Abstract
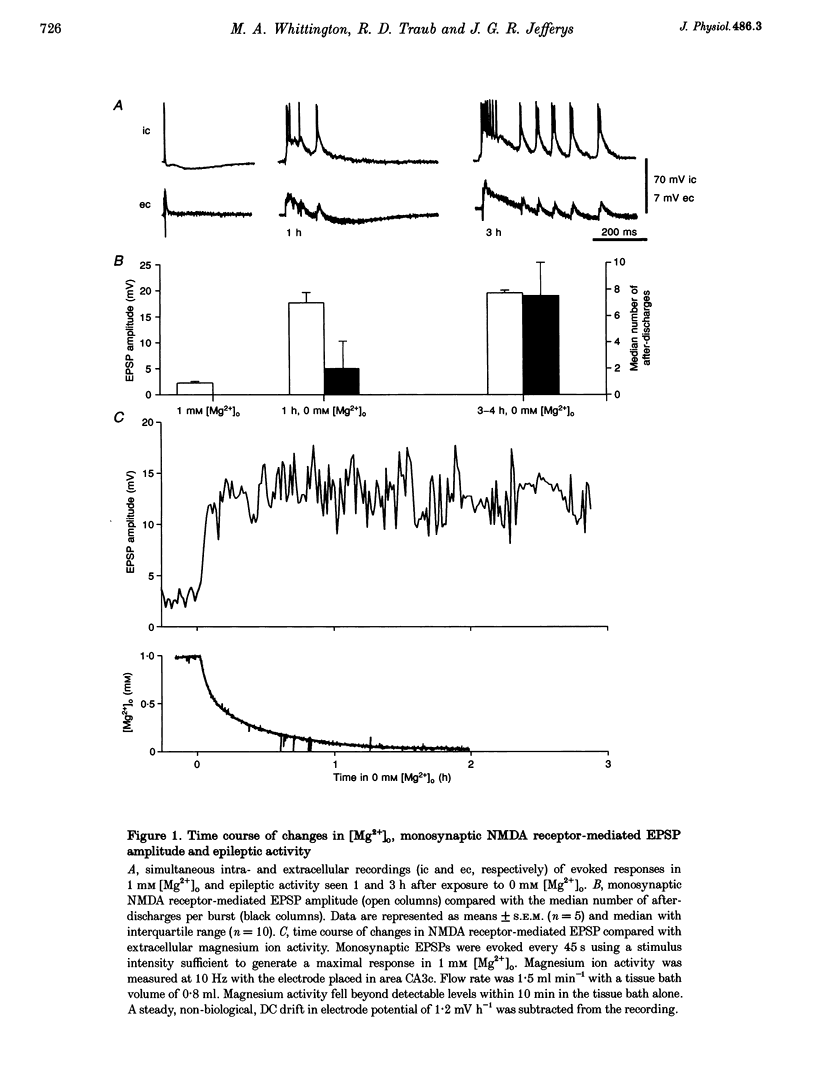
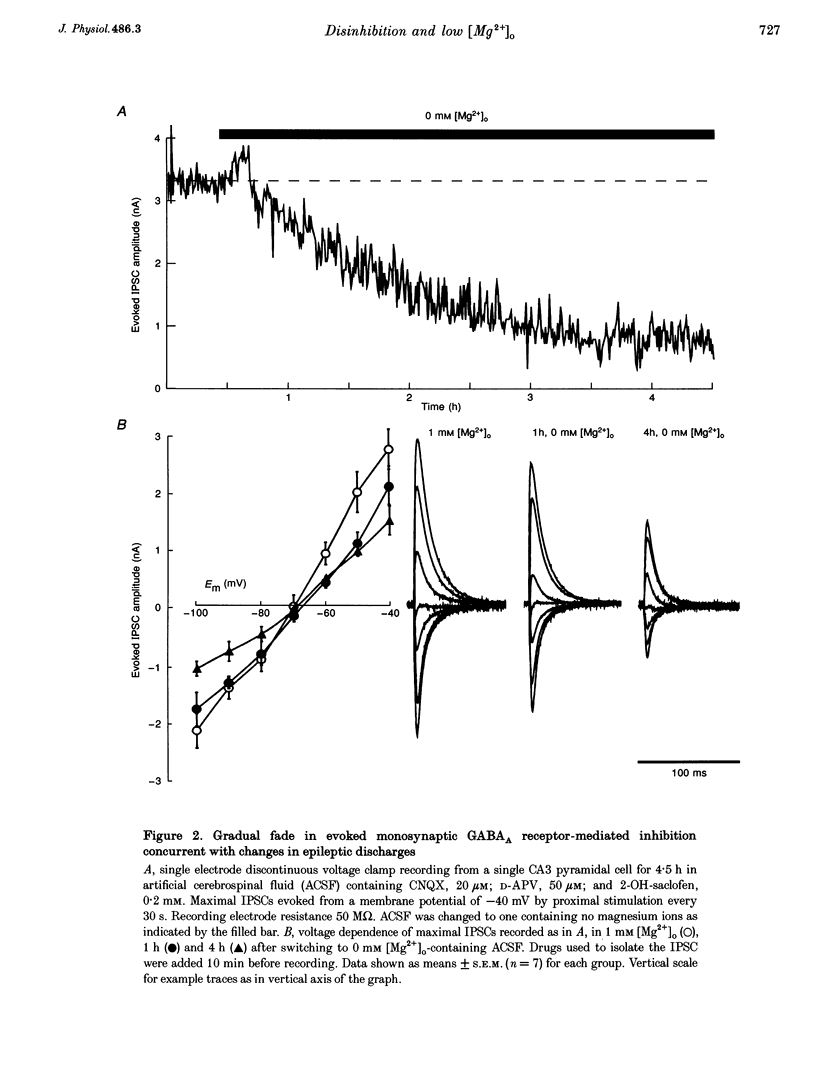
1. Bathing slices of rat hippocampus in media containing no magnesium ions results in epileptic discharges that originate in hippocampal area CA3. These discharges increase in severity gradually over periods of hours. 2. The progression of epileptic activity was much slower than the equilibration of extracellular magnesium activity and the resulting increase in strength of monosynaptic NMDA receptor-mediated excitation. Its time course matched that of a progressive decrease in pharmacologically isolated, evoked GABAA receptor-mediated inhibitory postsynaptic current (IPSC) in the CA3 pyramidal cells. Conductance decreased to 37 +/- 6% of control values after 4 h. Responses to exogenous GABA application decreased to 52 +/- 12%. 3. Maximal IPSC conductance in 0 mM extracellular Mg2+ ([Mg2+]o) also decreased gradually when epileptic activity was abolished by bath application of 20 microM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 microM D-2-amino-5-phosphonovaleric acid (D-APV) throughout the 4 h incubation period. It reached 61 +/- 8% of control values, a significantly smaller decline than that seen after 4 h of epileptic activity. 4. The decrease in mean IPSC conductance only partially reversed when the recording electrode contained 100 mM Mg2+. Complete recovery of IPSC strength occurred when electrodes also contained either 50 mM MgATP or 20 mM BAPTA. Reintroduction of 1 mM [Mg2+]o rapidly abolished epileptic activity and caused a slow, partial increase in IPSC conductance. 5. In the presence of 1 mM [Mg2+]o, GABAA receptor-mediated inhibition had to decrease to 17 +/- 11% of control values, in the presence of 4-7 microM bicuculline, to reach threshold for epileptic activity. 6. These data demonstrate a postsynaptic decrease in GABAA receptor-mediated inhibition in the in vitro low magnesium model of epilepsy. We propose that the apparent leaching of intracellular Mg2+ ([Mg2+]i) from cells leads to loss of ATP and consequent partial dephosphorylation of the GABAA receptor and that this is exacerbated by epileptic activity.
Full text
PDF


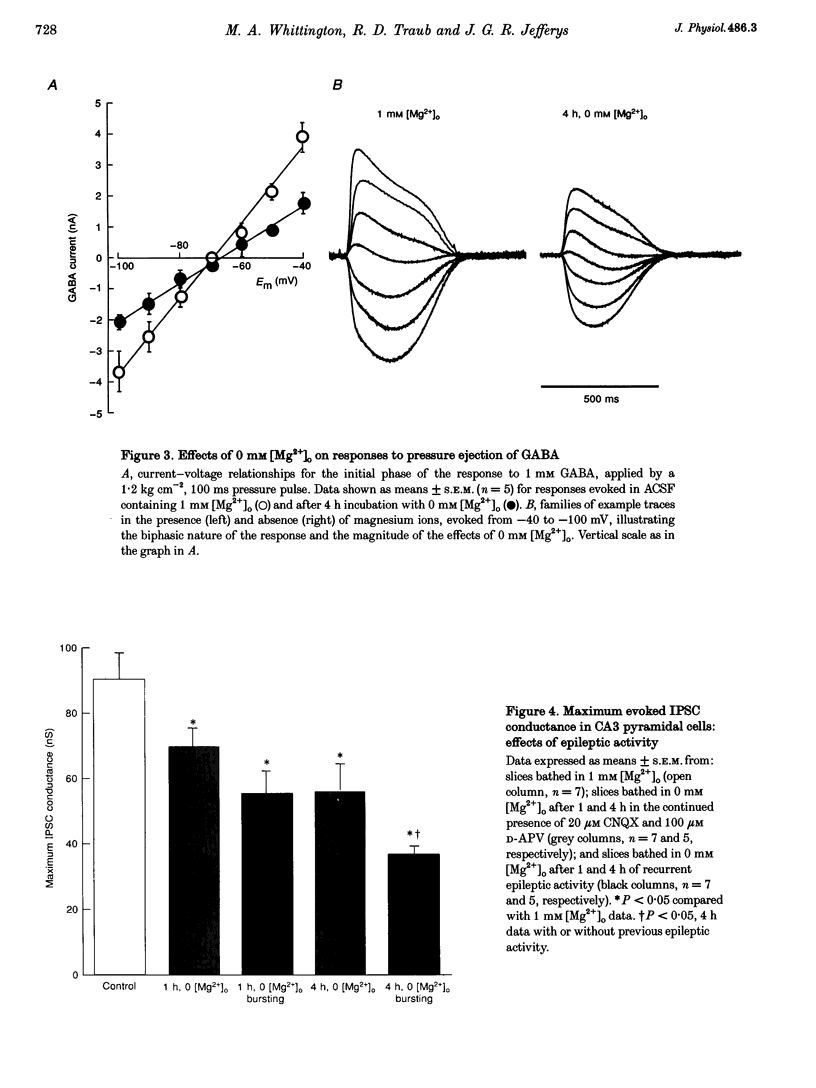
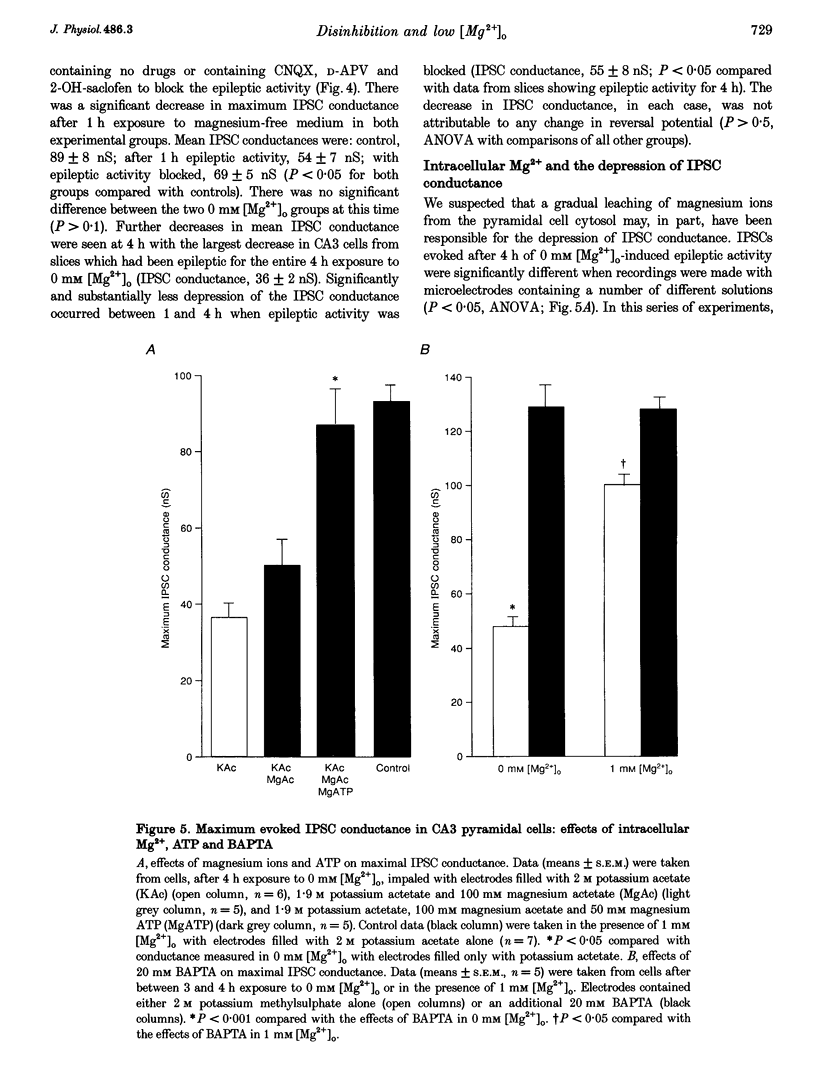
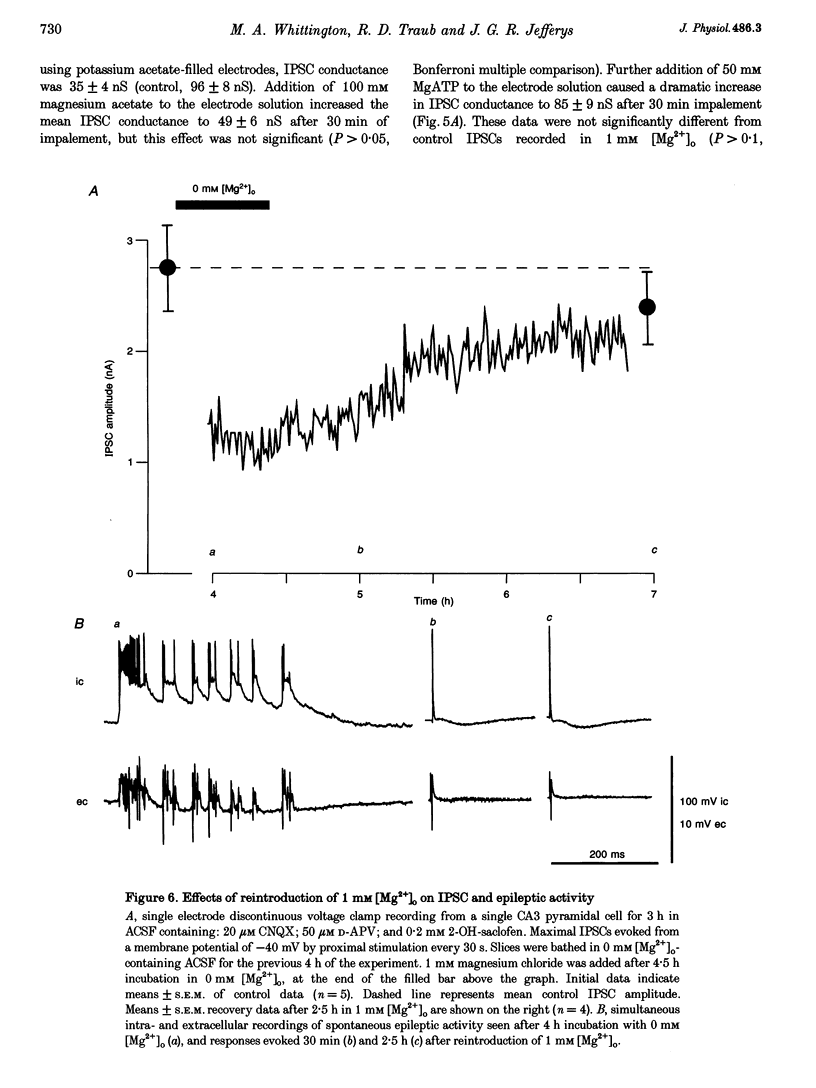
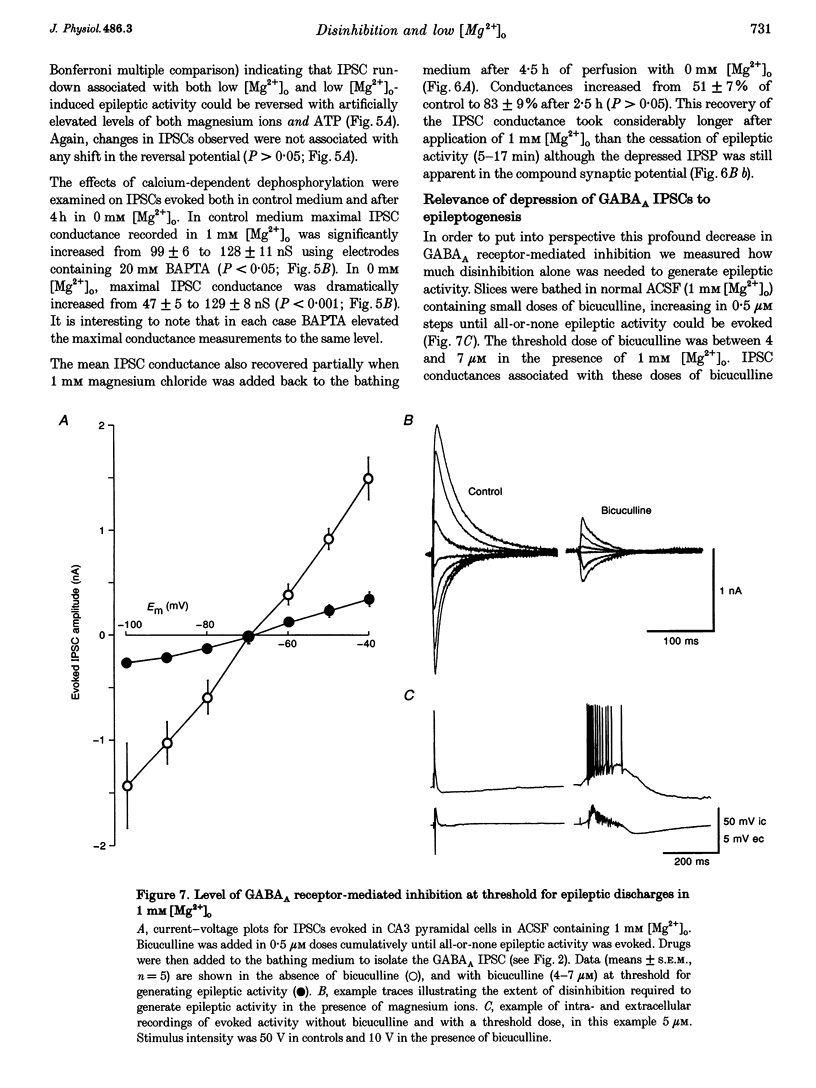



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. W., Lewis D. V., Swartzwelder H. S., Wilson W. A. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986 Nov 19;398(1):215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989 Nov;62(5):1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985 Jan 7;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier J. P., Heinemann U. Late low magnesium-induced epileptiform activity in rat entorhinal cortex slices becomes insensitive to the anticonvulsant valproic acid. Neurosci Lett. 1990 Oct 30;119(1):68–70. doi: 10.1016/0304-3940(90)90757-z. [DOI] [PubMed] [Google Scholar]
- Dreier J. P., Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87(3):581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Durlach J. Apropos d'un cas de forme "comitiale" de tétanie latente par déficit magnésien. Rev Neurol (Paris) 1967 Jul;117(1):189–196. [PubMed] [Google Scholar]
- Haefely W. Benzodiazepine interactions with GABA receptors. Neurosci Lett. 1984 Jun 29;47(3):201–206. doi: 10.1016/0304-3940(84)90514-7. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990 Sep;10(9):3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S. J., Jefferys J. G. Sustained and selective block of IPSPs in brain slices from rats made epileptic by intrahippocampal tetanus toxin. Epilepsy Res. 1992 Apr;11(2):119–129. doi: 10.1016/0920-1211(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Giacchino J. L., Chamberlin N. L., Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987 Jan;57(1):325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G., Bossi L., Morselli P. L., Munari C., Rougier M., Loiseau H. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- Lothman E. W., Hatlelid J. M., Zorumski C. F. Functional mapping of limbic seizures originating in the hippocampus: a combined 2-deoxyglucose and electrophysiologic study. Brain Res. 1985 Dec 23;360(1-2):92–100. doi: 10.1016/0006-8993(85)91224-7. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Michaelis E. K., Michaelis M. L., Chang H. H., Kitos T. E. High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. J Biol Chem. 1983 May 25;258(10):6101–6108. [PubMed] [Google Scholar]
- Mody I., Lambert J. D., Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987 Mar;57(3):869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980 Feb 3;183(1):61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Sessler F. M., Mouradian R. D., Cheng J. T., Yeh H. H., Liu W. M., Waterhouse B. D. Noradrenergic potentiation of cerebellar Purkinje cell responses to GABA: evidence for mediation through the beta-adrenoceptor-coupled cyclic AMP system. Brain Res. 1989 Oct 9;499(1):27–38. doi: 10.1016/0006-8993(89)91132-3. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Aibara K., Akaike N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. J Physiol. 1992 Apr;449:551–572. doi: 10.1113/jphysiol.1992.sp019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N. T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987 Apr 16;326(6114):698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. N-methylaspartate receptors mediate epileptiform activity evoked in some, but not all, conditions in rat neocortical slices. Neuroscience. 1986 Dec;19(4):1161–1177. doi: 10.1016/0306-4522(86)90130-2. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Dingledine R. Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. J Neurophysiol. 1990 Sep;64(3):1009–1018. doi: 10.1152/jn.1990.64.3.1009. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Jefferys J. G., Whittington M. A. Enhanced NMDA conductance can account for epileptiform activity induced by low Mg2+ in the rat hippocampal slice. J Physiol. 1994 Aug 1;478(Pt 3):379–393. doi: 10.1113/jphysiol.1994.sp020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Jefferys J. G. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol. 1993 Feb;461:525–547. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E., Ben-Ari Y. Usefulness of parenteral kainic acid as a model of temporal lobe epilepsy. Rev Electroencephalogr Neurophysiol Clin. 1984 Dec;14(3):241–246. doi: 10.1016/s0370-4475(84)80011-8. [DOI] [PubMed] [Google Scholar]
- Veruki M. L., Yeh H. H. Vasoactive intestinal polypeptide modulates GABAA receptor function in bipolar cells and ganglion cells of the rat retina. J Neurophysiol. 1992 Apr;67(4):791–797. doi: 10.1152/jn.1992.67.4.791. [DOI] [PubMed] [Google Scholar]
- Vink R., Faden A. I., McIntosh T. K. Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J Neurotrauma. 1988;5(4):315–330. doi: 10.1089/neu.1988.5.315. [DOI] [PubMed] [Google Scholar]
- Walther H., Lambert J. D., Jones R. S., Heinemann U., Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986 Aug 29;69(2):156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Walton N. Y., Treiman D. M. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988 Aug;101(2):267–275. doi: 10.1016/0014-4886(88)90010-6. [DOI] [PubMed] [Google Scholar]