Abstract
Jellyfishes of the order Rhizostomeae include ecologically and economically important species predisposed to forming massive aggregations. This study reports the first complete mitochondrial genome of two rhizostomes: Cephea cephea (Forskål, 1775) and Mastigias albipunctata Stiasny, 1920. The linear mitochondrial genomes are 16,667bp and 16,707bp in length and 65.5% and 68.4% AT respectively; each comprises 15 protein-coding genes (PCGs; dpo, orf314, cox1-3, nd1-6, nd4L, atp6, atp8 and cytB), two ribosomal RNAs (16S and 12S rRNA), and two tRNAs (trnM and trnW). The phylogenetic analysis reveals strong support for monophyly of the suborder Kolpophorae and Cephea cephea of the infraorder Actinomyaria as sister to Krykomyaria which includes Mastigias.
Keywords: Jellyfishes, Kolpophorae, Krykomyaria, linear mitochondrial genomes, phylogeny
Introduction
Jellyfishes of the order Rhizostomeae (class: Scyphozoa) have variable medusa morphologies, including the benthic ‘upside-down jellyfish’ Cassiopea, a model system (Ohdera et al. 2018), the pelagic Mastigias, and blooming species exploited for commercial fisheries and aquaculture (Omori and Nakano 2001; Brotz et al. 2017).
Rhizostomeae medusae lack tentacles on the umbrella margin but bear eight oral arms extending from the central region of the subumbrella. Some species have endosymbiotic relationships with Symbiodiniaceae, a family of dinoflagellates (Djeghri et al. 2019). Species of five genera, including Cassiopea and Netrostoma, a close relative of Cephea, are known to release motile, multicellular bodies comprising ciliated and stinging cells, termed ‘cassiosomes’, into the water column (Ames et al. 2020).
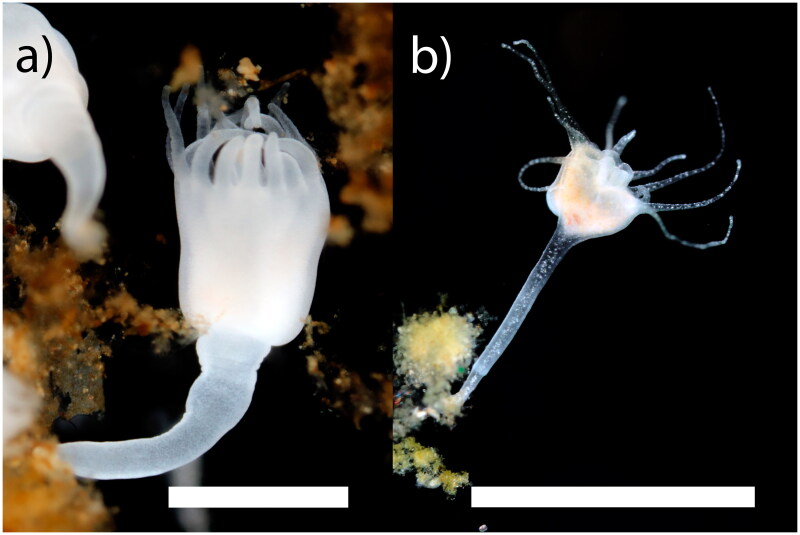
Rhizostomeae has 2 suborders, Dactyliophorae and Kolpophorae, with species in the latter often hosting Symbiodiniaceae. Apart from Kolpophorae genera Cassiopea and Mastigias, Dactyliophorae taxa have been sampled comparatively more thoroughly for molecular analysis. Accordingly, no genomic data are available for the Kolpophorae Cephea cephea or kin, precluding our understanding of the complete mitochondrial genome – a dynamic molecular marker for metazoan evolutionary studies. Hence, we sequenced the complete mitogenome of Cephea cephea (Forskål, 1775) and Mastigias albipunctata Stiasny, 1920 (Figure 1) and conducted phylogenetic analysis of the order Rhizostomeae.
Figure 1.
Photographs of the polyps of a) cephea cephea and b) Mastigias albipunctata reared in the AquaRoom, taken by Allen Collins. Scalebars represent 500 uM.
Materials and methods
Clonal polyp cultures of Cephea cephea and Mastigias albipunctata are kept live in the National Museum of Natural History (NMNH) Department of Invertebrate Zoology AquaRoom. Polyp specimens were deposited at the NMNH (https://naturalhistory.si.edu/, contact person: Allen Collins, e-mail: Allen.Collins@noaa.gov) under the vouchers USNM 1715474 and USNM 1715470 respectively. DNA from several polyps of each species was extracted using an AutoGenPrep 965 robot (AutoGen, Holliston, MA, USA) following the manufacturer’s tissue protocol. For whole genome shotgun sequencing, enzymatically sheared libraries were prepared with NEB Ultra II FS DNA library prep kit (New England Biolabs), targeting an insert size of approximately 400 bp. Libraries were amplified using six cycles of PCR following the kit manufacturer’s chemistry and thermocycler recommendations. We employed iTru y-yoke adapter stubs and iTru unique dual indices (Glenn et al. 2019) rather than NEB adapters and indices, tailoring the amount of adapter based on DNA concentrations specified in the NEB guidelines. Sequencing of equimolar pooled libraries (150 bp, paired end reads) was achieved using a NovaSeq 6000 (Illumina Inc., San Diego, CA, USA).
The resulting reads were trimmed of adapters and poor-quality sequences using BBDUK, part of BBTools (Bushnell B. – sourceforge.net/projects/bbmap/). To assemble mitochondrial genomes, we used the ‘Map to Reference’ function and built-in mapper of Geneious Prime 2022 (https://www.geneious.com), with sensitivity set to ‘medium/low’ and iterations set to 3 or 5, starting with GenBank published sequences for mitochondrial genes 16S rRNA (KY610618) and COX 1 (KU900928) for Cephea cephea and Mastigias albipunctata, respectively. Results of the pending mitochondrial genome assemblies were inspected and ends trimmed (up to 50 bp) where coverage was low (<5X). Consensus sequences were generated and used as subsequent reference seeds, and the ‘Map to Reference’ step was repeated until assemblies ceased to increase in size. Refer to Supplementary Figure 1 for read coverage plot.
Mitogenome annotation was performed in Geneious Prime 2024 (https://www.geneious.com), using genetic code 4, ‘Mold/Protozoan Mitochondrial’. Predicted open reading frames were checked manually by aligning to whole mitochondrial genomes of Rhizostomeae available in GenBank and annotated accordingly. tRNAs were identified using tRNAscan-SE v2.0 (http://lowelab.ucsc.edu/tRNAscan-SE/ (accessed 24 April 2024) with the default settings (Mold & Protozoa Mito genetic code) (Lowe and Chan 2016). rRNA genes were identified based on alignments with the most closely related species (Mastigias papua and Phyllorhiza punctata. Likewise, we annotated the UNVERIFIED mitogenomes of Pseudorhiza haeckeli (OZ032132), Catostylus mosaicus (OZ025205) and a Mastigias papua (OZ025288) from GenBank.
Phylogenetic analysis employed our two newly generated whole mitochondrial genomes, and 12 complete and four partial mitochondrial genomes from GenBank (Table S1). In the absence of genomes for Versuriga anadyomene, Thysanostoma thysanura and Lobonema smithii their mitochondrial genes 16S rRNA and COX 1 were substituted to ensure representation of all known Rhizostomeae families (validated in WoRMS, https://www.marinespecies.org); the outgroup was Semaeostome Aurelia aurita (DQ787873). A maximum likelihood phylogenetic tree (TIM2 + F + G + I) was constructed in IQ-TREE (Trifinopoulos et al. 2016) using concatenated alignments (MAFFT alignment; Katoh and Standley 2013) of each protein-coding and rRNA gene sequence. Nodes were assessed using aBayes test and 1000 ultrafast bootstrap replicates (Hoang et al. 2018).
Results
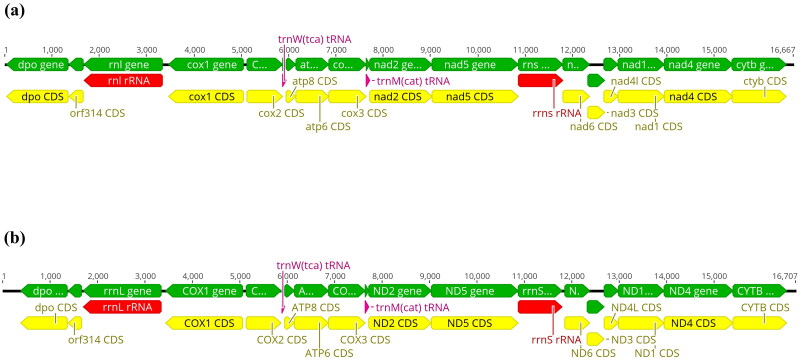
The complete mitogenomes of Cephea cephea and Mastigias albipunctata were 16,667 and 16,707bp in length, respectively, containing 15 protein-coding genes, two transfer RNA (tRNA) genes (trnW and trnM), and two ribosomal RNA (rRNA) genes (Figure 2a,b). Accessioned SRA datasets (BioSamples: SAMN41149076-7) for C. cephea and M. albipunctata contain 9,998,268 and 10,870,791 spots, respectively, with 101,434 and 182,567 reads mapping to the respective mitochondrial genomes with 860X and 1568X coverage.
Figure 2.
Graphic view of the linear mitochondrial genomes of a) Cephea cephea and b) Mastigias albipunctata. Annotated genes (n = 19) with their transcription direction indicated by arrowhead. Arrow colors: green = genes, yellow = coding regions (CDS), red = rRNA genes, pink = tRNA genes.
For C. cephea, start codons for the protein-coding genes comprise 3 TAC, 11 ATG and 1 GTG; stop codons are 2 ATT, 3 TAG and 10 TAA (Figure 2a), whereas for M. albipunctata, start codons include 3 TAC, 10 ATG and 2 GTG; stop codons comprised 1 ATT, 1 ATC, 4 TAG and 9 TAA (Figure 2b). Base compositions were A: 29.2%, C: 16.5%, G: 18.0%, T: 36.3% for C. cephea and A: 30%, C: 14.8%, G: 16.8%, T: 38.4% for M. albipunctata. 12S rRNA and 16S rRNA were predicted to be 934 bp and 1665 bp, and 935 bp and 1645 bp for C. cephea and M. albipunctata, respectively.
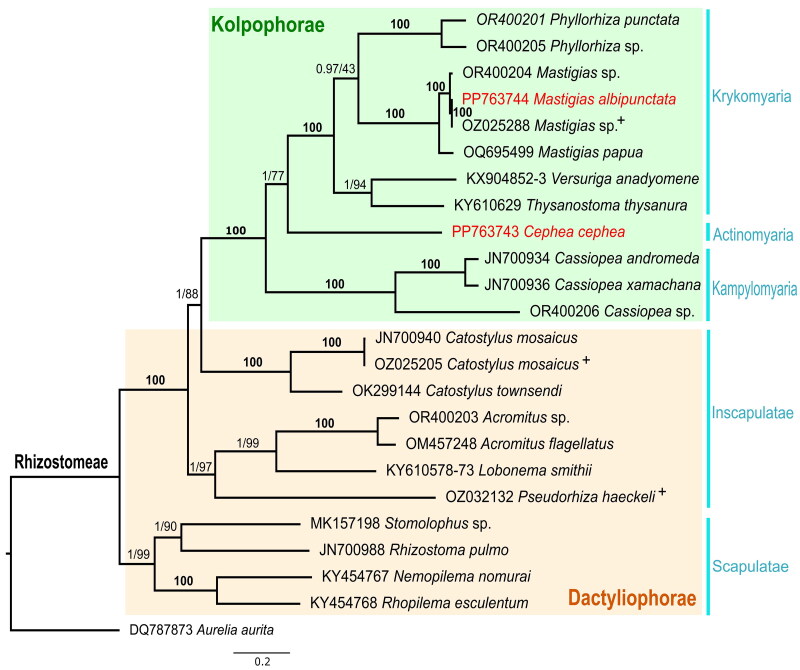
The resulting maximum likelihood tree (Figure 3) consisted of 24 rhizostome taxa with representatives from each family. Tree topology (maximal aBayes and bootstrap support) was consistent with the hypotheses of Uchida (1926) and Bayha et al. (2010) of monophyly of the suborder Kolpophorae derived from within Dactyliophorae. Monophyly of the infraorders Kampylomyaria, Krykomyaria, and Scapulatae were strongly supported (>95% bootstrap). C. cephea was placed as sister to the infraorder Krykomyaria.
Figure 3.
Maximum likelihood phylogenetic tree with aBayes and bootstrap support for the mitochondrial genomes of 24 rhizostomes and one semaeostomeae (Aurelia aurita) outgroup. Whole or partial mitochondrial genomes were not available for three families: Versurigidae, lobonematidae and thysanostomatidae. In this phylogenetic analysis the concatenated sequences of mitochondrial genes 16S rRNA and COX1 for Versuriga anadyomene (KX904852, KX904853) and Lobonema smithii (KY610578, KY610573) were used, while only 16S rRNA was used for Thysanostoma thysanura (KY610629). Cephea cephea (PP763743) and Mastigias albipunctata (PP763744) are highlighted in red and + denotes additional mtGenomes annotated in this study. aBayes support and ultrafast bootstrap support (%) (1000 replicates) were calculated for the nodes. Bold 100 represents indices of 100 for both criteria.
Discussions and conclusions
These two new mitochondrial genomes bring the number of accessioned annotated whole Rhizostomeae mitochondrial genomes to 14. Our mitogenome for Mastigias albipunctata fell into a distinct clade with so-called ‘M. papua’ (OZ025288) with 99.041% similarity, but only 90.861% identity to M. papua (OQ695499). A recent integrative analysis of Mastigias identified three phylogeographically and morphologically distinct lineages: M. papua, M. albipunctata, and Mastigias from Tufi (Souza and Dawson 2018). To assess the identities of our Mastigias exemplars, we reconstructed a COX 1 ML-phylogenetic tree (Supplementary Figure 2; Accession numbers in Table S2) in IQ-TREE with additional data from NCBI GenBank. Results corroborate the existence of three distinct clades, with NMNH live cultures in the M. albipunctata clade. Though OZ025288 is accessioned in GenBank under the species name M. papua, our findings support its proper identity as M. albipunctata.
Our analyses (Supplementary Figure 3) uncovered other likely errors in taxon identity. For instance, Ling et al. (2023) noted uncertainty in the identity of the juveniles from which they derived the sequences accessioned as Phyllorhiza punctata (OR400204) and Acromitus sp. (OR400205), which we discovered correspond to Mastigias and Phyllorhiza respectively.
Phylogenetic analysis corroborates previous studies that strongly support the monophyly of Kolpophorae, derived from within Dactyliophorae, highlighting the need for broader genomic sequencing of Rhizostomeae genera to fill data gaps. A similar phylogenetic approach conducted on the endosymbiotic Symbiodiniaceae DNA extracted from the rhizostome host offers a tractable method to explore patterns of Cnidaria-Dinoflagellate coevolution.
Supplementary Material
Funding Statement
This work was supported by the US National Science Foundation, Division Of Integrative Organismal Systems Awards 2227068-70; World Premier International Research Center Initiative (WPI) AIMEC, MEXT, Japan.
Author contributions
AGC and KCT conceived the project, AGC collected the jellyfish samples and conducted the experiments. AGC and KCT analyzed the data with support from CLA. All authors wrote and revised the paper and approved the final manuscript before submission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical approval
This study did not involve human participants or animals. The jellyfishes Cephea cephea (Forskål, 1775) and Mastigias albipunctata Stiasny, 1920 are not endangered or protected species. Therefore, ethical approval or permission was not necessary.
Data availability statement
The genome sequence data supporting this study’s findings are available at GenBank (https://www.ncbi.nlm.nih.gov/) under accession number PP763743-PP763744. The associated BioProject, SRA and Bio-Sample numbers are PRJNA1077907, SRR28865914-5 and SAMN41149076-7 respectively. Supplementary materials (https://doi.org/10.5281/zenodo.13961219) and our annotations of three other rhizostome mitochondrial genomes in GenBank are available here: https://doi.org/10.5281/zenodo.11580939.
References
- Ames CL, Klompen AML, Badhiwala K, Muffet K, Reft AJ, Kumar M, Janssen JD, Schultzhaus JN, Field LD, Muroski ME, et al. 2020. Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun Biol. 3(1):67. doi: 10.1038/s42003-020-0777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayha KM, , Dawson MN, , Collins AG, , Barbeitos MS, , Haddock SHD. 2010. Evolutionary relationships among scyphozoan jellyfish families based on complete taxon sampling and phylogenetic analyses of 18S and 28S ribosomal DNA. Integr. Comp. Biol. 50(3):436–455. doi: 10.1093/icb/icq074. [DOI] [PubMed] [Google Scholar]
- Brotz L, Schiariti A, López-Martínez J, Álvarez-Tello J, Peggy Hsieh Y-H, Jones RP, Quiñones J, Dong Z, Morandini AC, Preciado M, et al. 2017. Jellyfish fisheries in the Americas: origin, state of the art, and perspectives on new fishing grounds. Rev Fish Biol Fish. 27(1):1–29. doi: 10.1007/s11160-016-9445-y. [DOI] [Google Scholar]
- Djeghri N, Pondaven P, Stibor H, Dawson MN.. 2019. Review of the diversity, traits, and ecology of zooxanthellate jellyfishes. Mar Biol. 166(11):147. doi: 10.1007/s00227-019-3581-6. [DOI] [Google Scholar]
- Glenn TC, Nilsen RA, Kieran TJ, Sanders JG, Bayona-Vásquez NJ, Finger JW, Pierson TW, Bentley KE, Hoffberg SL, Louha S, et al. 2019. Adapterama I: universal stubs and primers for 384 unique dual-indexed or 147,456 combinatorially-indexed Illumina libraries (iTru & iNext). PeerJ. 7:e7755. doi: 10.7717/peerj.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS.. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling MK, Yap NWL, Iesa IB, Yip ZT, Huang D, Quek ZBR.. 2023. Revisiting mitogenome evolution in Medusozoa with eight new mitochondrial genomes. iScience. 26(11):108252. doi: 10.1016/j.isci.2023.108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Chan PP.. 2016. tRNAscan-SE On-line: search and contextual analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–57. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohdera AH, Abrams MJ, Ames CL, Baker DM, Suescún-Bolívar LP, Collins AG, Freeman CJ, Gamero-Mora E, Goulet TL, Hofmann DK, et al. . 2018. Upside-down but headed in the right direction: review of the highly versatile cassiopea xamachana system. Front Ecol Evol. 6. doi: 10.3389/fevo.2018.00035. [DOI] [Google Scholar]
- Omori M, , Nakano E. 2001. Jellyfish fisheries in southeast Asia. Hydrobiologia. 451(1/3):19–26. doi: 10.1023/A:1011879821323. [DOI] [Google Scholar]
- Souza MRD, Dawson MN.. 2018. Redescription of Mastigias papua (Scyphozoa, Rhizostomeae) with designation of a neotype and recognition of two additional species. Zootaxa. 4457(4):520–536. doi: 10.11646/zootaxa.4457.4.2. [DOI] [PubMed] [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ.. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T. 1926. The anatomy and development of a Rhizostome medusa Mastigias papua L. Agassiz, with observations on the phylogeny of Rhizostomeae. J Fac Sci, Tokyo Univ, Sect IV. Zool. 1:45–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequence data supporting this study’s findings are available at GenBank (https://www.ncbi.nlm.nih.gov/) under accession number PP763743-PP763744. The associated BioProject, SRA and Bio-Sample numbers are PRJNA1077907, SRR28865914-5 and SAMN41149076-7 respectively. Supplementary materials (https://doi.org/10.5281/zenodo.13961219) and our annotations of three other rhizostome mitochondrial genomes in GenBank are available here: https://doi.org/10.5281/zenodo.11580939.