Abstract
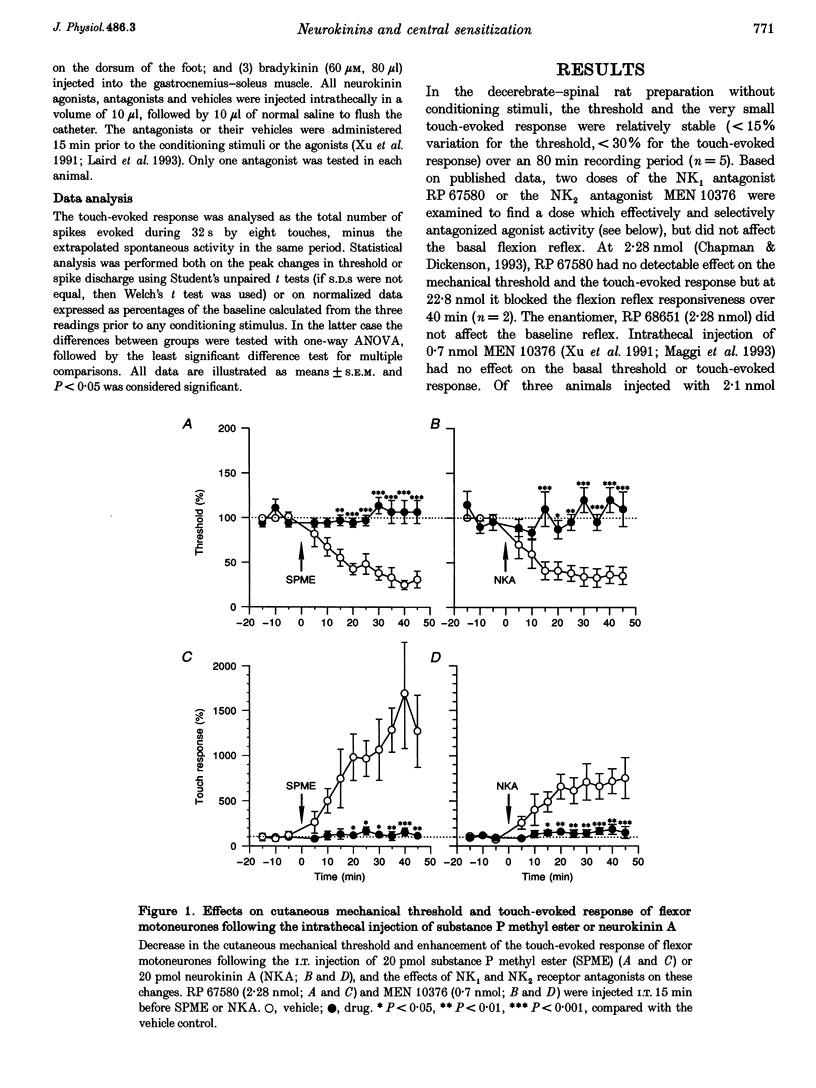
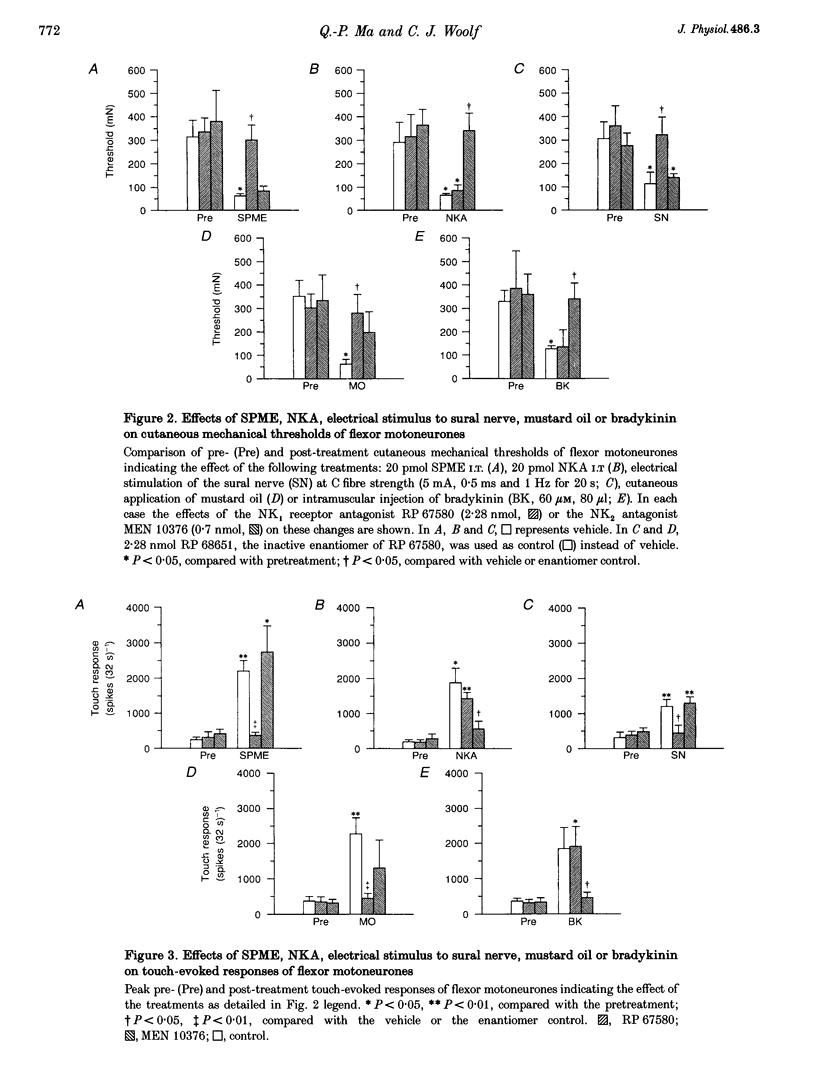
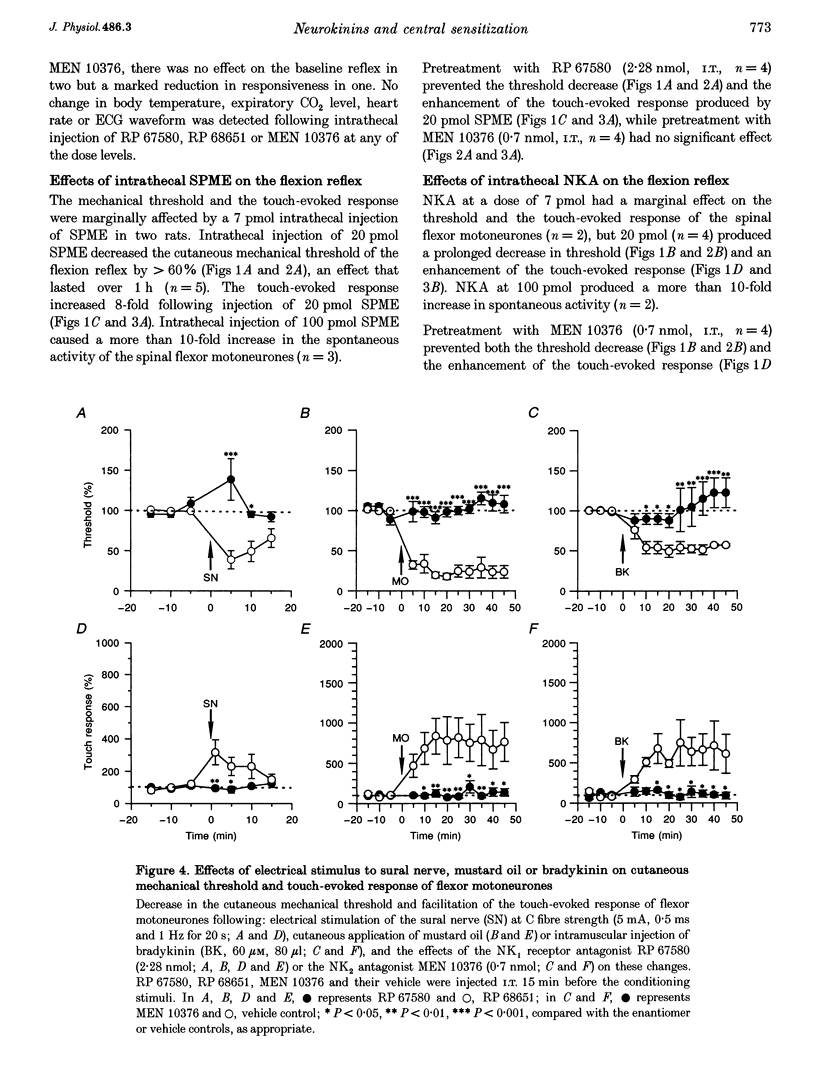
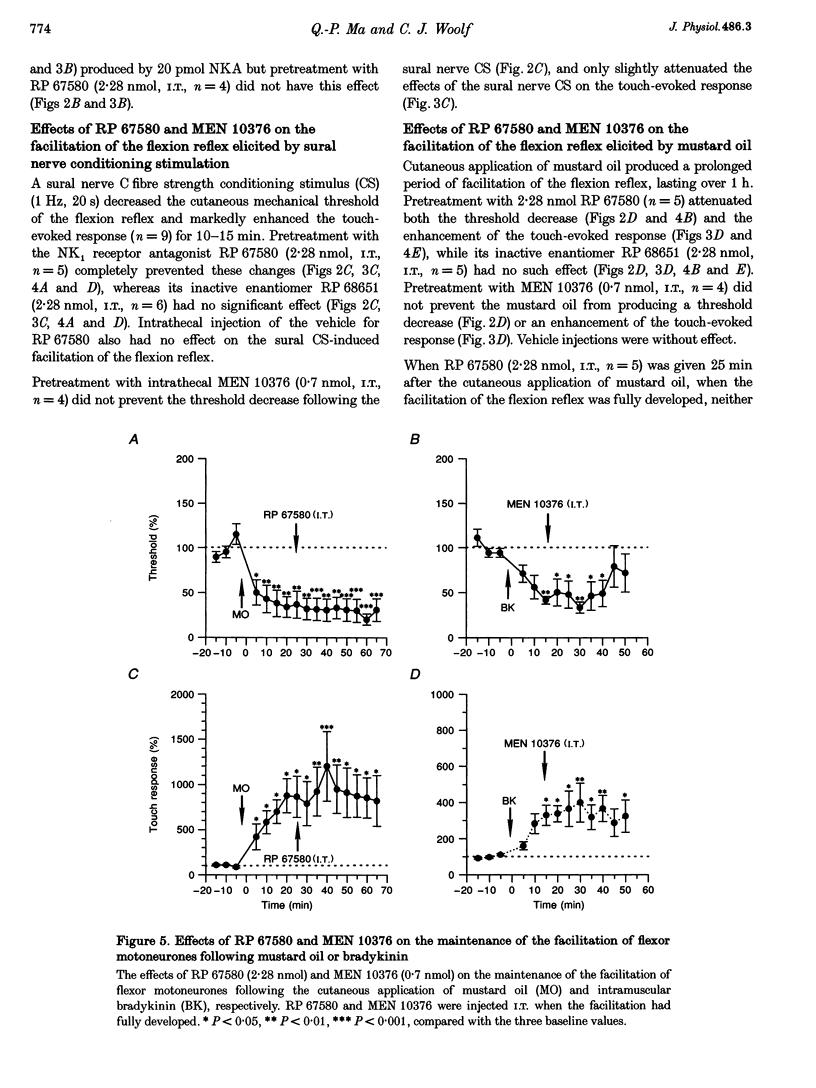
1. Intrathecal (i.t.) injections of the (tachykinin) NK1 receptor agonist, substance P methyl ester (SPME; 20 pmol), or the NK2 receptor agonist, neurokinin A (NKA; 20 pmol), substantially decreased the cutaneous mechanical threshold and markedly enhanced the touch-evoked response of posterior biceps femoris-semitendinosus (PBF-ST) spinal flexor motoneurones in decerebrate-spinal rats. This cutaneous mechanical reflex allodynia was prevented by pretreatment with the NK1 antagonist RP 67580 (2.28 nmol, i.t.) and the NK2 antagonist MEN 10376 (0.7 nmol, i.t.), respectively. 2. Electrical stimulation of the sural nerve at C fibre strength or cutaneous application of the irritant, mustard oil, produced prolonged cutaneous mechanical allodynia in PBF-ST motoneurones (15 min and > 1 h, respectively). Pretreatment with RP 67580 but not MEN 10376 prevented this, but when RP 67580 was administered 25 min after the application of mustard oil, the established hypersensitivity of the flexor motor reflex was not reversed. The enantiomer of RP 67580, RP 68651 was without effect. 3. Injection of bradykinin (60 microM, 80 microliters) into the gastrocnemius muscle increased the cutaneous mechanical hypersensitivity of PBF-ST flexor motoneurones for 40-50 min. MEN 10376, but not RP 67580, prevented this, but only when administered prior to the bradykinin injection. 4. These results suggest that the induction, but not the maintenance, of cutaneous mechanical allodynia in flexor motoneurones is NK receptor dependent, with cutaneous C fibre conditioning inputs acting via NK1 and muscle C fibre conditioning inputs via NK2 receptor subtypes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman V., Dickenson A. H. The effect of intrathecal administration of RP67580, a potent neurokinin 1 antagonist on nociceptive transmission in the rat spinal cord. Neurosci Lett. 1993 Jul 23;157(2):149–152. doi: 10.1016/0304-3940(93)90724-y. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lodge D. Evidence for involvement of N-methylaspartate receptors in 'wind-up' of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987 Oct 27;424(2):402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987 Aug;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hendry I. A., Morton C. R., Hutchison W. D., Zhao Z. Q. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988 Jun 7;451(1-2):261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hope P. J., Jarrott B., Schaible H. G., Fleetwood-Walker S. M. Release, spread and persistence of immunoreactive neurokinin A in the dorsal horn of the cat following noxious cutaneous stimulation. Studies with antibody microprobes. Neuroscience. 1990;35(1):195–202. doi: 10.1016/0306-4522(90)90134-p. [DOI] [PubMed] [Google Scholar]
- Kangrga I., Randic M. Tachykinins and calcitonin gene-related peptide enhance release of endogenous glutamate and aspartate from the rat spinal dorsal horn slice. J Neurosci. 1990 Jun;10(6):2026–2038. doi: 10.1523/JNEUROSCI.10-06-02026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird J. M., Hargreaves R. J., Hill R. G. Effect of RP 67580, a non-peptide neurokinin1 receptor antagonist, on facilitation of a nociceptive spinal flexion reflex in the rat. Br J Pharmacol. 1993 Jul;109(3):713–718. doi: 10.1111/j.1476-5381.1993.tb13632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderoth B., Brodin E. Tachykinin release from rat spinal cord in vitro and in vivo in response to various stimuli. Regul Pept. 1988 May;21(1-2):129–140. doi: 10.1016/0167-0115(88)90097-3. [DOI] [PubMed] [Google Scholar]
- Ma Q. P., Woolf C. J. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995 Jun;61(3):383–390. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Rovero P., Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993 Feb;13(1):23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- McNeill D. L., Westlund K. N., Coggeshall R. E. Peptide immunoreactivity of unmyelinated primary afferent axons in rat lumbar dorsal roots. J Histochem Cytochem. 1989 Jul;37(7):1047–1052. doi: 10.1177/37.7.2471724. [DOI] [PubMed] [Google Scholar]
- Mense S., Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol. 1985 Jun;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C., Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience. 1986 Sep;19(1):297–312. doi: 10.1016/0306-4522(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Molander C., Grant G. Spinal cord projections from hindlimb muscle nerves in the rat studied by transganglionic transport of horseradish peroxidase, wheat germ agglutinin conjugated horseradish peroxidase, or horseradish peroxidase with dimethylsulfoxide. J Comp Neurol. 1987 Jun 8;260(2):246–255. doi: 10.1002/cne.902600208. [DOI] [PubMed] [Google Scholar]
- Nagy I., Miller B. A., Woolf C. J. NK1 and NK2 receptors contribute to C-fibre evoked slow potentials in the spinal cord. Neuroreport. 1994 Oct 27;5(16):2105–2108. doi: 10.1097/00001756-199410270-00029. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kanazawa I., Kimura S. Regional distribution of substance P, neurokinin alpha and neurokinin beta in rat spinal cord, nerve roots and dorsal root ganglia, and the effects of dorsal root section or spinal transection. Brain Res. 1985 Dec 16;359(1-2):152–157. doi: 10.1016/0006-8993(85)91423-4. [DOI] [PubMed] [Google Scholar]
- Picard P., Boucher S., Regoli D., Gitter B. D., Howbert J. J., Couture R. Use of non-peptide tachykinin receptor antagonists to substantiate the involvement of NK1 and NK2 receptors in a spinal nociceptive reflex in the rat. Eur J Pharmacol. 1993 Mar 2;232(2-3):255–261. doi: 10.1016/0014-2999(93)90782-d. [DOI] [PubMed] [Google Scholar]
- Price D. D., Hull C. D., Buchwald N. A. Intracellular responses of dorsal horn cells to cutaneous and sural nerve A and C fiber stimuli. Exp Neurol. 1971 Nov;33(2):291–309. doi: 10.1016/0014-4886(71)90022-7. [DOI] [PubMed] [Google Scholar]
- Rupniak N. M., Boyce S., Williams A. R., Cook G., Longmore J., Seabrook G. R., Caeser M., Iversen S. D., Hill R. G. Antinociceptive activity of NK1 receptor antagonists: non-specific effects of racemic RP67580. Br J Pharmacol. 1993 Dec;110(4):1607–1613. doi: 10.1111/j.1476-5381.1993.tb14008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin K. I., Ryu P. D., Randic M. Modulation of excitatory amino acid responses in rat dorsal horn neurons by tachykinins. J Neurophysiol. 1992 Jul;68(1):265–286. doi: 10.1152/jn.1992.68.1.265. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Woolf C. J. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994 Jul;72(1):169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Thompson S. W., Gerber G., Sivilotti L. G., Woolf C. J. Long duration ventral root potentials in the neonatal rat spinal cord in vitro; the effects of ionotropic and metabotropic excitatory amino acid receptor antagonists. Brain Res. 1992 Nov 6;595(1):87–97. doi: 10.1016/0006-8993(92)91456-o. [DOI] [PubMed] [Google Scholar]
- Thompson S. W., King A. E., Woolf C. J. Activity-Dependent Changes in Rat Ventral Horn Neurons in vitro; Summation of Prolonged Afferent Evoked Postsynaptic Depolarizations Produce a d-2-Amino-5-Phosphonovaleric Acid Sensitive Windup. Eur J Neurosci. 1990;2(7):638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Thompson S. W., Woolf C. J., Sivilotti L. G. Small-caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal rat spinal cord in vitro. J Neurophysiol. 1993 Jun;69(6):2116–2128. doi: 10.1152/jn.1993.69.6.2116. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Lundberg L. E., LaMotte R. H. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992 Mar;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede R. D., Meyer R. A., Raja S. N., Campbell J. N. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tsuchida K., Shigemoto R., Yokota Y., Nakanishi S. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur J Biochem. 1990 Nov 13;193(3):751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 Dec 15;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res. 1981 Mar 30;209(2):491–495. doi: 10.1016/0006-8993(81)90176-1. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Thompson S. W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991 Mar;44(3):293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Wall P. D. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986 May;6(5):1433–1442. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. J., Dalsgaard C. J., Wiesenfeld-Hallin Z. Intrathecal CP-96,345 blocks reflex facilitation induced in rats by substance P and C-fiber-conditioning stimulation. Eur J Pharmacol. 1992 Jun 17;216(3):337–344. doi: 10.1016/0014-2999(92)90428-7. [DOI] [PubMed] [Google Scholar]
- Xu X. J., Maggi C. A., Wiesenfeld-Hallin Z. On the role of NK-2 tachykinin receptors in the mediation of spinal reflex excitability in the rat. Neuroscience. 1991;44(2):483–490. doi: 10.1016/0306-4522(91)90071-u. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989 Apr;37(1):111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Shimoyama N., Mizuguchi T. The effects of morphine, MK-801, an NMDA antagonist, and CP-96,345, an NK1 antagonist, on the hyperesthesia evoked by carageenan injection in the rat paw. Anesthesiology. 1993 Jan;78(1):124–133. doi: 10.1097/00000542-199301000-00018. [DOI] [PubMed] [Google Scholar]
- Yashpal K., Dam T. V., Quirion R. Quantitative autoradiographic distribution of multiple neurokinin binding sites in rat spinal cord. Brain Res. 1990 Jan 8;506(2):259–266. doi: 10.1016/0006-8993(90)91260-n. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]


