Abstract
Background
The BODY-Q is a widely used patient-reported outcome measure for comprehensive assessment of treatment outcomes specific to patients undergoing body contouring surgery (BCS). However, for the BODY-Q to be meaningfully interpreted and used in clinical practice, minimal important difference (MID) scores are needed. A MID is defined as the smallest change in outcome measure score that patients perceive as important.
Objectives
The aim of this study was to determine BODY-Q MID estimates for patients undergoing BCS to enhance the interpretability of the BODY-Q.
Methods
Data from an international, prospective cohort from Denmark, Finland, Germany, Italy, the Netherlands, and Poland were included. Two distribution-based methods were used to estimate MID: 0.2 standard deviations of mean baseline scores and the mean standardized response change of BODY-Q scores from baseline to 3 years postoperatively.
Results
A total of 12,554 assessments from 3237 participants (mean age 42.5 ± 9.3 years; BMI 28.9 ± 4.9 kg/m2) were included. Baseline MID scores ranged from 1 to 5 on the health-related quality of life (HRQL) scales and 3 to 6 on the appearance scales. The estimated MID scores from baseline to 3-year follow-up ranged from 4 to 5 for HRQL and from 4 to 8 on the appearance scales.
Conclusions
The BODY-Q MID estimates from before BCS to 3 years postoperatively ranged from 4 to 8 and are recommended for interpretation of patients’ BODY-Q scores, evaluation of treatment effects of different BCS procedures, and calculation of sample size for future studies.
Level of Evidence: 3

It is estimated that between 70% and 90% of patients who undergo massive weight loss (MWL) develop varying degrees of excess skin, potentially affecting any area of the body, where subsequent body contouring surgery (BCS) may be needed.1,2 BCS has the potential to improve or restore patients’ body image and health-related quality of life (HRQL).3 However, to assess the impact of BCS on HRQL, there is a need for valid and reliable patient-reported outcome measures (PROMs).4 One such PROM is the BODY-Q, rigorously developed to measure patient-reported outcomes (PRO), specifically for patients undergoing BCS.5 Recent studies have highlighted the BODY-Q as the PROM with the most robust psychometric properties for patients undergoing BCS.6,7 As a result, it has had widespread uptake in the plastic and aesthetic surgical literature for exploration of the impact of BCS on patients’ lives.8-12 However, a gap exists in the interpretation of BODY-Q scores, limiting the utility of the BODY-Q in clinical care and research. Although efforts have been undertaken to generate normative BODY-Q scores from the general population for comparison with patient-reported scores, it is pivotal that clinicians and researchers are able to meaningfully interpret the BODY-Q scores in patients who have had BCS to estimate the impact of clinical interventions.13,14
The minimal important difference (MID) is a measure of the smallest detectable change in a scale of interest that is considered important by patients.15,16 Two main methods exist to estimate the MID: the distribution-based method and the anchor-based method. The distribution-based method is based on the distribution of observed scores in a sample, examining score variability either through standard deviations (SD) or the change in SD that patients experience during a study period.17,18 The anchor-based method employs an external, patient-centered reference to compare changes in PRO scores against this anchor.19,20 The details of various anchor-based approaches have been described elsewhere.17,18,21
By determining MID for the BODY-Q to define a meaningful change or improvement, we can set more realistic expectations for outcomes, enabling clinicians to guide and advise patients more effectively.22 MID scores can serve as reference values for the magnitude of change that patients perceive important in each BODY-Q scale, because a statistically significant difference may not necessarily constitute a meaningful change for the patients undergoing BCS.23 Therefore, there is an imperative need for BODY-Q MID scores to provide a more accurate interpretation guide of changes in scores, to facilitate comparative effectiveness research and to assist patients in shared decision-making.24 The aim of this study was to establish BODY-Q MID values for patients undergoing BCS in a multicenter European cohort with the distribution-based method.
METHODS
In this study, data were derived from an international, multicenter prospective cohort study (ClinicalTrials.gov, https://clinicaltrials.gov, NCT05272215) assessing the change of HRQL and appearance through the weight loss trajectory from prebariatric surgery to post-BCS. For this analysis, data from patients undergoing BCS were included. The study was conducted according to the Declaration of Helsinki principles and was approved by the local ethics committee from the respective sites before study commencement. Before data were merged at the coordinating center in Denmark (Odense University Hospital, Odense, Denmark) approval was obtained from the Danish Data Protection Agency. The study period ranged from June 2015 to February 2022.
Data Collection
The cohort included the following countries and hospitals: Denmark (Odense University Hospital, Hospital of Southwest Jutland, and Printzlau Private Hospital); Finland (Tampere University Hospital); Germany (Johanniter-Krankenhaus und Waldkrankenhaus, Bonn); Italy (Universita Campus Bio-Medico Hospital, Rome); the Netherlands (OLVG West Hospital, Amsterdam, Catharina Hospital, Eindhoven, and St. Antonius Hospital, Nieuwegein); and Poland (Marciniaka Specialized Hospital, Wroclaw). Patients ages 18 years or older undergoing BCS were included. Patients with limited proficiency in the language of the study site (ie, Danish, Finnish, German, Italian, Dutch, or Polish) and patients with cognitive impairments were excluded.
The BODY-Q
The BODY-Q was developed and validated in accordance with the Rasch measurement theory; the development of the BODY-Q has been previously published.5 The BODY-Q consists of 32 independently functioning scales measuring HRQL, appearance, eating-related concerns, and experience of care.5,25-28 This study included 5 HRQL scales (physical function, body image, psychological, sexual, and social) and 11 appearance scales (abdomen, body, arms, back, buttocks, hips and outer thighs, inner thighs, chest, nipples, stretch marks, and scars). The chest and nipple scales were only developed for patients seeking masculinizing chest surgery. Each of the BODY-Q scale contained 4 to 10 items, rated on a Likert scale of 1 (very dissatisfied) to 4 (very satisfied). The raw scores from each item in a scale were summed and subsequently transformed by the Rasch conversion tables into a score ranging from 0 to 100, such that a higher score signified greater satisfaction or improved quality of life.
Administration of the BODY-Q
The BODY-Q was administered preoperatively (baseline) and postoperatively at the following time points: 3 to 6 months, 1 year, 2 years, and 3 years after surgery. In Denmark, patients were included from June 2015 to November 2021. A direct link was sent to the questionnaire through the research electronic data capture (REDCap) hosted by the Open Patient Data Explorative Network (OPEN), Odense University Hospital, through their secure electronic mailbox. Additionally, patients were able to fill out the questionnaire at their hospital appointments with an iPad (Apple, Cupertino, CA). In Finland, Italy, the Netherlands, and Poland participants were included directly in Castor EDC (Amsterdam, the Netherlands), in a secure web-based application by a URL link. This included Finnish participants from February 2019 to November 2021, Italian participants from February 2019 to November 2021, Dutch participants from December 2017 to November 2021, and Polish participants from May 2018 to February 2022. German participants were recruited for June 2018 to May 2021. Additionally, patients were asked to provide information regarding their age, weight, weight loss, height, marital status, educational level, type of weight loss treatment, area of BCS, and comorbidities.
Determination of the Minimal Important Difference
With the distribution-based method, a reasonable effect size must be determined.29 An effect size of 0.2 is considered an appropriate measure for MID based on a review by Samsa et al.30 Two distribution-based analyses were performed to estimate the MID. First, the baseline SD was the measure of sample variation. Second, the 0.2 standardized response mean (SRM) was employed to estimate a mean MID from baseline to 3 years postoperatively.21,22,31 Additionally, the MID was stratified based on body mass index (BMI) groups (<18.49, 18.5-24.9, 25.0-29.9, 30-34.9, 35-39.9, >40 kg/m2), gender (male and female), and age groups (18-29, 30-39, 40-49, 50-59, >60 years).
Statistical Analyses
All analyses were conducted with StataBE Version 17 (College Station, TX). Demographic data of the patients were summarized with descriptive statistics, which included mean, standard deviation (SD), and 95% CI for continuous variables, whereas proportions were utilized for categorical variables. The summed raw scores from each BODY-Q were transformed to Rasch converted scores, ranging from 0 to 100, with the Rasch converted scoring tables. For each scale, the median and interquartile range (consisting of the 25th percentile and 75th percentile) of the patient's scores were applied to devise a score interpretation tool.
RESULTS
Patient Demographics
The sample consisted of 12,554 assessments from 3237 patients. See Table 1 for participant characteristics, and Supplemental Table 1, available online at www.aestheticsurgeryjournal.com, for patients’ educational levels and marital status. The cohort consisted of 2536 (87.1%) females and 375 (12.9%) males, with a mean age of 42.5 ± 9.3 years (ranging from 18.9 to 75.4) and a baseline BMI of 28.9 ± 4.9 kg/m2. The mean follow-up was 37.0 months overall, with a mean follow-up of 30.6 months in Denmark, 49.0 months in Finland, 12.0 months in Germany, 64.9 months in Italy, 16.7 months in the Netherlands, and 49.4 months in Poland.
Table 1.
Participant Characteristics
| Characteristics | Total | Netherlands | Denmark | Italy | Germany | Poland | Finland |
|---|---|---|---|---|---|---|---|
| Patients, n (%) | 3237 | 712 (22.0) | 1782 (55.1) | 420 (13.0) | 192 (16.9) | 68 (2.1) | 63 (1.9) |
| Assessments, n (%) | 12,554 | 5938 (47.3) | 3372 (26.9) | 1750 (13.9) | 384 (3.1) | 565 (4.5) | 545 (4.3) |
| Gender, n (%) | 2912 | 712 | 1782 | 95 | 192 | 68 | 63 |
| Female | 2536 (87.1) | 638 (89.6) | 1504 (84.4) | 84 () | 192 (100) | 63 (92.6) | 55 (87.5) |
| Male | 375 (12.9) | 73 (10.3) | 278 (15.6) | 11 () | 0 () | 5 (7.4) | 8 (12.5) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 () | 0 (0) | 0 (0) |
| Age | 2597 | 477 | 1782 | 90 | 145 | 57 | 46 |
| Mean (SD) | 42.5 (9.31) | 44.7 (11.4) | 44.9 (10.4) | 42.3 (8.54) | 38.0 (9.62) | 39.8 (6.00) | 45.3 (9.90) |
| Minimum, maximum | 18.9, 75.4 | 22.4, 68.1 | 18.9, 75.4 | 20.1, 58.0 | 20.9,62.9 | 26.6, 60.6 | 26.1, 68.8 |
| Age group, n (%) | 2597 | 477 | 1782 | 90 | 145 | 57 | 46 |
| 17-29 | 223 (8.6) | 40 (8.4) | 138 (7.7) | 9 (10.0) | 30 (20.7) | 4 (7.0) | 2 (4.3) |
| 30-39 | 665 (25.6) | 77 (16.1) | 474 (26.6) | 19 (21.1) | 56 (38.6) | 25 (43.9) | 14 (30.4) |
| 40-49 | 866 (33.3) | 152 (31.9) | 589 (33.1) | 43 (47.8) | 39 (26.9) | 27 (47.4) | 16 (34.8) |
| 50-59 | 678 (26.1) | 171 (35.8) | 460 (25.8) | 19 (21.1) | 17 (11.7) | 0 (0) | 11 (23.9) |
| >60 | 165 (6.4) | 37 (7.8) | 121 (6.8) | 0 (0) | 3 (2.1) | 1 (1.8) | 3 (6.5) |
| Missing | 640 | 235 | 0 | 330 | 47 | 11 | 17 |
| BMI | 2568 | 484 | 1773 | 95 | 109 | 57 | 50 |
| Mean (SD) | 28.9 (4.94) | 29.7 (6.81) | 28.7 (4.7) | 25.6 (2.81) | 32.6 (7.50) | 27.6 (4.11) | 29.0 (3.72) |
| Minimum, maximum | 19.0, 64.5 | 19.0, 64.5 | 22.7, 55.6 | 19.5, 32.9 | 20.5,57.1 | 19.8, 41.5 | 18.0, 40.9 |
| BMI groups, n (%) | 2567 | 484 | 1773 | 95 | 109 | 57 | 49 |
| <18.49 | 2 (0.01) | 0 (0) | 2 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 18.5-24.9 | 826 (32.2) | 97 (20.0) | 637 (35.9) | 59 (62.1) | 15 (13.8) | 13 (22.8) | 5 (10.2) |
| 25-29.9 | 1296 (50.5) | 226 (46.7) | 951 (53.6) | 30 (31.6) | 30 (27.5) | 31 (54.4) | 28 (57.1) |
| 30-34.9 | 305 (11.9) | 91 (18.8) | 152 (8.6) | 6 (6.3) | 31 (28.4) | 11 (19.3) | 14 (28.6) |
| 35-39.9 | 55 (2.1) | 20 (4.1) | 17 (1.0) | 0 (0) | 17 (15.6) | 0 (0) | 1 (2.0) |
| >40 | 83 (3.2) | 50 (10.3) | 14 (0.7) | 0 (0) | 16 (14.7) | 2 (3.5) | 1 (2.0) |
| Missing | 670 | 228 | 9 | 325 | 83 | 11 | 14 |
| Comorbidities n (%) | 2708 | 711 | 1782 | 84 | — | 68 | 63 |
| Diabetes | 169 (6.2) | 62 (8.7) | 99 (5.5) | 0 (0) | — | 1 (1.5) | 7 (11.1) |
| Hypertension | 209 (7.7) | 124 (17.4) | 65 (3.6) | 8 (9.5) | — | 4 (5.9) | 8 (12.7) |
| Hyperlipidemia | 18 (0.7) | 6 (0.8) | 12 (0.7) | 0 (0) | — | 0 (0) | 0 (0) |
| Obstructive sleep apnea | 99 (3.7) | 84 (11.8) | 9 (0.5) | 0 (0) | — | 0 (0) | 6 (9.5) |
| Osteoarthritic disease | 227 (8.4) | 132 (18.6) | 81 (4.5) | 7 (8.3) | — | 3 (4.4) | 4 (6.3) |
| Cardiovascular or coagulation disease | 85 (3.1) | 55 (7.7) | 23 (1.3) | 4 (4.8) | — | 2 (2.9) | 1 (1.6) |
| Psychiatric | 191 (7.1) | 95 (13.4) | 83 (4.7) | 9 (10.7) | — | 1 (1.5) | 3 (4.8) |
| Reflux disease | 129 (4.8) | 103 (14.5) | — | 12 (14.3) | — | 9 (13.2) | 5 (7.9) |
| No medical condition | 1905 (70.3) | 365 (51.3) | 1441 (80.1) | 28 (33.3) | — | 54 (79.4) | 17 (27.0) |
| Missing | 337 | 1 | 0 | 336 | — | 0 | 0 |
| Type of weight loss surgery n (%) | 2111 | 552 | 1452 | 20 | — | 54 | 33 |
| LRYGB (gastric bypass) | 1554 (73.6) | 341 (61.7) | 1.141 (78.5) | 5 (6.0) | — | 37 (68.5) | 30 (90.9) |
| LSG (gastric sleeve) | 507 (24.0) | 198 (35.8) | 292 (20.1) | 7 (8.3) | — | 7 (13.0) | 3 (0.9) |
| Gastric banding | 41 (1.9) | 12 (2.2) | 17 (1.2) | 2 (2.4) | — | 10 (18.5) | 0 (0) |
| Other | 9 (0.4) | 1 (0.1) | 2 (0.1) | 6 (7.1) | — | 0 (0) | 0 (0) |
| No surgery | 25 (1.2) | 1 (0.1) | — | 11 (13.1) | — | 2 (3.7) | 11 (33.3) |
| Area of body contouring surgery n (%) | |||||||
| Abdomen | 1532 (51.2) | 345 (48.6) | 755 (49.1) | 180 (42.8) | 192 (100) | 31 (45.3) | 29 (46.2) |
| Breast | 459 (15.3) | 165 (23.2) | 189 (12.3) | 85 (20.2) | — | 6 (9.3) | 14 (22.0) |
| Back | 270 (9.0) | 67 (9.4) | 180 (11.7) | 15 (3.6) | — | 5 (6.8) | 3 (4.1) |
| Arms | 297 (9.9) | 106 (14.9) | 129 (8.41) | 45 (10.7) | — | 10 (14.0) | 7 (11.8) |
| Inner thighs | 346 (11.6) | 146 (20.6) | 162 (10.5) | 30 (7.1) | — | 5 (8.0) | 3 (4.1) |
| Hips and outer thighs | 182 (6.1) | 102 (14.3) | 28 (1.80) | 40 (9.5) | — | 7 (10.3) | 5 (7.9) |
| Buttocks | 231 (7.7) | 106 (14.8) | 94 (6.1) | 25 (6.0) | — | 4 (6.4) | 2 (2.7) |
BMI, body mass index; SD, standard deviation.
The Minimal Important Difference
BODY-Q baseline 0.2 SD MID scores are presented in Table 2. The MID estimate was between 1 and 5 in the HRQL domain and between 3 and 6 in the appearance domain. The MID baseline estimate was 2 for psychological, 1 for social, 2 for sexual, 2 for physical function, and 2 for body image on the HRQL scales. On the appearance scales, the MID estimates were 3 for body, 5 for abdomen, 6 for back, 5 for buttocks, 5 for arms, 5 for inner thighs, 6 for hips and outer thighs, 6 for nipples, 5 for chest, 6 for stretch marks, 4 for skin, and 5 for scars.
Table 2.
Baseline Minimal Important Difference Values
| Appearance | n | SD | 0.2 SD | MID estimate |
|---|---|---|---|---|
| Appearance | ||||
| Body | 2382 | 15.30 | 3.06 | 3 |
| Abdomen | 2408 | 27.21 | 5.44 | 5 |
| Back | 2389 | 29.82 | 5.96 | 6 |
| Buttocks | 2369 | 26.33 | 5.27 | 5 |
| Arms | 2391 | 27.29 | 5.46 | 5 |
| Inner thighs | 2355 | 26.82 | 5.36 | 5 |
| Hips and outer thighs | 2358 | 29.95 | 5.99 | 6 |
| Chest | 261 | 23.29 | 4.66 | 5 |
| Nipples | 259 | 28.75 | 5.75 | 6 |
| Stretch marks | 2066 | 28.48 | 5.70 | 6 |
| Skin | 2341 | 18.24 | 3.65 | 4 |
| Scars | 66 | 25.16 | 5.03 | 5 |
| Health-related quality of life | ||||
| Psychological | 1998 | 7.83 | 1.57 | 2 |
| Social | 1997 | 6.14 | 1.22 | 1 |
| Sexual | 1980 | 7.39 | 1.47 | 2 |
| Physical | 1235 | 11.89 | 2.38 | 2 |
| Body image | 2001 | 8.61 | 1.72 | 2 |
MID, minimal important difference; SD, standard deviation.
The 0.2 SRMs from baseline to 3 years postoperatively for the HRQL and appearance domains are presented in Tables 3-5. The MID estimates based on change from baseline to 3 years postoperatively were between 4 and 8. On the Rasch transformed 0 to 100 scale, the MID estimate based on change from baseline was 4 for psychological, 3 for social, 4 for sexual, 4 for physical function, and 5 for body image on the HRQL scales. On the appearance scales, the MID estimates were 4 for body, 7 for abdomen, 6 for back, 5 for buttocks, 5 for arms, 6 for inner thighs, 5 for hips and outer thighs, 5 for nipples, 4 for chest, 4 for stretch marks, 5 for skin, and 3 for scars. This meant, for each surgical site, BODY-Q scores equal to or above the MID estimate represented a meaningful change in scores.
Table 3.
Mean Minimal Important Difference Values From Baseline to 3 Years Follow-up on Health-related Quality of Life Scales
| BODY-Q BCS | Psychological | Social | Sexual | Physical | Body image | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate |
| 6 months post-BCS | 543 | 4.12 | 4 | 541 | 3.51 | 4 | 531 | 5.02 | 5 | 343 | 4.47 | 4 | 546 | 4.80 | 5 |
| 1 year post-BCS | 389 | 4.18 | 4 | 385 | 3.51 | 4 | 378 | 5.04 | 5 | 290 | 4.88 | 5 | 386 | 5.00 | 5 |
| 2 years post-BCS | 121 | 4.67 | 5 | 119 | 3.38 | 3 | 119 | 5.35 | 5 | 88 | 4.24 | 4 | 123 | 5.52 | 6 |
| 3 years post-BCS | 31 | 4.57 | 5 | 31 | 3.77 | 4 | 31 | 5.71 | 6 | 20 | 6.65 | 7 | 31 | 5.46 | 5 |
| Mean | 4 | 3 | 4 | 4 | 5 | ||||||||||
BCS, body contouring surgery; MID, minimal important difference; SRM, standardized response mean.
Table 5.
Mean Minimal Important Difference Values From Baseline to 3 Years Follow-up on Appearance Scales, Part 2
| BODY-Q BCS | Nipples | Chest | Stretch marks | Skin | Scars | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate |
| 6 months post-BCS | 46 | 5.97 | 6 | 46 | 5.64 | 6 | 475 | 5.03 | 5 | 530 | 5.73 | 6 | 7 | 4.43 | 4 |
| 1 year post-BCS | 28 | 4.80 | 5 | 30 | 5.56 | 6 | 272 | 4.93 | 5 | 354 | 5.74 | 6 | 45 | 4.54 | 5 |
| 2 years post-BCS | 7 | 11.6 | 12 | 8 | 6.30 | 6 | 100 | 4.73 | 5 | 116 | 5.84 | 6 | 2 | 8.34 | 8 |
| 3 years post-BCS | 1 | — | — | 1 | — | — | 29 | 4.92 | 5 | 31 | 5.71 | 6 | 0 | — | — |
| Mean | 5 | 4 | 4 | 5 | 3 | ||||||||||
BCS, body contouring surgery; MID, minimal important difference; SRM, standardized response mean.
Table 4.
Mean Minimal Important Difference Values From Baseline to 3 Years Follow-up on Appearance Scales, Part 1
| BODY-Q BCS | Body | Abdomen | Back | Buttocks | Arms | Inner thighs | Hips and outer thighs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate | n | 0.2 SRM | MID estimate |
| 6 months post-BCS | 552 | 3.87 | 4 | 552 | 8.01 | 8 | 548 | 6.24 | 6 | 542 | 5.75 | 6 | 552 | 5.29 | 5 | 543 | 5.33 | 5 | 542 | 6.45 | 6 |
| 1 year post-BCS | 402 | 4.25 | 4 | 402 | 7.72 | 8 | 396 | 6.43 | 6 | 391 | 4.91 | 5 | 400 | 5.95 | 6 | 390 | 6.42 | 6 | 392 | 6.44 | 6 |
| 2 years post-BCS | 120 | 4.42 | 4 | 123 | 7.88 | 8 | 119 | 7.01 | 7 | 117 | 5.74 | 6 | 120 | 6.98 | 7 | 118 | 6.64 | 7 | 117 | 7.34 | 7 |
| 3 years post-BCS | 31 | 3.82 | 4 | 31 | 8.21 | 8 | 30 | 9.01 | 9 | 31 | 6.23 | 6 | 36 | 7.04 | 7 | 31 | 7.99 | 8 | 31 | 5.58 | 6 |
| Mean | 4 | 7 | 6 | 5 | 5 | 6 | 5 | ||||||||||||||
BCS, body contouring surgery; MID, minimal important difference; SRM, standardized response mean.
Proportion of Patients Achieving Minimal Important Difference Scores
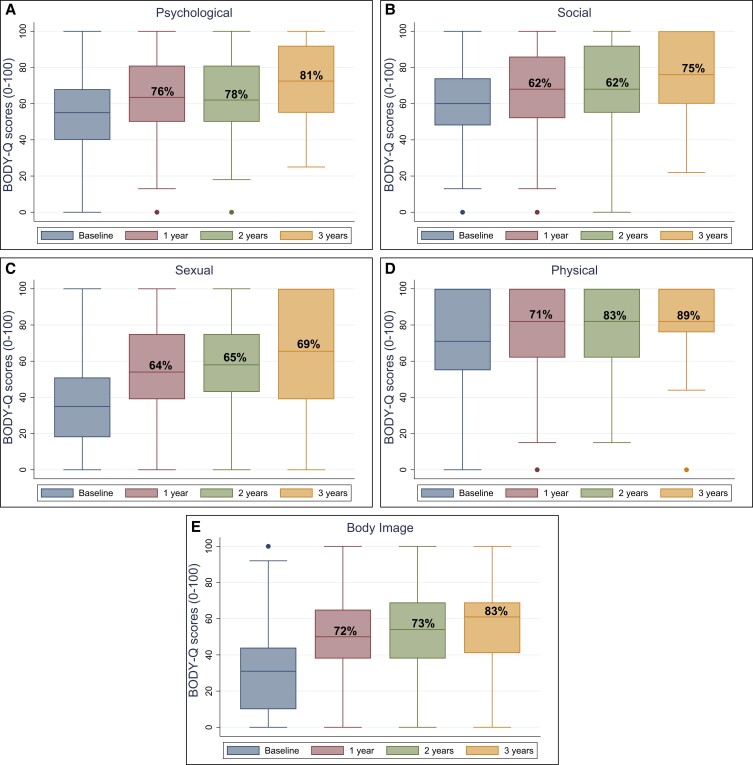
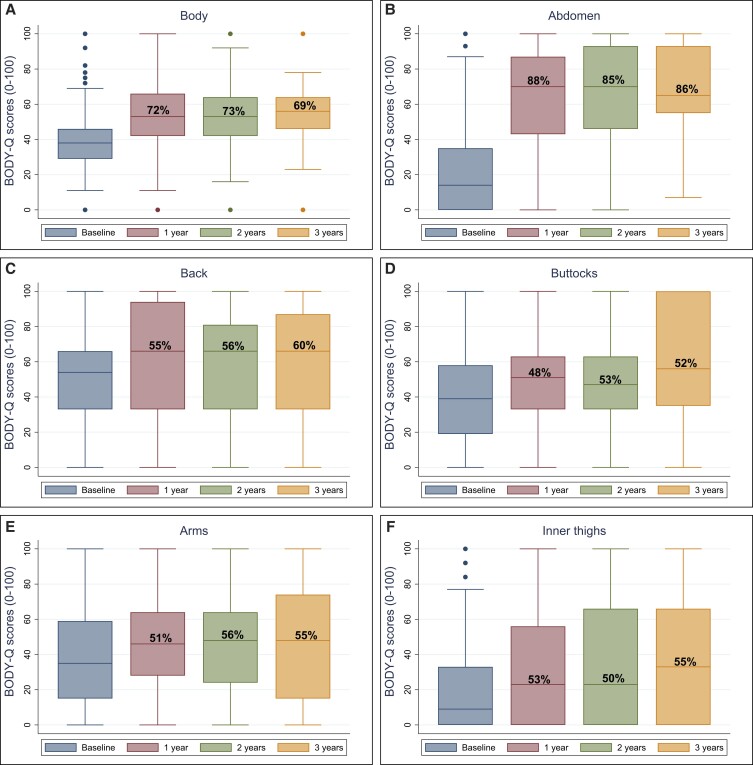
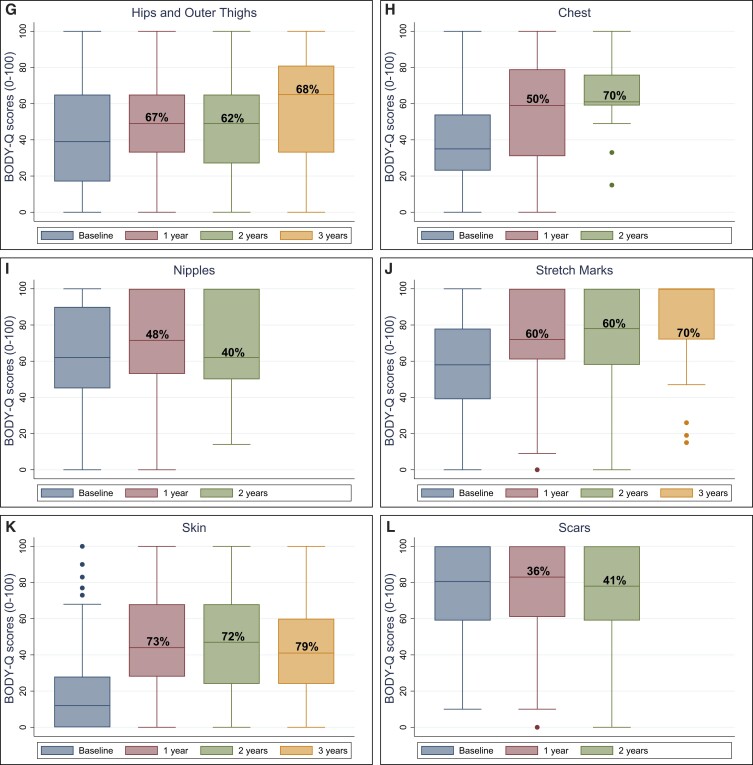
The median BODY-Q scores for each HRQL and appearance domain are presented in Figures 1 and 2, and in Supplemental Table 2 available online at www.aestheticsurgeryjournal.com, displaying the total number of patients on each scale and percentage of patients who achieved the estimated mean MID. All patients on average scored higher after BCS. The only exception was the nipple scale, for which the score declined 2 years after BCS.
Figure 1.
BODY-Q health-related quality of life scale scores (A-E) from baseline to 3 years after body contouring surgery.
Figure 2.
BODY-Q appearance scale scores (A-L) from baseline to 3 years after body contouring surgery.
BMI, Gender, and Age Stratified Minimal Important Difference
The 0.2 SDs from baseline stratified by age, gender, and BMI groups are presented in Supplemental Table 3, available online at www.aestheticsurgeryjournal.com. On all scales, age group 50 to 59 years had higher MID scores than younger and older age groups. Overall, there were no differences in BMI groups or between male and female participants.
DISCUSSION
In this study, BODY-Q MID scores were estimated based on 3237 patients undergoing BCS with the distribution-based method. At baseline, the MID estimates ranged from 1 to 5 on the HRQL scale and from 3 to 6 on the appearance scale. The mean change of MID from baseline to 3-year follow-up ranged from 4 to 5 on HRQL and from 4 to 8 on the appearance scale. BMI and gender stratified MIDs showed no differences, and the age group of 50 to 59 years scored higher MID scores than younger and older patients.
For all scales, the median BODY-Q scores improved and stabilized after BCS, except for the nipple scale, for which the median score declined 2 years postoperatively. This decline was probably due to the small sample size in the 2-year postoperative group, because only 10 patients were included at that time. The BODY-Q chest and nipple scales were relevant only for male participants. In a recent multinational longitudinal study of up to 10-year follow-up on patients undergoing bariatric surgery with or without subsequent BCS, all patients scored higher on all BODY-Q scales after BCS.32 However, surgery around the areola may disrupt normal appearance, cause asymmetry, or altered sensation, which may be undesirable for these participants. It is also known that post-MWL breast surgery is difficult to perform with long-lasting results due to skin quality.33
The distribution-based method was utilized to estimate the MID scores. Overall, there is no consensus regarding a “best-practice” approach for estimating a MID.24 The US Food and Drug Administration (FDA) recommends either the anchor-based method exclusively or in a combination with the distribution-based method, because the change in patient scores are linked to a meaningful external anchor, and so incorporate the patient's perspective.34 Because anchor-based approaches prioritize the patient's perspective, this recommendation is often made for an optimal estimation of MIDs.21,35 However, the anchor-based method is limited by the selection of appropriate anchors, because the subjective choice of individual patients may not be generalizable.36 Although the distribution-based method depends on the variability of the sample, it might not necessarily reflect the patient's perspective.36,37 Due to this limitation, the mean SRM was estimated, which is not dependent on the sample's baseline heterogeneity.21,38 For clinical care and research, assessing individuals or a larger group of patients undergoing BCS, we recommend the mean SRM as a MID reference value, because the mean change of scores is more representative than baseline values.21,36,37 Additionally, the outcomes of multiple centers from 6 European countries were combined in this study to increase the generalizability of the MID estimates. No other BCS-specific PROMs exist with established MID scores. In the plastic surgical literature, MIDs have been established for the BREAST-Q with the distribution-based method, as in our study.22,31,39 Similar to this study, they found MIDs ranging from 3 to 5 points.22,31
Without an established MID, it can be difficult to determine if a significant change in score represents a clinically meaningful change. Therefore clinicians can more accurately assess whether or not a treatment has resulted in a clinically meaningful improvement in a patient's HRQL and satisfaction with appearance with BODY-Q MIDs.21 The MIDs also enable exploration of results from previous and future BODY-Q studies. As an example, in a previous BODY-Q study by Poulsen et al, patients who underwent BCS had a score difference of 9.3 between preoperative and postoperative scores on the physical scale. Because our MID score based on the mean 0.2 SRM is 5, this change constitutes a meaningful change for the patients and not only a statistical significant difference.3 Or, in the opposite case, if a study shows a nonsignificant difference between preoperative and postoperative BODY-Q scores on an individual scale, for example, the physical scale, but the change in scores is above the 5, the mean MID for physical based on these results, the change can be interpreted as meaningful for the patients irrespective of the statistical significance.23
Previous research has demonstrated the negative impact of excess skin following MWL.40 Although this significantly improves after BCS, the healthcare budget remains limited for the reimbursement of BCS following MWL.3,32,41 This limitation is particularly notable when considering the reimbursement of BCS as an indispensable part of postbariatric surgical care.42 In this study, abdominal BCS was the most frequently performed procedure (51.2% of all BCS), with 86% of patients achieving the estimated MID for the BODY-Q abdomen scale postoperatively. Furthermore, between 69% and 89% of patients achieved the estimated MID across all HRQL scales. These results underscore the particular importance of abdominal BCS in improving HRQL and patient satisfaction with appearance during the post–weight loss period. These findings advocate for the incorporation of such procedures in holistic patient care following MWL and for the reimbursement of BCS by healthcare systems.
In a retrospective follow-up, Altieri et al demonstrated that only 6% of patients who had bariatric surgery subsequently underwent BCS, although 70% to 90% of patients following MWL report significant excess skin.1,2,43 The discrepancy between the number of patients desiring BCS and the uptake of BCS after bariatric surgery may be due to limited insurance coverage and income, which can influence the decision to undergo BCS, particularly in countries where BCS is not publicly funded.44,45 In the included countries in this study, BCS was either available through the public health system within specified criteria (Denmark and Finland), through insurance companies (the Netherlands, Germany, Italy), or a national healthcare insurer (Poland).46-50 However, in many countries, BCS is considered cosmetic surgery, and therefore is not reimbursed by insurance companies or national health systems.51 Utilizing the estimated MIDs in future BODY-Q BCS studies is crucial for demonstrating the positive impact that BCS has on patients’ HRQL following MWL and underscoring the need to include BCS as a reimbursed component of postbariatric surgery care.
To the best of our knowledge, this study is the first effort to establish BODY-Q MIDs for patients undergoing BCS. Strengths of this study included the large, multicenter cohort of BCS patients from 6 European countries gathered to estimate the MIDs. The relatively large sample size of 2253 patients should not influence the magnitude of the MIDs because it is expressed as an average variation around a mean value, but it is expected to improve the precision of the MIDs.21 In addition, we adjusted for potential clinical differences such as BMI, gender, and age by conducting subgroup analyses. These analyses confirmed that BMI and gender did not influence the MID estimates. Only the age group between 50-59 years was associated with higher MID scores when compared to younger and older patients.
These MID scores will enable sample size calculation when designing clinical research to obtain an adequately powered study to answer a research question.52 The MID is an essential component of estimating a required sample size and should be part of the design of future studies that incorporate the BODY-Q.
This study had several limitations. All data were solely patient reported; therefore, we were unable to characterize the specific BCS technique, number of previous BCSs, or complications during or after surgery. The sample sizes varied across countries, with the majority of participants from Denmark (55.1%) and the Netherlands (22.0%).
We only had baseline characteristics from Finland, Germany, Italy, and Poland. Therefore, we lacked follow-up data regarding patient characteristics such as BMI and change of BMI during the study period. Another limitation may be the use of the distribution-based method to estimate the MID. This method exclusively depends on statistical indicators, which might not reflect the clinical relevance of variations in the PRO sufficiently. This might yield MID estimates that lack sufficient relevance for patients. Furthermore, multiple approaches are described for the distribution-based method, including different effect sizes and SRM values.17,53 In this study, we chose a conservative approach (0.2 SD) for the BODY-Q MID scores in a BCS population, based on a review by Samsa et al.21,30,52,53 In the future, a combined approach with the distribution-based and anchor-based methods should be applied due to the limitations of each method. Therefore we acknowledge that future studies may report different MID values for patients undergoing BCS.36 However, until these values obtained with the anchor-based method become available, the estimates presented in this study are recommended for clinical and research use.
CONCLUSIONS
In this study, the estimated MID for the BODY-Q from baseline to 3 years postoperatively ranged from 4 to 5 on the HRQL scales and from 4 to 8 on the appearance scales. These findings provide meaningful insights and guidance for the interpretation of the BODY-Q scores in previous and future studies, improving the clinical and research utility of this PROM for understanding the impact of BCS on patients’ lives. In the future, a combined methodology comprising the distribution-based and anchor-based methods is recommended for the estimation of BODY MID scores.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Supplementary Material
Acknowledgments
The authors sincerely thank and appreciate the enormous contributions of Dr Andreas Printzlau and Printzlau Private Hospital, Virum, Denmark, for enrolling patients. They also thank all participants for their contributions through the completion of the BODY-Q questionnaire.
Disclosures
The BODY-Q is developed by Drs Klassen, Pusic, and Cano, and they all receive a share of any license revenues based on institutions’ inventor sharing policy. Dr Klassen is the owner of EVENTUM Research, which provides consulting services to the pharmaceutical industry. Dr Cano is CSO of Modus Outcomes, a Division of Thread (Cary, NC). The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This study was funded by the following grants: the Region of Southern Denmark (21/17592); the Odense University Hospital PhD Foundation (A5006); and the Jascha Fund (2021-0183). Dr Kaur is funded by the Canadian Institutes for Health Research Fellowship Award (2020-2024).
REFERENCES
- 1. Baillot A, Brais-Dussault E, Bastin A, et al. What is known about the correlates and impact of excess skin after bariatric surgery: a scoping review. Obes Surg. 2017;27(9):2488–2498. doi: 10.1007/s11695-017-2814-3 [DOI] [PubMed] [Google Scholar]
- 2. Aldaqal SM, Samargandi OA, El-Deek BS, Awan BA, Ashy AA, Kensarah AA. Prevalence and desire for body contouring surgery in postbariatric patients in Saudi Arabia. N Am J Med Sci. 2012;4(2):94–98. doi: 10.4103/1947-2714.93386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poulsen L, Rae C, Simonsen N, et al. Body contouring surgery improves long-term satisfaction with appearance and health-related quality of life after bariatric surgery: BODY-Q results from a Danish cohort study. Plast Reconstr Surg. 2023;151(6):1307–1316. doi: 10.1097/PRS.0000000000010165 [DOI] [PubMed] [Google Scholar]
- 4. University of Oxford Department of Public Health . Patient-reported outcome measurement: a structured review of patient-reported outcome measures for patients undergoing cosmetic surgical procedures. Accessed November 8, 2023. https://www.ndph.ox.ac.uk/files/research/cosmetic-surgery-proms-review2013.pdf. 2013.
- 5. Klassen AF, Cano SJ, Alderman A, et al. The BODY-Q: a patient-reported outcome instrument for weight loss and body contouring treatments. Plast Reconstr Surg Glob Open. 2016;4(4):e679. doi: 10.1097/GOX.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vries CEE, Kalff MC, Prinsen CAC, et al. Recommendations on the most suitable quality-of-life measurement instruments for bariatric and body contouring surgery: a systematic review. Obes Rev. 2018;19(10):1395–1411. doi: 10.1111/obr.12710 [DOI] [PubMed] [Google Scholar]
- 7. Barone M, Cogliandro A, Salzillo R, Tambone V, Persichetti P. Patient-reported satisfaction following post-bariatric surgery: a systematic review. Aesthetic Plast Surg. 2018;42(5):1320–1330. doi: 10.1007/s00266-018-1146-6 [DOI] [PubMed] [Google Scholar]
- 8. Poulsen L, Klassen A, Rose M, et al. Patient-reported outcomes in weight loss and body contouring surgery: a cross-sectional analysis using the BODY-Q. Plast Reconstr Surg. 2017;140(3):491–500. doi: 10.1097/PRS.0000000000003605 [DOI] [PubMed] [Google Scholar]
- 9. de Vries CEE, Klassen AF, Hoogbergen MM, Alderman AK, Pusic AL. Measuring outcomes in cosmetic abdominoplasty: the BODY-Q. Clin Plast Surg. 2020;47(3):429–436. doi: 10.1016/j.cps.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 10. Mou D, DeVries CEE, Pater N, et al. BODY-Q patient-reported outcomes measure (PROM) to assess sleeve gastrectomy vs. Roux-en-Y gastric bypass: eating behavior, eating-related distress, and eating-related symptoms. Surg Endosc. 2021;35(8):4609–4617. doi: 10.1007/s00464-020-07886-w [DOI] [PubMed] [Google Scholar]
- 11. Christopher AN, Morris MP, Patel V, Broach RB, Fischer JP. Abdominal body contouring: does body mass index affect clinical and patient reported outcomes? J Surg Res. 2021;270:348–358. doi: 10.1016/j.jss.2021.09.035 [DOI] [PubMed] [Google Scholar]
- 12. Elfanagely O, Mauch JT, Mellia JA, et al. Quality of life and concurrent procedures in truncal body contouring patients: a single-center retrospective study. Aesthetic Plast Surg. 2021;45(4):1620–1627. doi: 10.1007/s00266-021-02129-2 [DOI] [PubMed] [Google Scholar]
- 13. Dalaei F, de Vries CEE, Cano SJ, et al. BODY-Q normative scores: psychometric validation of the BODY-Q in the general population in Europe and North America. Plast Reconstr Surg Glob Open. 2023;11(11):e5401. doi: 10.1097/GOX.0000000000005401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalaei F, de Vries CEE, Poulsen L, et al. General population normative scores for interpreting the BODY-Q. Clin Obes. 2022;12(4):e12528. doi: 10.1111/cob.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. doi: 10.1001/jama.2014.13128 [DOI] [PubMed] [Google Scholar]
- 16. Brozek JL, Guyatt GH, Schünemann HJ. How a well-grounded minimal important difference can enhance transparency of labelling claims and improve interpretation of a patient reported outcome measure. Health Qual Life Outcomes. 2006;4(1):69. doi: 10.1186/1477-7525-4-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371 [DOI] [PubMed] [Google Scholar]
- 18. Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49(11):1215–1219. doi: 10.1016/S0895-4356(96)00206-5 [DOI] [PubMed] [Google Scholar]
- 19. Terwee CB, Peipert JD, Chapman R, et al. Minimal important change (MIC): a conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual Life Res. 2021;30(10):2729–2754. doi: 10.1007/s11136-021-02925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Vet HC, Terluin B, Knol DL, et al. Three ways to quantify uncertainty in individually applied “minimally important change” values. J Clin Epidemiol. 2010;63(1):37–45. doi: 10.1016/j.jclinepi.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 21. Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1 [DOI] [PubMed] [Google Scholar]
- 22. Chu JJ, Tadros AB, Gallo L, et al. Interpreting the BREAST-Q for breast-conserving therapy: minimal important differences and clinical reference values. Ann Surg Oncol. 2023;30(7):4075 4084. doi: 10.1245/s10434-023-13222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mouelhi Y, Jouve E, Castelli C, Gentile S. How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health Qual Life Outcomes. 2020;18(1):136. doi: 10.1186/s12955-020-01344-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voineskos SH, Nelson JA, Klassen AF, Pusic AL. Measuring patient-reported outcomes: key metrics in reconstructive surgery. Annu Rev Med. 2018;69(1):467–479. doi: 10.1146/annurev-med-060116-022831 [DOI] [PubMed] [Google Scholar]
- 25. Klassen AF, Cano SJ, Kaur M, Breitkopf T, Pusic AL. Further psychometric validation of the BODY-Q: ability to detect change following bariatric surgery weight gain and loss. Health Qual Life Outcomes. 2017;15(1):227. doi: 10.1186/s12955-017-0802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poulsen L, Pusic A, Robson S, et al. The BODY-Q stretch marks scale: a development and validation study. Aesthet Surg J. 2018;38(9):990–997. doi: 10.1093/asj/sjy081 [DOI] [PubMed] [Google Scholar]
- 27. de Vries CEE, Mou D, Poulsen L, et al. Development and validation of new BODY-Q scales measuring expectations, eating behavior, distress, symptoms, and work life in 4004 adults from 4 countries. Obes Surg. 2021;31(8):3637–3645. doi: 10.1007/s11695-021-05462-2 [DOI] [PubMed] [Google Scholar]
- 28. Klassen AF, Kaur M, Poulsen L, et al. Development of the BODY-Q chest module evaluating outcomes following chest contouring surgery. Plast Reconstr Surg. 2018;142(6):1600–1608. doi: 10.1097/PRS.0000000000004978 [DOI] [PubMed] [Google Scholar]
- 29. Cohen J. Statistical Power Analysis for the Behavioral Science, 2nd ed. Academic Press; 1988. [Google Scholar]
- 30. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15(2):141–155. doi: 10.2165/00019053-199915020-00003 [DOI] [PubMed] [Google Scholar]
- 31. Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ. Giving meaning to differences in BREAST-Q scores: minimal important difference for breast reconstruction patients. Plast Reconstr Surg. 2020;145(1):11e–20e. doi: 10.1097/PRS.0000000000006317 [DOI] [PubMed] [Google Scholar]
- 32. Dalaei F, de Vries CEE, Poulsen L, et al. Body contouring surgery after bariatric surgery improves long-term health-related quality of life and satisfaction with appearance: an international longitudinal cohort study using the BODY-Q. Ann Surg. 2024;279(6):1008–1017. doi: 10.1097/SLA.0000000000006244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barone M, Cogliandro A, Tsangaris E, et al. Treatment of severe gynecomastia after massive weight loss: analysis of long-term outcomes measured with the Italian version of the BODY-Q. Aesthetic Plast Surg. 2018;42(6):1506–1518. doi: 10.1007/s00266-018-1232-9 [DOI] [PubMed] [Google Scholar]
- 34. Administration . UFaD 2020;Pages. https://www.fda.gov/about-fda/cdrh-patient-science-and-engagement-program/clinical-outcome-assessments-coas-medical-device-decision-making on 28.06 2023. Accessed December 2, 2023.
- 35. Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14(2):285–295. doi: 10.1007/s11136-004-0705-2 [DOI] [PubMed] [Google Scholar]
- 36. Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 37. US Food and Drug Administration . (FDA) USFD 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims on 04.05.2023, 2023.
- 38. Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health-related quality of life. J Clin Epidemiol. 2004;57(11):1153–1160. doi: 10.1016/j.jclinepi.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 39. Mundy LR, Homa K, Klassen AF, Pusic AL, Kerrigan CL. Breast cancer and reconstruction: normative data for interpreting the BREAST-Q. Plast Reconstr Surg. 2017;139(5):1046e–1055e. doi: 10.1097/PRS.0000000000003241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berkane Y, Saget F, Lupon E, et al. Abdominoplasty and lower body lift surgery improves the quality of life after massive weight loss: a prospective multicenter study. Plast Reconstr Surg. 2024;153(6):1101e–1110e. doi: 10.1097/PRS.0000000000010683 [DOI] [PubMed] [Google Scholar]
- 41. Mocquard C, Pluvy I, Chaput B, et al. Medial thighplasty improves patient's quality of life after massive weight loss: a prospective multicentric study. Obes Surg. 2021;31(11):4985–4992. doi: 10.1007/s11695-021-05654-w [DOI] [PubMed] [Google Scholar]
- 42. Alvarez AH, Valentine L, Stearns S, et al. A national analysis of socioeconomic variables of access to inpatient body contouring procedures after bariatric surgery. Obes Surg. 2023;33(8):2428–2433. doi: 10.1007/s11695-023-06683-3 [DOI] [PubMed] [Google Scholar]
- 43. Altieri MS, Yang J, Park J, et al. Utilization of body contouring procedures following weight loss surgery: a study of 37,806 patients. Obes Surg. 2017;27(11):2981–2987. doi: 10.1007/s11695-017-2732-4 [DOI] [PubMed] [Google Scholar]
- 44. ElAbd R, Samargandi OA, AlGhanim K, et al. Body contouring surgery improves weight loss after bariatric surgery: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45(3):1064–1075. doi: 10.1007/s00266-020-02016-2 [DOI] [PubMed] [Google Scholar]
- 45. Dreifuss SE, Rubin JP. Insurance coverage for massive weight loss panniculectomy: a national survey and implications for policy. Surg Obes Relat Dis. 2016;12(2):412–416. doi: 10.1016/j.soard.2015.08.509 [DOI] [PubMed] [Google Scholar]
- 46. Repo JP, Homsy P, Uimonen MM, Roine RP, Jahkola T, Popov P. Validation of the Finnish version of the BODY-Q patient-reported outcome instrument among patients who underwent abdominoplasty. J Plast Reconstr Aesthet Surg. 2019;72(6):933–940. doi: 10.1016/j.bjps.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 47. Poulsen L, Roessler KK, Rose M, Bakholdt V, Sørensen JA. [Quality of life of bariatric and body contouring]. Ugeskr Laeger. 2015;177(20):V12140668. [PubMed] [Google Scholar]
- 48. Sadeghi P, Duarte-Bateman D, Ma W, et al. Post-bariatric plastic surgery: abdominoplasty, the state of the art in body contouring. J Clin Med. 2022;11(15):4315. doi: 10.3390/jcm11154315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Vries CEE. Patient-centered outcomes research in bariatric and body contouring surgery. PhD-Thesis - Research and graduation internal, Vrije Universiteit Amsterdam. 2022.
- 50. Paul MA, Opyrchał J, Knakiewicz M, et al. The long-term effect of body contouring procedures on the quality of life in morbidly obese patients after bariatric surgery. PLoS One. 2020;15(2):e0229138. doi: 10.1371/journal.pone.0229138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lo Torto F, Frattaroli JM, Kaciulyte J, et al. Is body-contouring surgery a right for massive weight loss patients? A survey through the European Union National Health Systems. Eur J Plast Surg. 2021;44(4):459–466. doi: 10.1007/s00238-020-01779-w [DOI] [Google Scholar]
- 52. Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant. 2010;25(5):1388–1393. doi: 10.1093/ndt/gfp732 [DOI] [PubMed] [Google Scholar]
- 53. King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):171–184. doi: 10.1586/erp.11.9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.