Abstract
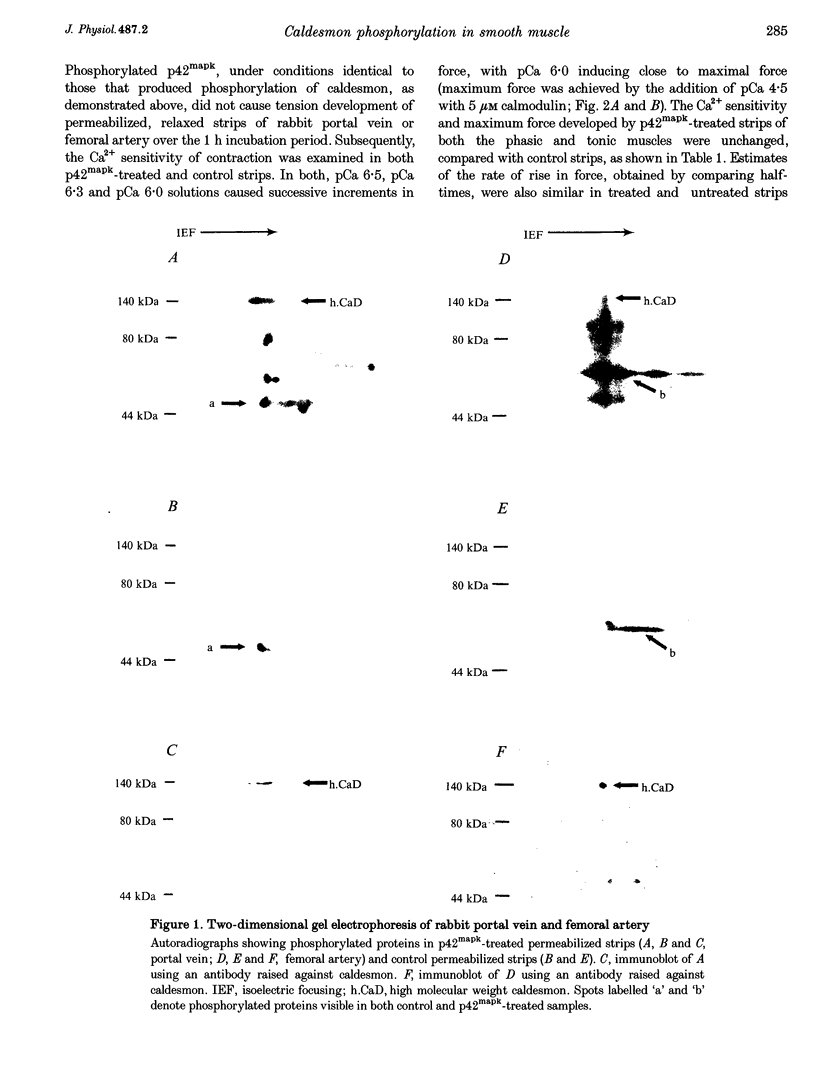
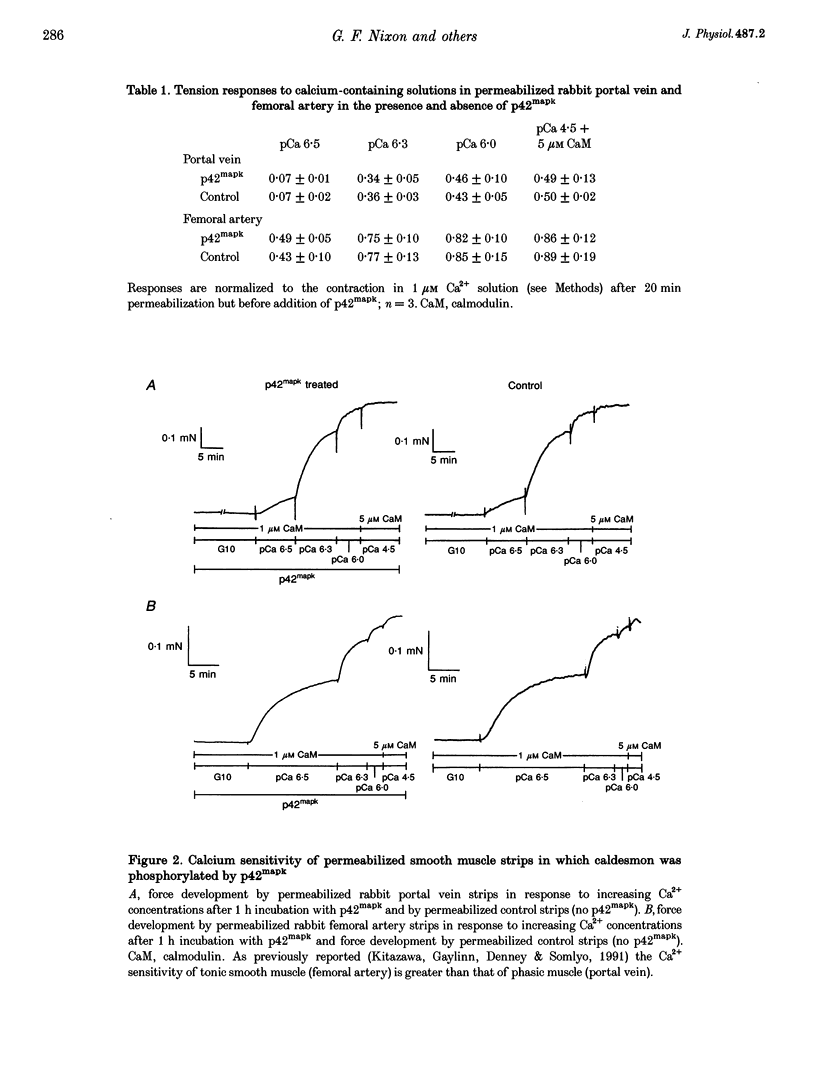
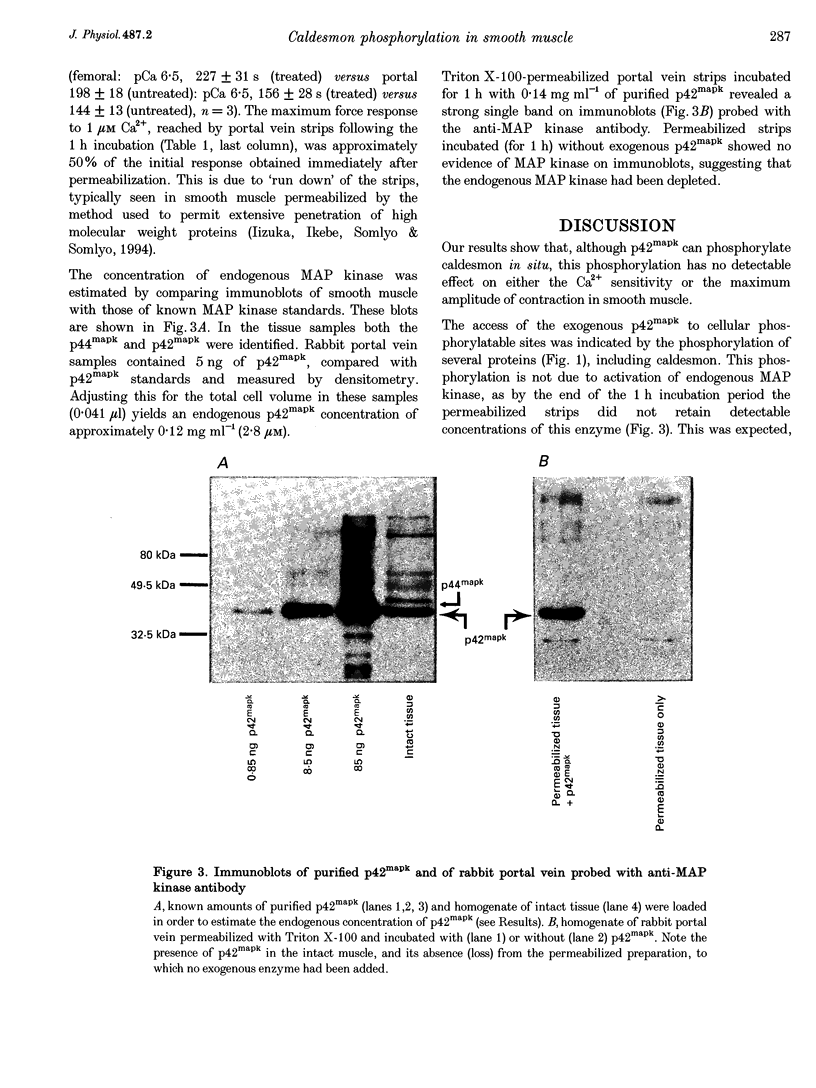
1. Recombinant, activated mitogen-activated protein kinase (3.3 microM; p42mapk) phosphorylated caldesmon in phasic (rabbit portal vein) and tonic (rabbit femoral artery) smooth muscle strips permeabilized with Triton X-100. 2. Phosphorylation of caldesmon by p42mapk neither induced contraction of relaxed smooth muscle nor affected the Ca2+ sensitivity of submaximally contracted permeabilized phasic or tonic smooth muscle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam L. P., Gapinski C. J., Hathaway D. R. Phosphorylation sequences in h-caldesmon from phorbol ester-stimulated canine aortas. FEBS Lett. 1992 May 18;302(3):223–226. doi: 10.1016/0014-5793(92)80446-n. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Adam L. P., Hathaway D. R. Identification of mitogen-activated protein kinase phosphorylation sequences in mammalian h-Caldesmon. FEBS Lett. 1993 May 3;322(1):56–60. doi: 10.1016/0014-5793(93)81110-l. [DOI] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Chalovich J. M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs T. J., Mak A. S. Smooth-muscle mitogen-activated protein (MAP) kinase: purification and characterization, and the phosphorylation of caldesmon. Biochem J. 1993 Dec 15;296(Pt 3):745–751. doi: 10.1042/bj2960745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs T. J., Watson M. H., Sanghera J. S., Campbell D. L., Pelech S. L., Mak A. S. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22853–22859. [PubMed] [Google Scholar]
- Haeberle J. R., Hathaway D. R., Smith C. L. Caldesmon content of mammalian smooth muscles. J Muscle Res Cell Motil. 1992 Feb;13(1):81–89. doi: 10.1007/BF01738431. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Dent P., Wu J., Haystead C. M., Sturgill T. W. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 1992 Jul 13;306(1):17–22. doi: 10.1016/0014-5793(92)80828-5. [DOI] [PubMed] [Google Scholar]
- Iizuka K., Ikebe M., Somlyo A. V., Somlyo A. P. Introduction of high molecular weight (IgG) proteins into receptor coupled, permeabilized smooth muscle. Cell Calcium. 1994 Dec;16(6):431–445. doi: 10.1016/0143-4160(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Kasturi R., Vasulka C., Johnson J. D. Ca2+, caldesmon, and myosin light chain kinase exchange with calmodulin. J Biol Chem. 1993 Apr 15;268(11):7958–7964. [PubMed] [Google Scholar]
- Katsuyama H., Wang C. L., Morgan K. G. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992 Jul 25;267(21):14555–14558. [PubMed] [Google Scholar]
- Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991 Jan 25;266(3):1708–1715. [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Pato M. D., Sutherland C., Winder S. J., Walsh M. P. Smooth-muscle caldesmon phosphatase is SMP-I, a type 2A protein phosphatase. Biochem J. 1993 Jul 1;293(Pt 1):35–41. doi: 10.1042/bj2930035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzer G., Zeugner C., Troschka M., Chalovich J. M. Caldesmon and a 20-kDa actin-binding fragment of caldesmon inhibit tension development in skinned gizzard muscle fiber bundles. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5904–5908. doi: 10.1073/pnas.90.13.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter K., Marston S. B. Phosphorylation of vascular smooth muscle caldesmon by endogenous kinase. FEBS Lett. 1992 Jul 6;305(3):192–196. doi: 10.1016/0014-5793(92)80665-4. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Walsh M. P. The Ayerst Award Lecture 1990. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem Cell Biol. 1991 Dec;69(12):771–800. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]





