Abstract
Background:
The NOVA study (NCT01847274) compared niraparib with placebo as a maintenance treatment for patients with recurrent ovarian cancer (OC) but was not powered to detect an overall survival (OS) improvement.
Objective:
To compare OS in a real-world population of patients with BRCA wild-type (BRCAwt) recurrent OC who received second-line maintenance (2LM) niraparib monotherapy versus active surveillance (AS).
Design:
A retrospective study using a US-based nationwide deidentified electronic health record-derived database.
Methods:
Patients diagnosed with epithelial OC (January 1, 2011–May 31, 2021) who completed second-line (2L) therapy (January 1, 2017–March 2, 2022) and were BRCAwt were included. A NOVA study-like subpopulation included patients with an Eastern Cooperative Oncology Group performance status score of 0–1 and platinum-sensitive disease. Patients were assigned to 2LM niraparib or AS cohorts. Follow-up was measured from the index date (2L non-maintenance therapy end) until the first of study end (May 31, 2022), last clinical activity, or death. Median OS (mOS) and hazard ratios were estimated with an emulated trial methodology.
Results:
The overall population comprised 199 patients in the 2LM niraparib monotherapy cohort and 707 patients in the AS cohort; the NOVA study-like subpopulation included 123 patients in the 2LM niraparib monotherapy cohort and 143 in the AS cohort. Demographic and clinical characteristics were similar in both populations. Overall, adjusted mOS was 24.1 months for the 2LM niraparib monotherapy cohort versus 18.4 months for the AS cohort (hazard ratio, 0.8; 95% confidence interval [CI]: 0.7–0.9). In the NOVA study-like subpopulation, adjusted mOS was 28.1 months for the 2LM niraparib monotherapy cohort versus 21.5 months for the AS cohort (hazard ratio, 0.6; 95% CI: 0.5–0.9).
Conclusion:
These results provide important real-world OS data for patients with recurrent BRCAwt OC who received niraparib monotherapy compared with patients receiving AS.
Keywords: active surveillance, BRCA wild-type, niraparib, observational study, recurrent ovarian cancer, second-line maintenance
Plain language summary
Niraparib maintenance treatment versus monitoring for recurrent ovarian cancer without BRCA mutation
This study examined the real-life survival of patients with ovarian cancer (OC) who received two lines of chemotherapy for OC, known as recurrent OC. We compared two groups: one patient group received a subsequent oral medication called niraparib (a type of maintenance therapy) to delay recurrence, whereas the other group was monitored by their physicians without receiving any medication (also known as active surveillance). This study analyzed health records from across the United States, focusing on patients who were diagnosed with OC between January 2011 and May 2021. Importantly, these patients completed their second line of therapy between January 2017 and March 2022 and had BRCA wild-type disease (meaning that they did not have a specific gene mutation known as BRCA). Patients then received maintenance therapy with niraparib or active surveillance. To assess how long patients lived, patient records were reviewed until the study ended in May 2022, or for patients who did not have data available through that date, until the date of their last visit or death. The study found a significant difference in how long the two groups of patients survived on average. Those patients who received the medication niraparib survived for 24.1 months, compared with 18.4 months for patients in the group that was monitored by their physicians. These results provide valuable insights into the real-life benefits of using niraparib to treat OC outside of a clinical trial. Importantly, these results support the positive outcomes seen in clinical trials, indicating that this medication is a promising option for patients with recurring OC.
Introduction
Ovarian cancer (OC) is the eighth most common cancer among women worldwide. 1 In 2023, there were an estimated 19,710 new OC cases and 13,270 OC-related deaths projected in the United States. 2 Treatment for OC typically involves primary cytoreductive surgery with adjuvant chemotherapy or neoadjuvant chemotherapy with subsequent interval cytoreductive surgery and additional chemotherapy.3,4 Although most patients respond to the initial treatment, approximately 70% of patients with advanced disease experience disease progression 5 and frequently require subsequent therapies. 6 Nevertheless, advances in supportive care and therapeutic options have had a measurable impact on increasing patient survival and consequently the disease prevalence in the United States, which has steadily risen over the past 20 years and accounts for approximately 236,000 patients alive with this diagnosis. 7
After response to chemotherapy, treatment options include active surveillance (AS) or maintenance therapy. In the maintenance setting, poly(ADP-ribose) polymerase (PARP) inhibitors are often prescribed. 6 Niraparib, an oral PARP-1 and PARP-2 inhibitor, has been shown to improve progression-free survival (PFS) in patients with OC while demonstrating a consistent safety profile in first-line (1L) maintenance and recurrent settings.8,9
In NOVA, a randomized, placebo-controlled phase III trial, patients who received niraparib monotherapy in the second-line maintenance (2LM) or later setting had significantly longer median PFS than patients who received placebo, regardless of biomarker status. 8 Specifically, the hazard ratio comparing median PFS for niraparib versus placebo in a germline BRCA (gBRCA) cohort was 0.27 (95% confidence interval [CI]: 0.17–0.41; niraparib, 21.0 months; placebo, 5.5 months); in a non-gBRCA cohort with homologous recombination-deficient tumors, the hazard ratio was 0.38 (95% CI: 0.24–0.59; niraparib, 12.9 months; placebo, 3.8 months); and in an overall non-gBRCA cohort, the hazard ratio was 0.45 (95% CI: 0.34–0.61; niraparib, 9.3 months; placebo, 3.9 months). Although this trial demonstrated a clinical benefit of niraparib maintenance for recurrent OC, it was not powered to detect improvement between cohorts in the secondary efficacy endpoint, overall survival (OS). Real-world studies investigating OS in patients with platinum-sensitive OC treated with niraparib monotherapy in the recurrent setting could help address this gap. It is important to note that in the United States, the country in which this study was conducted, niraparib was initially approved in 2017 as a 2LM or later therapy in patients with platinum-sensitive recurrent OC, including patients with deleterious or suspected deleterious gBRCA-mutated and BRCA wild type (BRCAwt) recurrent OC.
The objective of this retrospective observational study was to compare OS in a US-based, real-world population of patients with BRCAwt recurrent OC who received 2LM niraparib monotherapy with patients who had their care managed with AS. This association was additionally examined in a NOVA study-like subpopulation of patients.
Methods
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. 10 The order of the STROBE checklist items has been modified to group similar items together. This file is found in the Supplemental Materials.
Data source
This study was conducted using data from the Flatiron Health database, a US-based nationwide database of deidentified electronic health record (EHR)-derived data from approximately 280 cancer clinics representing an estimated 800 sites of care.11,12 The database contains patient-level structured and unstructured data, curated via technology-enabled abstraction from physician notes and other unstructured documents.11,12 Data consisted of a random sample of the broad OC population in the real-world database, plus a custom-curated dataset of all patients diagnosed with OC who received niraparib but were not included in the deidentified database. The deidentified data were subject to obligations to prevent reidentification and protect patient confidentiality.
Study population
Female patients were included if they had a diagnosis of the ovarian, fallopian tube, or peritoneal cancer (collectively referred to as OC) as defined by International Classification of Diseases (ICD) codes 183x and 158x (ICD 9) and C56x, C57.0x, C48x (ICD-10) and at least two documented clinical visits between January 1, 2011 and May 31, 2022 (hereafter referred to as the study period). All patients were aged 18 years or older at index, were diagnosed with epithelial OC during the patient selection period (January 1, 2011–May 31, 2021), had received two prior lines of therapy, and were BRCAwt. BRCA status was defined as “BRCA mutated” for patients who at any time had mutations in either or both BRCA1 or BRCA2 or unspecified BRCA mutations; “BRCAwt” for patients without BRCA mutations (which includes genetic variants favoring polymorphism, or genetic variants of unknown significance); and “unknown” for patients who were untested or with undetermined biomarker status (patients without documented BRCA mutation or wild-type status). The index date was defined as the last treatment date of second-line (2L) non-maintenance therapy occurring between January 1 2017, and March 2, 2022. Patients were required to have received either niraparib monotherapy as 2LM or not have received any maintenance therapy within 120 days after the index date and were required to have 2 or more days of follow-up after the index date. Patients were excluded if they had missing data (i.e., a more than 90-day gap in clinical activity after initial diagnosis and any structured activity such as records for any visits or non-canceled medication orders). Patients who were recorded as receiving any PARP inhibitor monotherapy (niraparib, olaparib, and/or rucaparib) as non-maintenance 1L or 2L treatment were excluded, as these patients may have been incorrectly assigned because of a database-specific, oncologist-defined, rule-based line of therapy. Patients were also excluded if they initiated maintenance therapy within a predefined grace period (defined as 120 days after the index date) with a regimen other than niraparib monotherapy. Patients were excluded if they initiated 2LM niraparib monotherapy and did not have a non-canceled medication order for niraparib. Patients who met the selection criteria above were included in the overall study population.
A NOVA study-like subpopulation was created from the overall study population by applying additional inclusion criteria of an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1, known histology, and platinum-sensitive disease, defined as 6 months or more between the end of 1L platinum-based treatment and the start of 2L platinum-based treatment. Platinum-based treatment was defined as chemotherapy treatment lines that include carboplatin, cisplatin, and oxaliplatin. These criteria were based on similar inclusion criteria in the NOVA clinical trial. 8
Patients who met the inclusion criteria from both the overall population and the NOVA study-like subpopulation were assigned to either the 2LM niraparib monotherapy cohort or the AS cohort. AS was not recorded in the database but rather was derived from the line of therapy available in the database as patients who did not initiate 2LM during a grace period after the end of 2L non-maintenance therapy. Patients were followed from index date to date of death, last clinical activity, or study period end (May 31, 2022), whichever occurred first.
In contrast to the NOVA study 8 that classified patients as either gBRCA or non-gBRCA (which included patients with somatic BRCA (sBRCA) mutations and BRCAwt tumors), this study excluded patients with any mutations (gBRCA or sBRCA). This difference was due to a lack of available data in this dataset. Also, unlike the NOVA study 8 that assessed patients who received 2LM and later lines of maintenance therapy, this study focused on patients within the 2L setting.
Study outcome
The study outcome was OS, defined as the time from index date to death from any cause. Patients who did not have a death event during the study period were censored at the earliest occurrence of the last clinical activity or the end of the study period.
Statistical methods
To adjust for immortal time bias associated with a potential artificial increase in the follow-up time for the maintenance cohort (e.g., when the start of follow-up and treatment initiation do not coincide), a target trial emulation with a cloning-weighting-censoring approach was selected a priori. 13 Inverse-probability-of-censoring weighting (IPCW) was used to control for informative censoring bias arising from the cloning approach, and weights were stabilized to reduce the size of extreme weights. The methods used in this study have been previously described in detail in a prior report. 14
Patient demographic and clinical characteristics were summarized descriptively for the overall study and the NOVA study-like subpopulation before cloning. The balance of key baseline covariates between both cohorts was assessed using standardized mean differences with a threshold of less than 15% chosen as the midpoint between acceptable ranges.15,16 These variables were defined a priori because of their potential to affect treatment decisions and were as follows: age at index, race, region of residence, practice type (academic and community), epithelial histology, stage at initial diagnosis, ECOG performance status score, BRCA status, and duration between last treatment of 1L non-maintenance and initiation of 2L non-maintenance.
Survival curves for the 2LM niraparib monotherapy cohort and the AS cohort were estimated using an inverse-probability-of-censoring (IPC)-weighted nonparametric Kaplan–Meier estimator. Median OS (mOS) in months, survival rate at 24 months, and associated 95% CIs were generated. IPC-weighted Cox proportional hazards regression models with a robust variance estimator to account for within-person correlation were used to compare OS between both cohorts. Hazard ratios and 95% CIs were generated. All survival analyses were conducted for the overall study and the NOVA study-like subpopulation. All analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC, USA).
Results
Participants
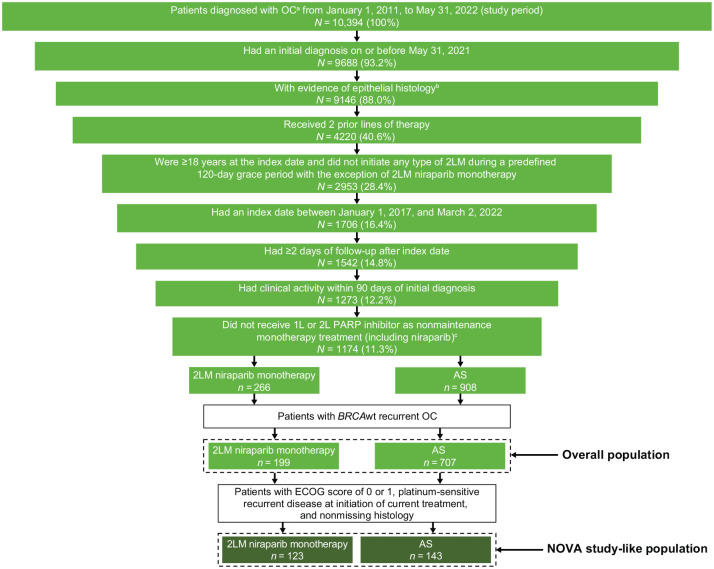
In this real-world database, 10,394 patients were diagnosed with OC during the study period. Of these, 906 and 266 patients met eligibility criteria for the overall population and the NOVA study-like subpopulation, respectively, and were included in analyses (Figure 1). In the overall population, 199 patients were assigned to the 2LM niraparib monotherapy cohort and 707 patients to the AS cohort. In the NOVA study-like subpopulation, 123 patients were assigned to the 2LM niraparib monotherapy and 143 to the AS cohort.
Figure 1.
Study population attrition.
aBased on the presence of ICD versions 9 and 10 codes for ovarian, fallopian tube, and/or peritoneal cancer (ICD-9: 183x, 158x; ICD-10: C56x, C57.0x, C48x) with two or more documented clinical visits.
bPatients with borderline histology were excluded.
cPatients with niraparib 2LM who did not have a non-canceled medication order for niraparib were excluded.
1L, first-line; 2L, second-line; 2LM, second-line maintenance; AS, active surveillance; ECOG, Eastern Cooperative Oncology Group; ICD, International Classification of Disease; OC, ovarian cancer; PARP, poly(ADP-ribose) polymerase; wt, wild type.
Patient demographic and clinical characteristics before adjustment are shown in Table 1. In the overall population, the median age at index was 68 years (interquartile range [IQR], 61–75 years) for the 2LM niraparib monotherapy cohort and 69 years (IQR, 60–76 years) for the AS cohort. Most patients were White (66.3% in the 2LM niraparib monotherapy cohort and 71.9% in the AS cohort) and from a community setting (84.4% in the 2LM niraparib monotherapy cohort and 81.2% in the AS cohort). In the 2LM niraparib monotherapy and AS cohorts, 83.9% and 71.4% of patients had an ECOG performance status score of 0 or 1, respectively. The median duration between the last treatment date of 1L and the start of 2L was 13.6 months (IQR, 7.6–20.9 months) for the 2LM niraparib monotherapy cohort and 6.2 months (IQR, 2.1–12.5 months) for the AS cohort. Demographic and clinical characteristics for the NOVA study-like subpopulation were similar to the overall population in most instances (Table 1). Two exceptions were by study design and involved all patients in both cohorts having an ECOG performance status score of 0 or 1 and epithelial histology not otherwise specified or unknown. Key characteristics that may affect treatment decisions were balanced after cloning and stabilized IPCW (Table 2).
Table 1.
Baseline demographic, clinical, and tumor characteristics before adjustment.
| Characteristic | Overall population | NOVA study-like subpopulation | ||
|---|---|---|---|---|
| 2LM niraparib monotherapy (N = 199) | AS (N = 707) | 2LM niraparib monotherapy (N = 123) | AS (N = 143) | |
| Age at index, median (IQR), years | 68 (61–75) | 69 (60–76) | 68.0 (61.0–74.0) | 69.0 (60.0–78.0) |
| Age group at index, n (%) | ||||
| 18–74 years | 146 (73.4) | 498 (70.4) | 94 (76.4) | 94 (65.7) |
| ⩾75 years | 53 (26.6) | 209 (29.6) | 29 (23.6) | 49 (34.3) |
| Race, n (%) | ||||
| White | 132 (66.3) | 508 (71.9) | 87 (70.7) | 112 (78.3) |
| Other a | 61 (30.7) | 157 (22.2) | 32 (26.0) | 24 (16.8) |
| Unknown | 6 (3.0) | 42 (5.9) | 4 (3.3) | 7 (4.9) |
| Practice type, n (%) | ||||
| Academic b | 18 (9.0) | 118 (16.7) | 14 (11.4) | 8 (5.6) |
| Community | 168 (84.4) | 574 (81.2) | 99 (80.5) | 129 (90.2) |
| Both | 13 (6.5) | 15 (2.1) | 10 (8.1) | 6 (4.2) |
| Region, n (%) c | ||||
| Midwest | 20 (10.1) | 76 (10.7) | 14 (11.4) | 22 (15.4) |
| Northeast | 17 (8.5) | 73 (10.3) | 8 (6.5) | 19 (13.3) |
| South | 107 (53.8) | 308 (43.6) | 64 (52.0) | 64 (44.8) |
| West | 22 (11.1) | 94 (13.3) | 12 (9.8) | 18 (12.6) |
| Other/unknown | 33 (16.6) | 156 (22.1) | 25 (20.3) | 20 (14.0) |
| Weight, n (%) d | ||||
| <77 kg | 120 (60.3) | 464 (65.6) | 68 (55.3) | 95 (66.4) |
| ⩾77 kg | 79 (39.7) | 243 (34.4) | 55 (44.7) | 48 (33.6) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ECOG performance status, n (%) | ||||
| 0–1 | 167 (83.9) | 505 (71.4) | 123 (100.0) | 143 (100.0) |
| 2–4 | 18 (9.0) | 93 (13.2) | 0 (0.0) | 0 (0.0) |
| Unknown | 14 (7.0) | 109 (15.4) | 0 (0.0) | 0 (0.0) |
| Disease stage at diagnosis, n (%) | ||||
| I–II | 21 (10.6) | 77 (10.9) | 14 (11.4) | 21 (14.7) |
| III | 103 (51.8) | 353 (49.9) | 66 (53.7) | 87 (60.8) |
| IV | 56 (28.1) | 203 (28.7) | 36 (29.3) | 28 (19.6) |
| Unknown or missing | 19 (9.5) | 74 (10.5) | 7 (5.7) | 7 (4.9) |
| Epithelial histology, n (%) e | ||||
| Serous | 156 (78.4) | 545 (77.1) | 98 (79.7) | 116 (81.1) |
| Other | 20 (10.1) | 68 (9.6) | 14 (11.4) | 14 (9.8) |
| Epithelial NOS/unknown | 23 (11.6) | 94 (13.3) | 11 (8.9) | 13 (9.1) |
| HRD status, n (%) f | ||||
| HRD status known | 17 (8.5) | 94 (13.3) | 9 (7.3) | 20 (14.0) |
| Unknown | 182 (91.5) | 613 (86.7) | 114 (92.7) | 123 (86.0) |
| Treatment lines after index, n (%) | ||||
| 0 | 69 (34.7) | 185 (26.2) | 47 (38.2) | 43 (30.1) |
| 1 | 64 (32.2) | 221 (31.3) | 37 (30.1) | 37 (25.9) |
| 2 | 25 (12.6) | 137 (19.4) | 14 (11.4) | 33 (23.1) |
| 3+ | 41 (20.6) | 164 (23.2) | 25 (20.3) | 30 (21.0) |
| Used bevacizumab before 2L maintenance therapy, n (%) g | 73 (36.7) | 310 (43.8) | 41 (33.3) | 60 (42.0) |
| Platinum-based therapy during 2L treatment, n (%) | 195 (98.0) | 277 (39.2) | 123 (100.0) | 143 (100.0) |
| Duration between end of 1L and start of 2L, median (IQR), months | 13.6 (7.6–20.9) | 6.2 (2.1–12.5) | 16.1 (10.8–26.6) | 14.2 (10.8–28.7) |
| Duration of follow-up, median (IQR), months | 15.6 (9.1–27.1) | 9.3 (3.2–21.0) | 16.8 (10.4–28.7) | 10.2 (4.1–23.7) |
Other may include race categories provided in the database of “Black or African American,” “Asian,” “Hispanic or Latino,” and “Other Race.”
Academic may include both university and non-university academic settings.
Patients from academic practices had unknown geographic regions. Patients in Puerto Rico were grouped into other/unknown because of low numbers.
Defined as the most recent body weight recorded from initial diagnosis to index date.
Patients with unknown histology were excluded in the NOVA study-like subpopulation.
HRD status was defined as patients who ever had a positive or negative result were grouped as status known, remaining patients who only had an unknown result or were never tested were grouped as unknown. Positive/negative categories with less than five patients were combined to protect patient confidentiality.
Defined as the use of bevacizumab as part of a line of therapy prior to index, including 1L, 1L maintenance, and/or 2L.
1L, first line; 2L, second line; 2LM, second-line maintenance; AS, active surveillance; ECOG, Eastern Cooperative Oncology Group; HRD, homologous recombination deficiency; IQR, interquartile range; NOS, not otherwise specified.
Table 2.
Balance of patient characteristics before and after IPCW, using a 15% threshold.
| Characteristic | Absolute standardized mean difference, % a | |||||
|---|---|---|---|---|---|---|
| Overall population | NOVA study-like subpopulation | |||||
| Before IPCW | After IPCW | After stabilized IPCW | Before IPCW | After IPCW | After stabilized IPCW | |
| Age group at index | 26.4 | 6.4 | 6.9 | 30.0 | 2.0 | 4.2 |
| Race | 28.4 | 11.2 | 11.0 | 25.8 | 13.8 | 14.0 |
| Practice type b | 30.6 | 15.8 | 15.3 | 27.8 | 14.7 | 14.7 |
| Region | 24.2 | 15.1 | 14.2 | 47.0 | 10.7 | 12.6 |
| ECOG performance status | 31.7 | 12.9 | 12.2 | – | – | – |
| Disease stage at diagnosis | 6.8 | 6.3 | 5.5 | 27.7 | 2.2 | 4.7 |
| Epithelial histology | 5.3 | 2.8 | 2.2 | 5.2 | 1.3 | 0.5 |
| Duration between the end of 1L and the start of 2L | 73.1 | 5.1 | 10.6 | 3.2 | 4.8 | 9.3 |
Standardized mean difference is calculated as the absolute difference in means, mean ranks, or proportions divided by the pooled standard deviation. For categorical variables, the overall standardized difference was calculated using Mahalanobis distance. 17
Practice type had a standardized mean difference of 15.3% in the overall population after stabilized IPCW, but the balance was achieved at the categorical level.
1L, first-line; 2L, second-line; ECOG, Eastern Cooperative Oncology Group; IPCW, inverse-probability-of-censoring weights.
IPCW overall survival
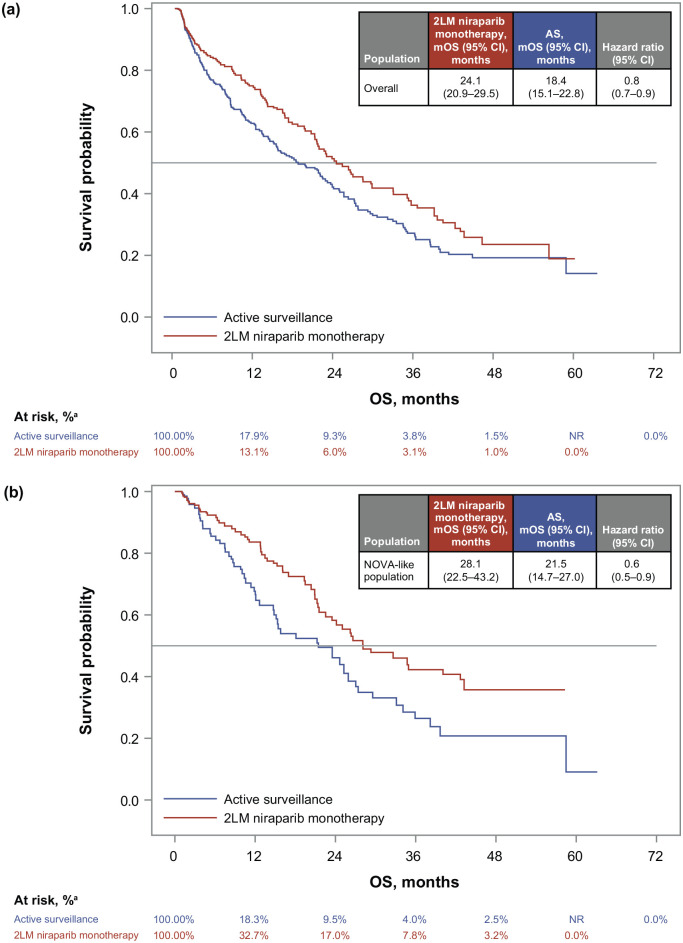
For the overall population, mOS was significantly longer for the 2LM niraparib monotherapy cohort (24.1 months; 95% CI: 20.9–29.5 months) than the AS cohort (18.4 months; 95% CI: 15.1–22.8 months; hazard ratio, 0.8; 95% CI: 0.7–0.9; Figure 2(a)) after adjustment. Survival rates at 24 months were 50.6% (95% CI: 42.5%–58.1%) for the 2LM niraparib monotherapy cohort and 41.6% (95% CI: 35.4%–47.6%) for the AS cohort.
Figure 2.
Adjusted Kaplan–Meier curves and adjusted hazard ratios for the (a) overall population and (b) the NOVA study-like subpopulation.
The survival curves were estimated using a stabilized IPC-weighted nonparametric Kaplan–Meier estimator, and hazard ratios were estimated using stabilized IPC-weighted Cox proportional hazards regression models.
aPercentage at risk is reported in percentages because of the weighting approach that can result in non-whole numbers.
2LM, second-line maintenance; AS, active surveillance; IPC, inverse probability of censoring; mOS, median overall survival; NR, not reported; OC, ovarian cancer; OS, overall survival; wt, wild type.
OS results were similar for the NOVA study-like subpopulation after adjustment (Figure 2(b)). The mOS was 28.1 months (95% CI: 22.5–43.2 months) and 21.5 months (95% CI: 14.7–27.0 months) for the 2LM niraparib monotherapy and AS cohorts, respectively (hazard ratio, 0.6; 95% CI: 0.5–0.9). The survival rate at 24 months was 58.2% (95% CI: 47.5%–67.6%) for the 2LM niraparib monotherapy cohort and 46.1% (95% CI: 33.6%–57.7%) for the AS cohort.
Discussion
Given a lack of real-world data reporting outcomes for patients with OC who receive niraparib maintenance monotherapy in the recurrent setting, this retrospective observational study provides an informative real-world comparison of OS for patients with BRCAwt OC who received 2LM niraparib monotherapy versus those whose care was managed with AS. In both the overall population and a NOVA study-like subpopulation, patients who received 2LM niraparib monotherapy had a longer adjusted mOS than patients whose care was managed with AS.
These real-world results support niraparib’s clinical benefit in treating recurrent OC. 8 These results also support preplanned exploratory NOVA analyses that demonstrated a continued clinical benefit of niraparib in the maintenance setting beyond the first progression. After a median follow-up of more than 75 months, the mOS in a gBRCA mutation cohort was 40.9 months for niraparib versus 38.1 months for placebo, and in a non-gBRCA mutation cohort, was 31.0 months for niraparib versus 34.8 months for placebo. 18 However, OS data from that study should be interpreted with caution because the NOVA study was not powered to evaluate between-group differences in OS.
The results from this study should be interpreted within the context of some potential limitations. Real-world, retrospective, EHR database analyses are limited by the type of data collected and the quality control of data contained within the database.19,20 However, the database used in this study is considered to have a high degree of accuracy in the essential dataset required when evaluating real-world OS, namely all-cause mortality data. 21 The study population is limited to data from the real-world database, collected primarily from community practices, and may not represent the entire OC population in the United States. The study included a broad patient cohort to be reflective of the real-world OC population, which included patients who were not treated with platinum-based 2L therapy or patients who may have had platinum-resistant or -refractory disease (which is inconsistent with the niraparib label), who were therefore at a higher risk of having poorer outcomes. However, in the NOVA study-like subpopulation, all patients received a platinum-based 2L therapy and were required to have at least 6 months between the 1L and 2L, to exclude patients with platinum-resistant or -refractory disease. For both cohorts, patients who were treated with 2LM had longer OS than patients who were under AS. Relative to the NOVA study, this study excluded sBRCA because data distinguishing between gBRCA and sBRCA mutations were unavailable, and homologous recombination deficiency results were limited (unknown status ranged from 86.0% to 92.7% per cohort). Therefore, the findings of this study were limited to patients with BRCAwt, and direct comparisons with results of the NOVA study may be limited. Unlike randomized clinical trials, treatments are not assigned at random in real-world clinical practice. These analyses did not account for post-index events (i.e., initiation of 2LM after the predefined grace period or subsequent lines of therapy). The follow-up period in this study may have been limited for patients whose index date occurred toward the end of the study period. Furthermore, treatment response was not captured in the database. Therefore, the AS cohort could have included patients with stable disease or non-responders who were ineligible for 2LM niraparib monotherapy, and those patients were more likely to have poorer outcomes. To account for this possible limitation, patients who initiated third-line therapy within 60 days of the index were censored both in the AS and maintenance cohorts. However, this adjustment still did not capture 2L treatment non-responders who did not have subsequent treatment. Finally, niraparib is currently approved for patients with deleterious or suspected deleterious gBRCA mutations in the recurrent setting (i.e., 2L or later), based on results from NOVA, 8 where among all patients who received niraparib (regardless of gBRCA status), 39.2% of patients received 3 or more prior lines of therapy. However, because of methodological complexities, this real-world study focused on BRCAwt OC in the 2L setting and did not include patients who were treated with niraparib or AS in the third-line or later setting, so patients in NOVA were more heavily pretreated. Since the completion of the study, the approval of niraparib as 2LM therapy was amended in the United States to include only patients with deleterious or suspected deleterious gBRCA mutations, 22 while the approval in Europe remained unchanged. 23 Despite this label change, the results from this real-world study demonstrated an OS benefit from 2LM niraparib monotherapy among patients with BRCAwt OC, compared with AS, suggesting the potential value of niraparib in these patients.
Conclusion
The findings of this study provide important real-world OS data for patients with recurrent OC who received niraparib monotherapy in the 2LM setting. In this real-world study from a clinical practice population in the United States, results provide evidence of an OS benefit from using 2LM niraparib monotherapy over AS that supports data generated in the NOVA clinical trial, specifically in patients with BRCAwt OC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241292272 for Real-world overall survival in second-line maintenance niraparib monotherapy versus active surveillance in patients with BRCA wild-type recurrent ovarian cancer by Robert L. Coleman, Jessica A. Perhanidis, Linda Kalilani, Nicole M. Zimmerman, Amanda Golembesky and Kathleen N. Moore in Therapeutic Advances in Medical Oncology
Acknowledgments
Statistical programming support was provided by Pratyk Gomez, BA, and Warsha Kumari Singh, BTech, both of GSK. Medical writing and editorial assistance, funded by GSK (Waltham, MA, USA) and coordinated by Chun Zhou, PhD, CMPP, of GSK, were provided by Tafara T.R. Kunota, PhD, and Kathleen Blake, PhD, of Ashfield MedComms, an Inizio Company.
Footnotes
ORCID iD: Jessica A. Perhanidis  https://orcid.org/0000-0001-6943-8500
https://orcid.org/0000-0001-6943-8500
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Robert L. Coleman, Texas Oncology, US Oncology Network and GOG Foundation, 9180 Pinecroft Drive, Suite 600, The Woodlands, TX 77380, USA.
Jessica A. Perhanidis, GSK, Waltham, MA, USA
Linda Kalilani, GSK, Durham, NC, USA.
Nicole M. Zimmerman, GSK, Upper Providence, PA, USA
Amanda Golembesky, GSK, Durham, NC, USA.
Kathleen N. Moore, Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with all applicable patient privacy laws. The deidentified data were subject to obligations to prevent reidentification and protect patient confidentiality. There was no direct patient contact or primary collection of individual human participant data. Study results and analyses omit patient identification. Therefore, informed consent and ethics committee or institutional review board approval were not required.
Consent for publication: Not applicable.
Author contributions: Robert L. Coleman: Conceptualization; Writing – review & editing.
Jessica A. Perhanidis: Conceptualization; Formal analysis; Methodology; Software; Validation; Writing – original draft; Writing – review & editing.
Linda Kalilani: Conceptualization; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Nicole M. Zimmerman: Conceptualization; Formal analysis; Methodology; Software; Validation; Writing – original draft; Writing – review & editing.
Amanda Golembesky: Conceptualization; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Kathleen N. Moore: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study (OneCDP219306) was supported by GSK.
Competing interests: Dr R.L.C. reports an advisory role for AbbVie, Aravive, AstraZeneca, Clovis Oncology, Eisai, GSK, Janssen, Merck, Mersana Therapeutics, Novocure, OncoQuest, OncXerna, Onxeo, Roche/Genentech, and Zentalis; receives research grants from Genentech, Genmab, ImmunoGen, and Merck; is a member of the board of directors for GOG Foundation and a co-director for GOG Partners. Ms J.A.P. is a GSK employee and holds stocks/shares in Boston Scientific and GSK. Drs L.K. and A.G. are employees of GSK. Ms N.M.Z. is a GSK employee and holds stocks/shares in GSK. Dr K.N.M. reports an advisory role for Aadi, Alkermes, Aravive, AstraZeneca, Blueprint Pharma, Clovis Oncology, Eisai, Elevar, Genentech/Roche, GSK, Hengrui, I-Mab, ImmunoGen, Lilly, Merck, Mereo, Mersana Therapeutics, Myriad Genetics, Novartis, OncXerna, Onconova, Sorrento, VBL Therapeutics, and Verastem Oncology; receives research grants from Lilly, Merck, PTC Therapeutics, and Verastem Oncology; is a member of the board of directors for GOG Foundation and an associate director for GOG Partners.
Availability of data and materials: The data that support the findings of this study have been originated by Flatiron Health, Inc. Requests for data sharing by licenses or by permission for the specific purpose of replicating results in this manuscript can be submitted to PublicationsDataAccess@flatiron.com.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023; 73(1): 17–48. [DOI] [PubMed] [Google Scholar]
- 3. Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl. 6): vi24–vi32. [DOI] [PubMed] [Google Scholar]
- 4. Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 2019; 30(5): 672–705. [DOI] [PubMed] [Google Scholar]
- 5. Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus 2016; 5(1): 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ray-Coquard I, Mirza MR, Pignata S, et al. Therapeutic options following second-line platinum-based chemotherapy in patients with recurrent ovarian cancer: comparison of active surveillance and maintenance treatment. Cancer Treat Rev 2020; 90: 102107. [DOI] [PubMed] [Google Scholar]
- 7. SEER*Explorer. An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute, https://seer.cancer.gov/statistics-network/explorer/ (2024, accessed 7 June 2024).
- 8. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016; 375(22): 2154–2164. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019; 381(25): 2391–2402. [DOI] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 11. Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv 2020: 2020.2003.2016.20037143. [Google Scholar]
- 12. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv preprint arXiv:200109765, 2020. [Google Scholar]
- 13. Maringe C, Benitez Majano S, Exarchakou A, et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int J Epidemiol 2020; 49(5): 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perhanidis JA, Kalilani L, Zimmerman NM, et al. An emulated target trial case study of real-world overall survival with second-line maintenance niraparib versus active surveillance in patients with recurrent ovarian cancer. Pharmacoepidemiol Drug Saf 2024; 33(9): e70001. [DOI] [PubMed] [Google Scholar]
- 15. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25(1): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28(25): 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. https://support.sas.com/resources/papers/proceedings12/335-2012.pdf (2012, accessed 14 May 2024).
- 18. Matulonis U, Herrstedt J, Oza A, et al. Final overall survival and long-term safety in the ENGOT-OV16/NOVA phase III trial of niraparib in patients with recurrent ovarian cancer (LBA 6). Gynecol Oncol 2023; 176: S31–S32. [Google Scholar]
- 19. Nordo AH, Levaux HP, Becnel LB, et al. Use of EHRs data for clinical research: historical progress and current applications. Learn Health Syst 2019; 3(1): e10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rudrapatna VA, Butte AJ. Opportunities and challenges in using real-world data for health care. J Clin Invest 2020; 130(2): 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Q, Gossai A, Monroe S, et al. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res 2021; 56(6): 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. GSK. GSK provides an update on Zejula (niraparib) US prescribing information November 11, 2022. https://www.gsk.com/en-gb/media/press-releases/gsk-provides-an-update-on-zejula-niraparib-us-prescribing-information (2022, accessed 29 February 2024).
- 23. European Medicines Agency. Zejula (niraparib): an overview of zejula and why it is authorised in the EU November 16, 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/zejula (2017, accessed 30 July 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241292272 for Real-world overall survival in second-line maintenance niraparib monotherapy versus active surveillance in patients with BRCA wild-type recurrent ovarian cancer by Robert L. Coleman, Jessica A. Perhanidis, Linda Kalilani, Nicole M. Zimmerman, Amanda Golembesky and Kathleen N. Moore in Therapeutic Advances in Medical Oncology