cells were exposed to ISO (10μM) for indicated time periods. AUF1, HUR, and NCL protein levels were analyzed by WB. E HEK293T cells expressing GFP or GFP-NCL were subjected to RNA-IP using anti-GFP antibody. The binding of BECN1 mRNA to NCL was analyzed by PCR. F The stable shRNA transfectants (UMUC3/Nonsense and UMUC3/shNCL) were identified by WB. G Indicated cells were exposed to 5 or 10μM of ISO for 24 h BECN1 levels were analyzed by WB. H Indicated cells were pretreated with ISO (10μM) for 6 h. BECN1 mRNA stability was determined with Act D (20μM) for 12 and 24 h *Significant decrease from vehicle control. ♣Significant increase from cells with Act D alone (n = 3). I The anchorage-independent growth of indicated cells were accessed by soft-agar assay. The number of colonies containing >32 cells were counted and presented. *Significant decrease from vehicle control. ♣Significant increase from nonsense group (n = 3).
AU-rich element RNA-binding protein 1 (AUF1), human antigen R (HuR), and Nucleolin (NCL) are RNA-binding proteins to modulate their target mRNA stability [[38], [39], [40]]. Thus, we inspected whether any of these proteins participated in ISO stabilization of BECN1 mRNA. The analysis revealed a notable increase in NCL expression following ISO treatment, while AUF1 and HuR levels remained unchanged (Fig. 3D), suggesting that NCL might mediate ISO-induced BECN1 mRNA stability. Moreover, RNA-IP assay revealed a direct binding of NCL to BECN1 mRNA in the absence of ISO treatment (Fig. 3E). NCL knockdown in UMUC3 cells (Fig. 3F) decreased ISO-stabilized BECN1 protein (Fig. 3G) and mRNA (Fig. 3H). Notably, NCL knockdown reversed the inhibitory effect of ISO on anchorage-independent growth in human BC cells (Fig. 3I). Collectively, ISO treatment upregulates NCL expression, facilitating NCL-mediated BECN1 mRNA stabilization, which subsequently contributes to its anti-cancer activity.
Inhibition of miR-613 mediated ISO-induced upregulation of NCL and BECN1
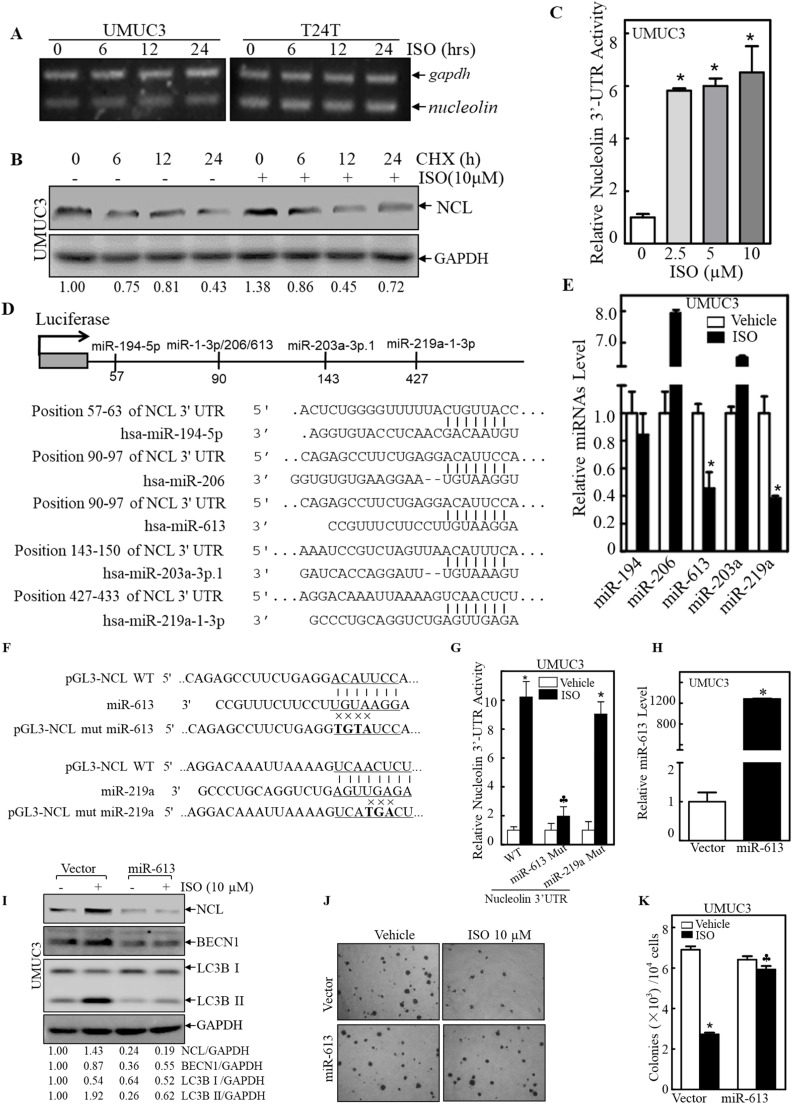
NCL dysregulation has been reported in human cancers, but the mechanism controlling its expression are largely unknown. To identify the mechanism underlying ISO-induced NCL expression, we analyzed NCL mRNA level in ISO-treated UMUC3 and T24T cells. Although NCL protein significantly increased in ISO-treated UMUC3 cells (Fig. 3D), its mRNA levels in two cells remained unchanged following ISO treatment (Fig. 4A), suggesting that ISO modulates NCL protein levels translationally or post-translationally. To investigate whether ISO affected NCL protein stability, UMUC3 cells were pretreated with ISO for 6 h and followed by CHX, a protein synthesis inhibitor. The half-life of NCL protein was analyzed at 6, 12, and 24 h after CHX treatment. Pretreatment with ISO did not affect NCL protein degradation compared to those treated with CHX alone (Fig. 4B). We next evaluated the potential effect of ISO on NCL protein translation by transfecting NCL mRNA 3′-UTR luciferase reporter into UMUC3 cells with various doses of ISO. The activity of NCL mRNA 3′UTR-driven luciferase reporter significantly increased in UMUC3 cells following ISO treatment (Fig. 4C), suggesting that ISO modulates NCL protein translation via targeting its mRNA 3′-UTR sequence.
Fig. 4.
miR-613 mediated ISO-enhanced NCL mRNA stability. A UMUC3 and T24T cells were treated with ISO (10μM) for indicated time periods. NCL mRNA expression was measured by RT-PCR. B UMUC3 cells pretreated with ISO (10μM) for 6 h Protein half-life of NCL was determined with CHX (100 μg/ml) for indicated time periods. C UMUC3 cells stably transfected with NCL 3′UTR luciferase reporter was treated with various concentrations ISO for 12 h. The result was presented as relative NCL 3′UTR activity compared with vehicle control. *Significant increase from vehicle control (n = 3). D Diagram of multiple miRNA consensus-binding site in NCL 3′UTR. E UMUC3 cells were exposed to ISO (10μM) for 24 h and analyzed for indicated miRNA levels by qPCR (n = 3). F Diagram of WT and mutant NCL 3′-UTR reporter constructs. G UMUC3 cells were stably transfected with WT or mutant NCL 3′-UTR luciferase reporters. The reporter activity was measured in cells following ISO (10μM) for 12 h. *Significant decrease from WT construct (n = 3). H The stable transfectants (UMUC3/Vector and UMUC3/ miR-613) were identified by qPCR (n = 3). I Indicated cells were treated with ISO (10μM) for 24 h. The levels of NCL, BECN1 and conversion of LC3B-I/II were assessed by WB. J, K Results were presented as representative images (J) and the number (K) of colonies grow in soft agar. *Significant decrease from vehicle control. ♣Significant increase from vector control group (n = 3).
Further analysis of the NCL 3′-UTR sequence identified potential binding sites for miR-194–5p, miR-206, miR-613, miR-203a and miR-219a (Fig. 4D). Expression miR-613 and miR-219a decreased significantly in ISO-treated UMUC3 cells, while miR-206 and miR-203a showed notable upregulation after ISO treatment (Fig. 4E). To investigate the impact of miR-613 and miR-219a on NCL protein translation, we generated NCL 3′-UTR mutants that disrupted the putative binding sites for either miR-613 or miR-219a (Fig. 4F). Luciferase reporter assays in UMUC3 cells revealed that mutation at the miR-613 binding site abolished ISO-induced NCL 3′-UTR activity, suggesting a role for miR-613 in mediating ISO-induced NCL expression (Fig. 4G). Ectopic expression of miR-613 in UMUC3 cells (Fig. 4H) attenuated the induction of NCL and BECN1, as well as autophagic responses following ISO treatment (Fig. 4I). Additionally, miR-613 overexpression reversed ISO inhibition of anchorage-independent cell growth in UMUC3 cells (Fig. 4J-K). These findings indicate that downregulation of miR-613 by ISO leads to increased NCL and BECN1 expression, promoting autophagy and inhibiting cancer.
Reduced c-Myc mRNA stability by ISO contributed to miR-613 downregulation in human BC cells
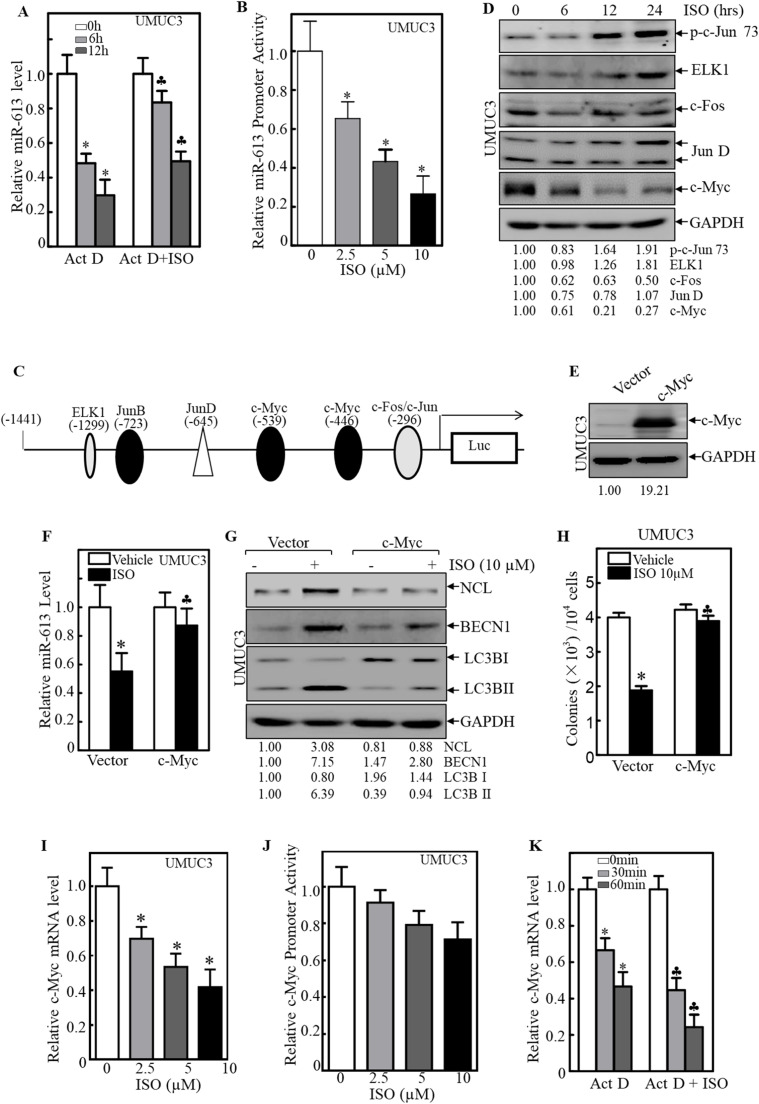
To explore whether ISO reduced miR-613 mRNA levels by transcriptional or post-transcriptional modulation, we analyzed miR-613 mRNA stability and its promoter-driven luciferase reporter activity following ISO treatment. Pretreatment UMUC3 cells with ISO followed by Act D co-treatment showed that ISO reduced miR-613 degradation compared to Act D treatment alone, indicating that mRNA stability is not the primary cause of miR-613 reduction (Fig. 5A). Transfection of a miR-613 promoter-driven luciferase reporter into UMUC3 cells revealed a significant decrease in promoter activity upon ISO treatment, suggesting transcriptional suppression of miR-613 by ISO (Fig. 5B). Bioinformatics analysis identified potential transcription factors involved in ISO-mediated inhibition of miR-613 transcription (Fig. 5C). Further examination showed increased phosphorylation of ELK1 and c-Jun at Ser73, as well as decreased c-Myc protein levels following ISO treatment, implicating c-Myc in the transcriptional inhibition of miR-613 by ISO (Fig. 5D). Overexpression of c-Myc in UMUC3 cells rescued miR-613 levels suppressed by ISO and attenuated ISO-induced expression changes in NCL, BECN1, and LC3B-I to LC3B-II conversion (Fig. 5E-G). Consistently, c-Myc overexpression significantly abolished the inhibitory effect of ISO on anchorage-independent growth (Fig. 5H). Moreover, ISO treatment dramatically downregulated c-Myc mRNA expression without significantly affecting its promoter activity (Fig. 5I, J), suggesting that ISO inhibits c-Myc via transcription-independent manner. Further study with Act D treatment revealed a reduced c-Myc mRNA stability following ISO treatment (Fig. 5K). Thus, our results demonstrate that ISO treatment reduces c-Myc mRNA stability and consequently attenuates its downstream targeted miR-613 transcription.
Fig. 5.
Induction of c-Myc mRNA degradation by ISO contributed to miR-613 downregulation in human BC cells. A UMUC3 cells were pretreated with ISO (10μM) for 6 h. The miR-613 stability was analyzed with Act D (20μM) for indicated periods. *Significant decrease from vehicle control. ♣Significant increase from Act D treatment alone (n = 3). B UMUC3 cells were stably transfected with miR-613-promoter luciferase reporter and then treated with various doses of ISO for 12 h. The luciferase activity was measured and presented as relative promoter activity. *Significant decrease from vehicle control (n = 3). C The potential transcription factor binding site in miR-613 promoter was analyzed using the TRANSFAC 8.3. D UMUC3 cells were treated with ISO (10μM) for indicated times. The levels of potential transcription factors were analyzed by WB. E The stable transfectants (UMUC3/Vector and UMUC3/c-Myc) identified by WB. F Indicated cells were treated with ISO (10μM) for 24 h. MiR-613 level was measured by RT-qPCR. *Significant decrease from vehicle control. ♣Significant increase from vector transfectants (n = 3). G Indicated cells were treated with ISO (10μM) for 24 h. NCL, BECN1, and LC3B levels were analyzed by WB. H The anchorage-independent growth of indicated cells were assessed by the number of colonies in soft-agar. *Significant decrease from vehicle control. ♣Significant increase from vector transfectant (n = 3). I UMUC3 cells were treated with ISO (10μM) for indicated time periods. C-Myc mRNA expression was determined by RT-qPCR. J UMUC3 cells stably transfected with c-Myc-promoter luciferase reporter were treated with various concentrations ISO for 12 h. The luciferase activity was measured and presented as relative promoter activity. K UMUC3 cells were pretreated with ISO (10μM) for 6 h. C-Myc mRNA stability was determined with Act D (20μM) for indicated periods. *Significant decrease from vehicle control. ♣Significant decrease from Act D alone group (n = 3).
Discussion
Bladder cancer (BC), particularly HGIBC, is a significant threat to men and women, with 20,000 deaths annually. Identifying new alternative medications targeting BC and understanding the mechanisms of their anti-cancer effects are crucial for reducing BC mortality. ISO exhibits multiple anti-cancer effects in human HGIBC cells and mouse BC models [4]. Our previous studies demonstrate the importance of autophagy in mediating ISO cancer inhibition, as depletion of SESN2 in BC cells not only impairs ISO-induced autophagy, but also abolishes its inhibiting BC cell growth. Our current study investigated the potential role of BECN1, another key autophagy regulator, in ISO anti-cancer activities. Reduced BECN1 expression were found in both human and mouse BCs. Treatment with ISO countered BECN1 downregulation in mouse bladder chemical carcinogenic models and reactivated BECN1 in cultured human BC cells, leading to significantly reduced bladder tumor formation in BBN-treated mice as well as decreased colony formation in soft agar. Moreover, our results indicated that c-myc/miR-613/NCL axis mediated BECN1 induction by ISO. Any alteration in this axis had a great effect on BECN1 expression and ISO cancer inhibition. Together, our results not only demonstrate the participation of BECN1 in ISO-induced autophagy, but also provide a novel mechanism on modulating cellular BECN1 levels in human BC cells as illustrated in Graphical Abstract.
Autophagy has been reported to be a key regulator involved in the pathogenesis of a wide range of human disorders [41]. In human cancer, autophagy functions as a double-edged sword that either inhibits or promotes cell proliferation and tumor progression [42]. As an important of autophagy regulator, BECN1 plays a dual role in tumor development, acting as either a tumor suppressor or an oncogenic factor [43,44]. Monoallelic loss or reduced expression of BECN1 in various human cancers, along with increased incidence of spontaneous tumor in BECN1 Heterozygous mice, suggest BECN1 as a haploinsufficient tumor suppressor [44,45]. However, recent study reveals a distinct role of BECN1 in the maintenance and tumorigenicity of breast cancer stem-like/progenitor cells [43]. Thus, the role of BECN1 in cancer is rather complicated and context-dependent.
Various evidences suggest that BECN1 functions as a crucial tumor suppressor in BC [46,47]. First, current study revealed decreased levels of BECN1 protein in human BC tissue compared to adjacent normal bladder tissues (Fig. 1A), consistent with previous findings of frequent BECN1 loss in higher-grade bladder tumors [26]. Moreover, a mouse model of BC induced by BBN mirrored the downregulation of BECN1 seen in human BC, and revealed an inverse correlation between BECN1 expression and bladder tumor development. Treatment with ISO not only prevented BECN1 downregulation and invasive BC formation induced by BBN, but also promoted an increase in BECN1 protein levels and inhibited anchorage-independent growth in human BC cells in vitro. Depletion of BECN1 impaired ISO-induced autophagy and abolished its inhibitory effects on anchorage-independent growth. Interestingly, recent reports have shown that BECN1 is upregulated in cisplatin-treated BC cells [47], suggesting that the induction of BECN1 by chemopreventive or chemotherapeutic agents may represent a novel mechanism underlying their anti-cancer activities.
Either tumor suppression or oncogenic effect of BECN1 has been closely related to autophagy-promoting activity [48]. Depletion of BECN1 in cancer cells led to defective autophagy in ISO- or cisplatin-treated BC cells, as evidenced by significantly reduced conversion of LC3-I to LC3-II (Fig. 2 and [47]). The impaired autophagy is crucial for cancer inhibition by ISO, since knockdown BECN1 or SESN2 in BC cells impeded ISO anti-cancer activity (Fig. 1A, [6]). Notably, despite both involved in ISO-induced autophagy, SESN2 and BECN1 may have different downstream targets leading to autophagic responses. For example, the conversion of LC3-I/II in the early phase was affected only in SESN2 knockdown cells, not in BECN1 knockdown cells [6]. Moreover, ISO-induced LC3A puncta formation was mainly observed in early phase (3–12 hrs) after ISO treatment, while LC3B puncta formation occurred in late phase (12–36 hrs) after ISO treatment (Fig. 2). BECN1 knockdown specific abolished LC3B puncta formation and LC3B-I/II conversion, but not LC3A puncta formation in human BC cells. Above results reveal that BECN1 and SESN2 may modulate distinct downstream processes leading to autophagic responses in human BC following ISO treatment. Studies have shown that autophagosomes are formed by one of three LC3 proteins, each with unique subcellular localization [49], implying different biological functions. Investigating the downstream targets of SESN2 and BECN1, along with their involvement in autophagic responses to ISO treatment and other stimuli, will provide valuable insights into the anticancer effect of ISO and the dual role of autophagy in cancer progression and treatment.
Our study elucidated the mechanism by which ISO induces BECN1 expression through a novel cascade involving c-Myc/miR-613/NCL. Initially, we demonstrated that NCL, upregulated by ISO, directly binds to BECN1 mRNA, enhancing its stability. NCL knockdown abolished ISO-induced BECN1 mRNA stabilization, protein expression, and inhibition of anchorage-independent growth. Subsequently, we observed that ISO downregulated miR-613, which binds to NCL 3′-UTR, suppressing its activity. Ectopic expression of miR-613 counteracted ISO-induced NCL expression, autophagy, and anti-cancer effects. Additionally, ISO treatment decreased the mRNA stability of c-Myc, a transcription factor that suppresses miR-613 transcription. Overexpression of c-Myc in BC cells reversed ISO-induced miR-613 downregulation, leading to decreased NCL/BECN1 expression, impaired autophagy, and cancer inhibition. Therefore, our study highlights the crucial role of the c-Myc/miR-613/NCL axis in ISO-induced BECN1 expression, autophagy, and anti-cancer activity.
In summary, our findings suggest that ISO treatment enhances BECN1 mRNA stability, elevating protein levels through the c-Myc/miR-613/NCL axis, ultimately contributing to ISO-mediated autophagy and cancer inhibition in human BCs. These findings, combined with our previous results on SESN2, emphasize the crucial role of autophagy in cancer inhibition, and provide new insights for potential utilization of ISO to develop a better therapeutic strategy for HGIBC.
Fundings
This work was partially supported by grants from Natural Science Foundation of China (NSFC81773391 and NSFC81702530) and Oujiang Research Project OJQD2022006.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval statement
Approval of the research protocol by an Institutional Reviewer Board and Informed Consent: All specimens were obtained with appropriate informed consent from the patients and a supportive grant obtained from the Medical Ethics Committee of the Union Hospital of Tongji Medical College, China, and was carried out in accordance with The Code of Ethics of the World Medical Association for experiments involving humans.
Registry and the registration no. of the study/trial
N/A.
Animal studies
All animal studies were performed according to protocols approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University (ID no. wydw2017–0073; approval date: March 4, 2017).
CRediT authorship contribution statement
Xiaohui Hua: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Daimin Xiang: Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Jiheng Xu: Methodology, Investigation. Shouyue Zhang: Methodology, Investigation. Shuai Wu: Methodology, Investigation. Zhongxian Tian: Software, Investigation. Junlan Zhu: Resources, Investigation. Chuanshu Huang: Writing – review & editing, Supervision, Resources, Project administration, Conceptualization.
Declaration of competing interest
Neither this paper nor any similar paper has been or will be submitted to or published in any other scientific journal. All authors are aware and agree to the content of the paper and to their being listed as an author on the manuscript. There is no conflict of interest or competing financial interests for all authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102178.
Contributor Information
Xiaohui Hua, Email: huaxiaohui0328@163.com.
Chuanshu Huang, Email: huangchuanshu@hotmail.com.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Tran L., Xiao J.F., Agarwal N., Duex J.E., Theodorescu D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer. 2021;21(2):104–121. doi: 10.1038/s41568-020-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang K.S., Wang Y.H., Li R.L., Lin M. Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry. 2000;54(8):875–881. doi: 10.1016/s0031-9422(00)00151-5. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y., Cao Z., Hou Q., Ma C., Yao C., Li J., et al. Cyclin d1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol. Cancer Ther. 2013;12(8):1492–1503. doi: 10.1158/1535-7163.MCT-12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y., Yu Y., Hou Q., Zheng X., Zhang M., Zhang D., et al. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J. Biol. Chem. 2012;287(42):35234–35243. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Y., Zhu J., Huang H., Xiang D., Li Y., Zhang D., et al. SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy. 2016;12(8):1229–1239. doi: 10.1080/15548627.2016.1179403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang G., Wu A.D., Huang C., Gu J., Zhang L., Huang H., et al. Isorhapontigenin (ISO) Inhibits Invasive Bladder Cancer Formation In Vivo and Human Bladder Cancer Invasion In Vitro by Targeting STAT1/FOXO1 Axis. Cancer Prev. Res. 2016;9(7):567–580. doi: 10.1158/1940-6207.CAPR-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky D.J., Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science (1979) 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–453. doi: 10.1016/j.immuni.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao X., Shiromoto Y., Sakurai M., Towers M., Zhang Q., Wu S., et al. ADAR1 downregulation by autophagy drives senescence independently of RNA editing by enhancing p16 (INK4a) levels. Nat. Cell Biol. 2022;24(8):1202–1210. doi: 10.1038/s41556-022-00959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitada M., Koya D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021;17(11):647–661. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 12.Xia H., Green D.R., Zou W. Autophagy in tumour immunity and therapy. Nat. Rev. Cancer. 2021;21(5):281–297. doi: 10.1038/s41568-021-00344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N., Levine B. Autophagy in Human Diseases. N. Engl. J. Med. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 14.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., Abdellatif M., Abdoli A., Abel S., et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2021;17(1):1–382. doi: 10.1080/15548627.2020.1797280. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu N., Zheng W., Zhou Y., Tian Y., Tang M., Feng X., et al. Autophagy in aging-related diseases and cancer: principles, regulatory mechanisms and therapeutic potential. Ageing Res. Rev. 2024;100 doi: 10.1016/j.arr.2024.102428. [DOI] [PubMed] [Google Scholar]
- 17.Codogno P., Mehrpour M. Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nature Rev. Mol. Cell Biol. 2011;13(1):7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- 18.Wirawan E., Lippens S., Vanden Berghe T., Romagnoli A., Fimia G.M., Piacentini M., et al. Beclin1: a role in membrane dynamics and beyond. Autophagy. 2012;8(1):6–17. doi: 10.4161/auto.8.1.16645. [DOI] [PubMed] [Google Scholar]
- 19.Marino G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang R., Zeh H.J., Lotze M.T., Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death. Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez R.T., Xu L. Bcl-2:beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012;2(2):214–221. [PMC free article] [PubMed] [Google Scholar]
- 22.Yue Z., Jin S., Yang C., Levine A.J. Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U S A. 2003;100(25):15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368(19):1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 25.Liang X.H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 26.Liu G.H., Zhong Q., Ye Y.L., Wang H.B., Hu L.J., Qin Z.K., et al. Expression of beclin 1 in bladder cancer and its clinical significance. Int. J. Biol. Markers. 2013;28(1):56–62. doi: 10.5301/JBM.2012.9769. [DOI] [PubMed] [Google Scholar]
- 27.Hua X., Xu J., Deng X., Xu J., Li J., Zhu D.Q., et al. New compound ChlA-F induces autophagy-dependent anti-cancer effect via upregulating Sestrin-2 in human bladder cancer. Cancer Lett. 2018;436:38–51. doi: 10.1016/j.canlet.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song L., Li J., Ye J., Yu G., Ding J., Zhang D., et al. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol. Cell Biol. 2007;27(7):2713–2731. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao G., Chen L., Li J., Zhang D., Fang Y., Huang H., et al. Isorhapontigenin (ISO) inhibited cell transformation by inducing G0/G1 phase arrest via increasing MKP-1 mRNA Stability. Oncotarget. 2014;5(9):2664–2677. doi: 10.18632/oncotarget.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D., Li J., Zhang M., Gao G., Zuo Z., Yu Y., et al. The requirement of c-Jun N-terminal kinase 2 in regulation of hypoxia-inducing factor-1alpha mRNA stability. J. Biol. Chem. 2012;287(41):34361–34371. doi: 10.1074/jbc.M112.365882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo X., Huang H., Jin H., Xu J., Risal S., Li J., et al. ISO, via Upregulating MiR-137 Transcription, Inhibits GSK3beta-HSP70-MMP-2 Axis, Resulting in Attenuating Urothelial Cancer Invasion. Mol. Therapy Nucleic Acids. 2018;12:337–349. doi: 10.1016/j.omtn.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Q., Chen C., Li H., Xu J., Wu L., Yu Y., et al. miR-3687 Overexpression Promotes Bladder Cancer Cell Growth by Inhibiting the Negative Effect of FOXP1 on Cyclin E2 Transcription. Mol. Therapy. 2019 doi: 10.1016/j.ymthe.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Yang K.B., Chen W., Mai J., Wu X.Q., Sun T., et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. 2021;17(12):4323–4340. doi: 10.1080/15548627.2021.1912270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia J., Zhang H.B., Shi Q., Yang C., Ma J.B., Jin B., et al. KLF5 downregulation desensitizes castration-resistant prostate cancer cells to docetaxel by increasing BECN1 expression and inducing cell autophagy. Theranostics. 2019;9(19):5464–5477. doi: 10.7150/thno.33282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matye D., Leak J., Woolbright B.L., Taylor J.A. Preclinical models of bladder cancer: BBN and beyond. Nature Rev. Urol. 2024 doi: 10.1038/s41585-024-00885-9. [DOI] [PubMed] [Google Scholar]
- 36.Lijinsky W., Epstein S.S. Nitrosamines as environmental carcinogens. Nature. 1970;225(5227):21–23. doi: 10.1038/225021a0. [DOI] [PubMed] [Google Scholar]
- 37.Korkmaz G., le Sage C., Tekirdag K.A., Agami R., Gozuacik D. miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy. 2012;8(2):165–176. doi: 10.4161/auto.8.2.18351. [DOI] [PubMed] [Google Scholar]
- 38.Abdelmohsen K., Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley. Interdiscip. Rev. RNa. 2010;1(2):214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Audic Y., Hartley R.S. Post-transcriptional regulation in cancer. Biol. Cell. 2004;96(7):479–498. doi: 10.1016/j.biolcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Ginisty H., Sicard H., Roger B., Bouvet P. Structure and functions of nucleolin. J. Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. Pt 6. [DOI] [PubMed] [Google Scholar]
- 41.Shintani T., Klionsky D.J. Autophagy in health and disease: a double-edged sword. Science (1979) 2004;306(5698):990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White E. The role for autophagy in cancer. J. Clin. Invest. 2015;125(1):42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong C., Bauvy C., Tonelli G., Yue W., Delomenie C., Nicolas V., et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene. 2013;32(18):1–11. doi: 10.1038/onc.2012.252. 2261-72, 72e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toton E., Lisiak N., Sawicka P., Rybczynska M. Beclin-1 and its role as a target for anticancer therapy. J. Physiol Pharmacol. 2014;65(4):459–467. [PubMed] [Google Scholar]
- 45.Hu F., Song D., Yan Y., Huang C., Shen C., Lan J., et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 2021;12(1):3651. doi: 10.1038/s41467-021-23923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baspinar S., Bircan S., Yavuz G., Kapucuoglu N. Beclin 1 and bcl-2 expressions in bladder urothelial tumors and their association with clinicopathological parameters. Pathol. Res. Pract. 2013;209(7):418–423. doi: 10.1016/j.prp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Lin J.F., Lin Y.C., Tsai T.F., Chen H.E., Chou K.Y., Hwang T.I. Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des. Devel. Ther. 2017;11:1517–1533. doi: 10.2147/DDDT.S126464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Y., Zou Z., Becker N., Anderson M., Sumpter R., Xiao G., et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154(6):1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koukourakis M.I., Kalamida D., Giatromanolaki A., Zois C.E., Sivridis E., Pouliliou S., et al. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS. One. 2015;10(9) doi: 10.1371/journal.pone.0137675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.