Abstract
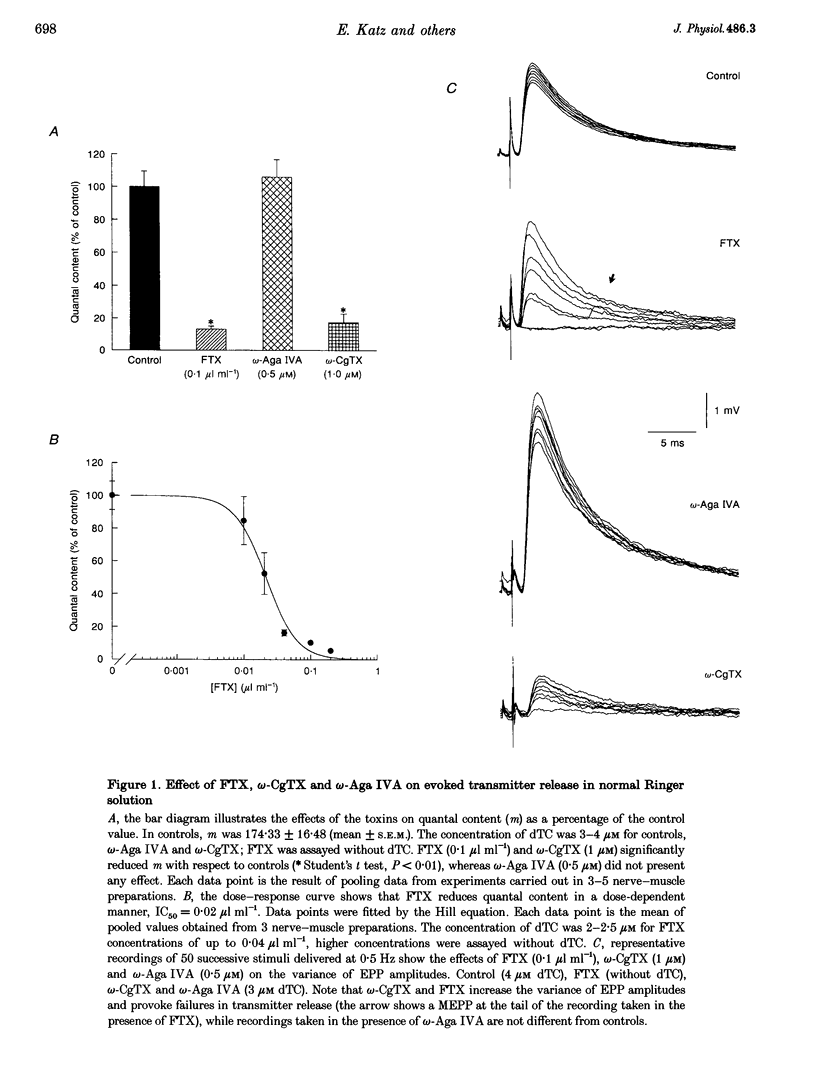
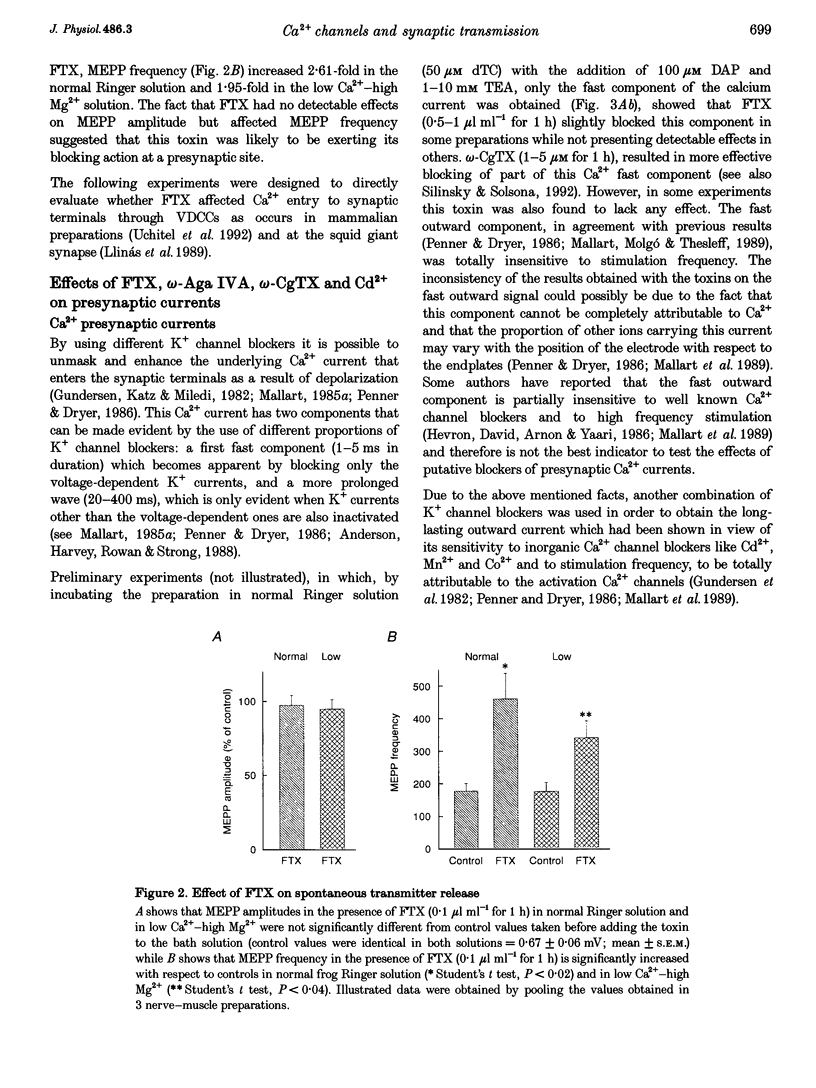
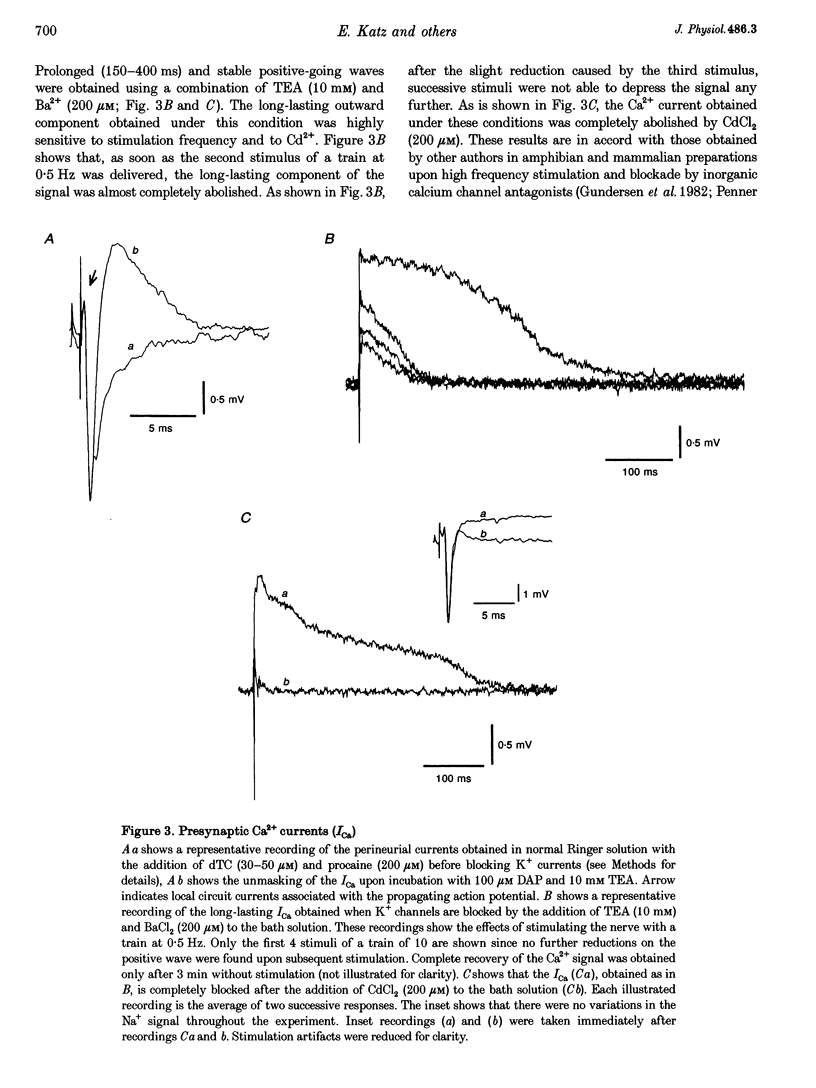
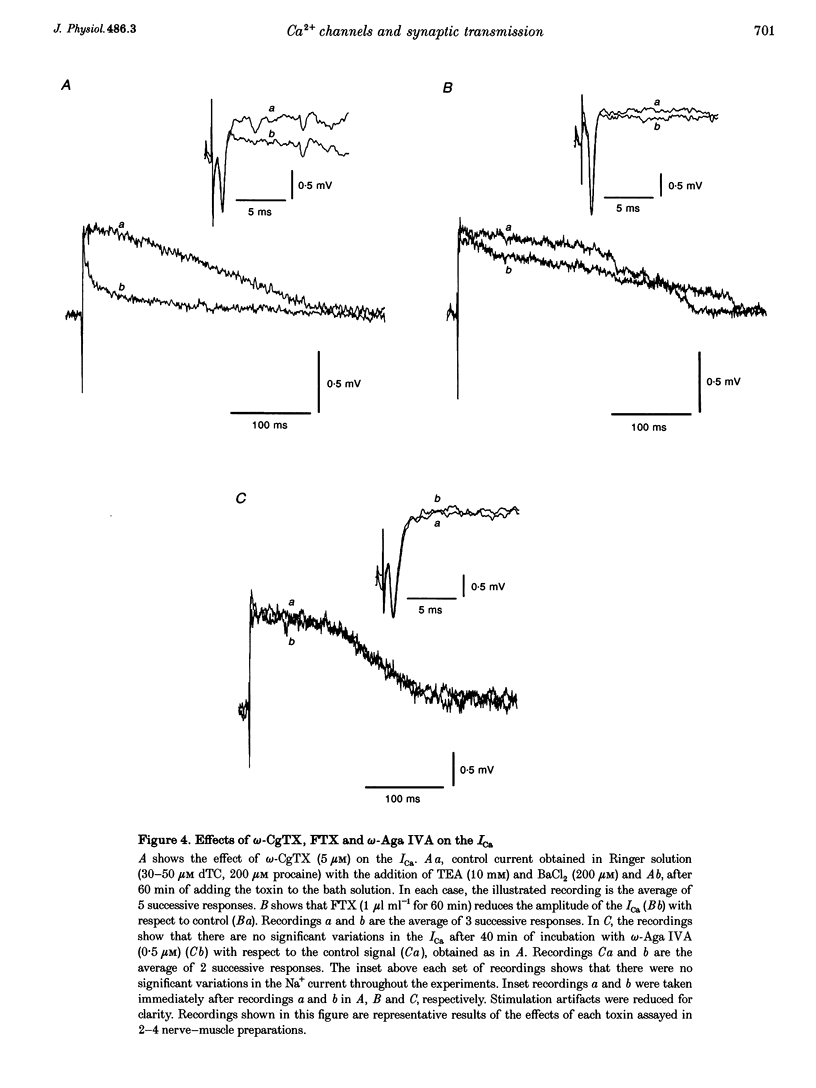
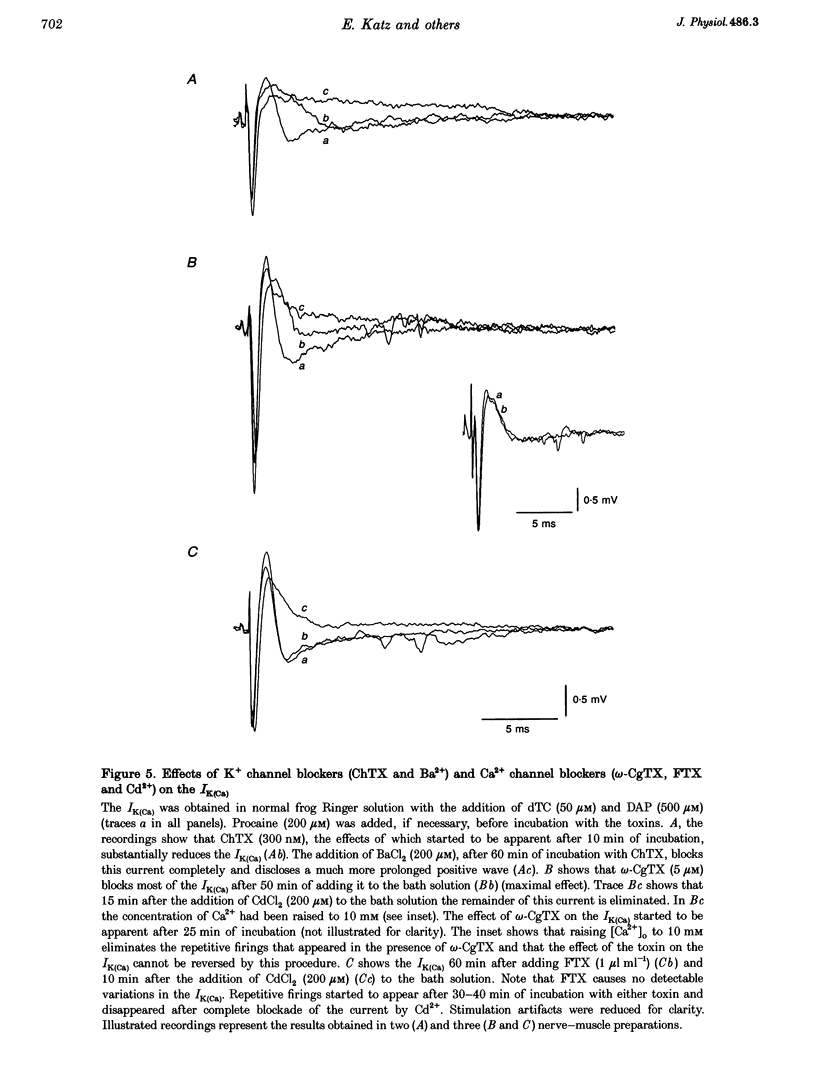
1. The effects of the calcium channel blockers, funnel-web spider toxin (FTX), omega-agatoxin IVA (omega-Aga IVA) and omega-conotoxin GVIA (omega-CgTX), were tested on transmitter release and presynaptic currents in frog motor nerve endings. 2. Evoked transmitter release was blocked by FTX (IC50 = 0.02 microliter ml-1) and omega-CgTX (1 microM) but was not affected by omega-Aga IVA (0.5 microM). When FTX (0.1 microliter ml-1) was assayed on spontaneous release either in normal Ringer solution or in low Ca(2+)-high Mg2+ solution, it was found not to affect miniature endplate potential (MEPP) amplitude but to increase MEPP frequency by approximately 2-fold in both conditions. 3. Presynaptic calcium currents (ICa), measured by the perineurial technique in the presence of 10 mM tetraethylammonium chloride (TEA) and 200 microM BaCl2 to block K+ currents, were blocked by omega-CgTX (5 microM), partially blocked by FTX (1 microliter ml-1) and not affected by omega-Aga IVA (0.5 microM). 4. The presynaptic calcium-activated potassium current (IK(Ca)) measured by the perineurial technique in the presence of 0.5 microM 3,4-aminopyridine (DAP) to block voltage-dependent K+ currents, was strongly affected by charybdotoxin (ChTX) (300 nM) and completely abolished by BaCl2 (200 microM). This current was also blocked by omega-CgTX (5 microM) and by CdCl2 (200 microM) but was not affected by FTX (1 microliter ml-1). The blockade by omega-CgTX could not be reversed by elevating [Ca]o to 10 mM. 5. The results suggest that in frog synaptic terminals two omega-CgTX-sensitive populations might coexist. The transmitter release process seems to be mediated by calcium influx through a omega-CgTX- and FTX-sensitive population.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Olivera B. M. Neurotoxins: overview of an emerging research technology. Trends Neurosci. 1994 Apr;17(4):151–155. doi: 10.1016/0166-2236(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Alvarez Maubecin V., Sanchez V. N., Rosato Siri M. D., Cherksey B. D., Sugimori M., Llinás R., Uchitel O. D. Pharmacological characterization of the voltage-dependent Ca2+ channels present in synaptosomes from rat and chicken central nervous system. J Neurochem. 1995 Jun;64(6):2544–2551. doi: 10.1046/j.1471-4159.1995.64062544.x. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J., Statham H. E. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1977 Jun;268(2):319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Pawson P. A. Dependence of spontaneous release at frog junctions on synaptic strength, external calcium and terminal length. J Physiol. 1989 Nov;418:397–410. doi: 10.1113/jphysiol.1989.sp017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Katz B., Miledi R. The antagonism between botulinum toxin and calcium in motor nerve terminals. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):369–376. doi: 10.1098/rspb.1982.0080. [DOI] [PubMed] [Google Scholar]
- Hevron E., David G., Arnon A., Yaari Y. Acetylcholine modulates two types of presynaptic potassium channels in vertebrate motor nerve terminals. Neurosci Lett. 1986 Dec 3;72(1):87–92. doi: 10.1016/0304-3940(86)90624-5. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F., Miyamoto M. Reduction of transmitter release by D-tubocurarine. Nature. 1969 Aug 2;223(5205):531–533. doi: 10.1038/223531a0. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Koyano K., Abe T., Nishiuchi Y., Sakakibara S. Effects of synthetic omega-conotoxin on synaptic transmission. Eur J Pharmacol. 1987 Mar 31;135(3):337–343. doi: 10.1016/0014-2999(87)90683-2. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Walton K., Bohr V. Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys J. 1976 Jan;16(1):83–86. doi: 10.1016/S0006-3495(76)85664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy P. M., Hamilton M. G., Frew R. Pharmacological identification of a novel Ca2+ channel in chicken brain synaptosomes. Brain Res. 1994 Apr 18;643(1-2):204–210. doi: 10.1016/0006-8993(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Molgó J., Angaut-Petit D., Thesleff S. Is the internal calcium regulation altered in type A botulinum toxin-poisoned motor endings? Brain Res. 1989 Feb 6;479(1):167–171. doi: 10.1016/0006-8993(89)91348-6. [DOI] [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984 Jan;400(1):8–13. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. On the effect of calcium on the frequency of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1977 Mar;266(1):91–101. doi: 10.1113/jphysiol.1977.sp011757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Cruz L. J., de Santos V., LeCheminant G. W., Griffin D., Zeikus R., McIntosh J. M., Galyean R., Varga J., Gray W. R. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry. 1987 Apr 21;26(8):2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Imperial J. S., Cruz L. J., Bindokas V. P., Venema V. J., Adams M. E. Calcium channel-targeted polypeptide toxins. Ann N Y Acad Sci. 1991;635:114–122. doi: 10.1111/j.1749-6632.1991.tb36486.x. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Uchitel O. D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993 Dec 13;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Publicover S. J., Duncan C. J. The action of verapamil on the rate of spontaneous release of transmitter at the frog neuromuscular junction. Eur J Pharmacol. 1979 Feb 15;54(1-2):119–127. doi: 10.1016/0014-2999(79)90414-x. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Charlton M. P. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992 Jan;12(1):297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Garcia M. L., Kaczorowski G. J., Charlton M. P. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993 Oct;11(4):645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C. S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J Physiol. 1992 Nov;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P., Reiner P. B. Ca2+ channels: diversity of form and function. Curr Opin Neurobiol. 1992 Jun;2(3):247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Tabti N., Bourret C., Mallart A. Three potassium currents in mouse motor nerve terminals. Pflugers Arch. 1989 Feb;413(4):395–400. doi: 10.1007/BF00584489. [DOI] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz M. M., Sugimori M., Cherksey B., Llinás R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992 Dec;9(6):1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- Wang G., Thorn P., Lemos J. R. A novel large-conductance Ca(2+)-activated potassium channel and current in nerve terminals of the rat neurohypophysis. J Physiol. 1992 Nov;457:47–74. doi: 10.1113/jphysiol.1992.sp019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Sosa M. A., Poage R. E. omega-Conotoxin reduces facilitation of transmitter release at the frog neuromuscular junction. Brain Res. 1993 May 14;611(1):25–30. doi: 10.1016/0006-8993(93)91772-k. [DOI] [PubMed] [Google Scholar]


