Abstract
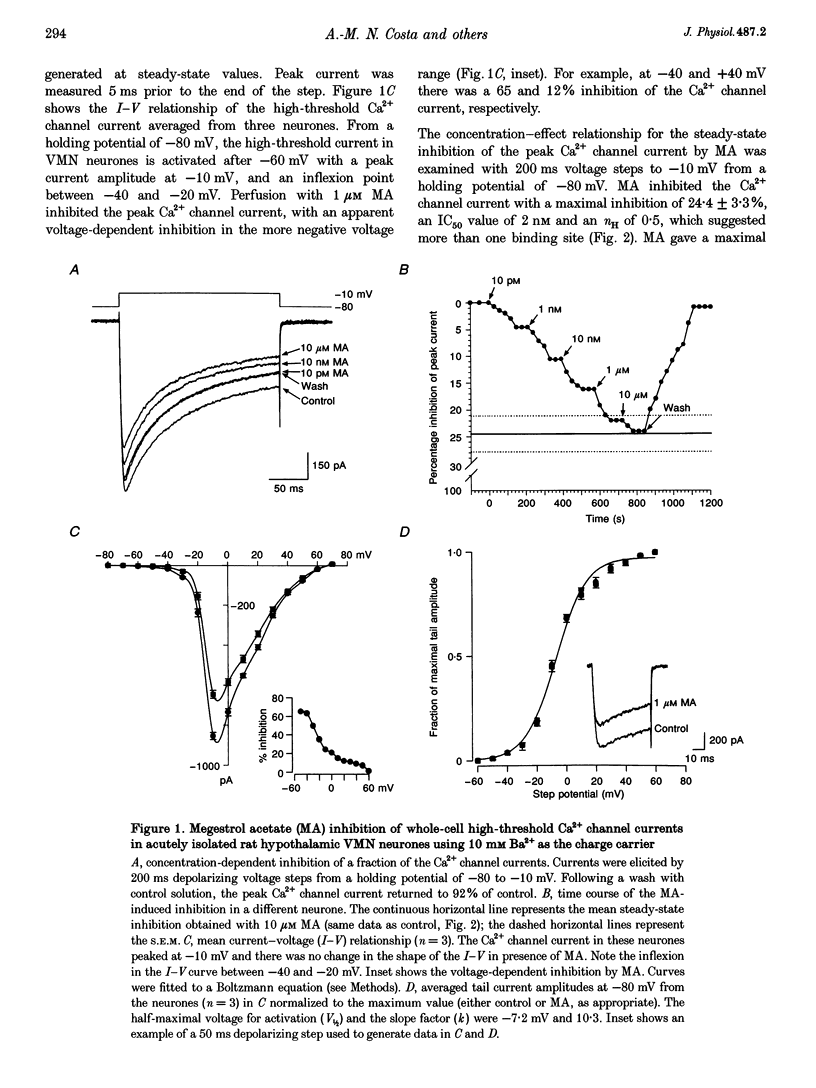
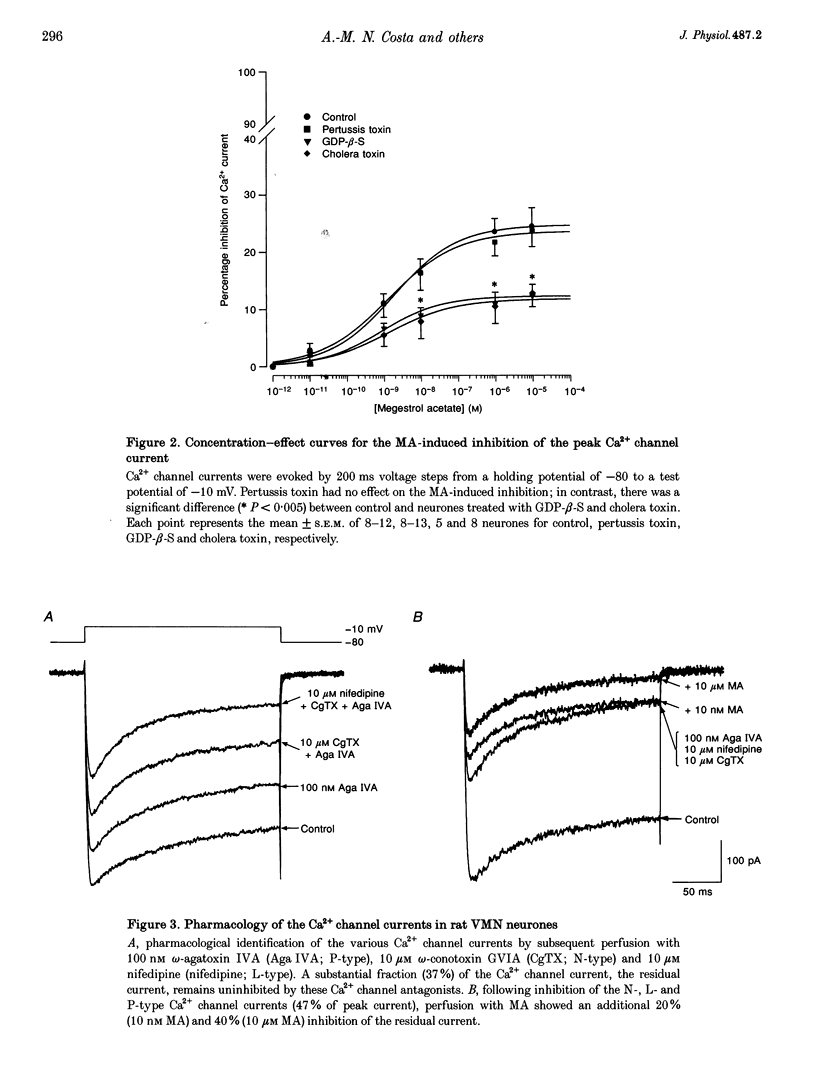
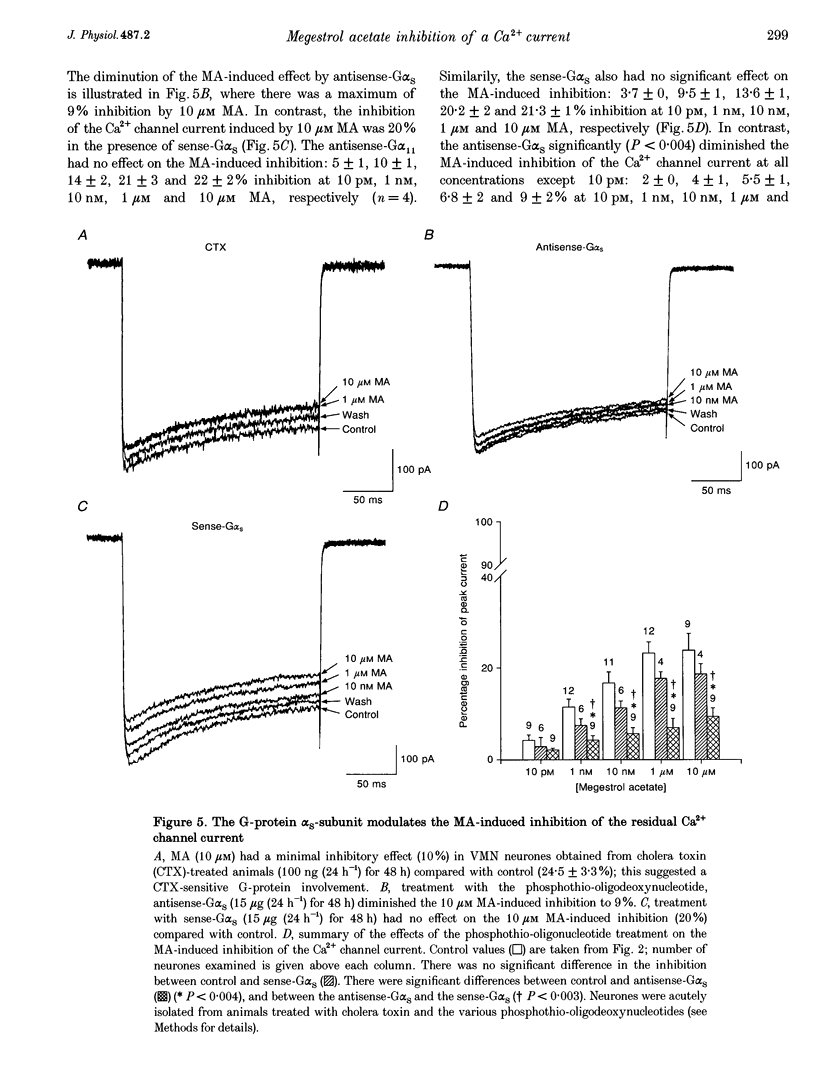
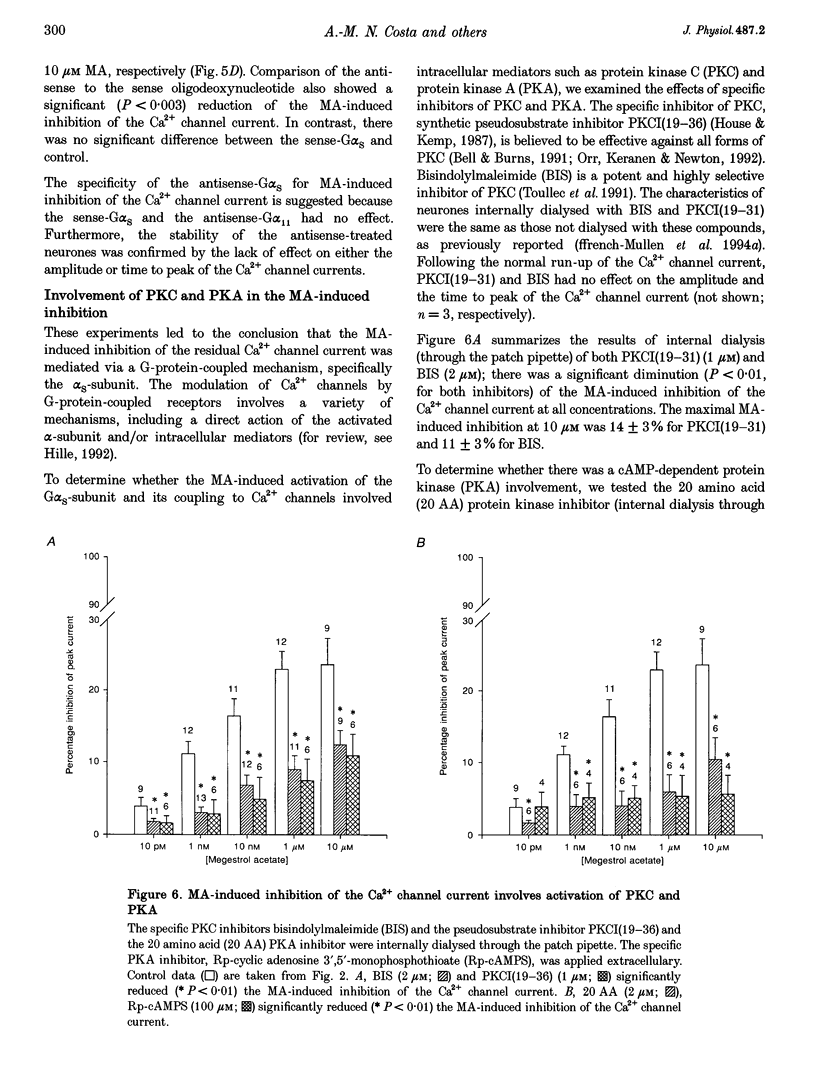
1. The inhibition of voltage-activated Ca2+ channel currents by the orally active progesterone derivative, megestrol acetate (MA), was examined in freshly dissociated rat ventromedial hypothalamic nucleus (VMN) neurones using the whole-cell voltage-clamp technique with 10 mM Ba2+ as the charge carrier. 2. The steady-state inhibition of the peak high-threshold Ca2+ channel current evoked by depolarization from -80 to -10 mV by MA increased in a concentration-dependent fashion. MA inhibited a fraction of the whole-cell Ca2+ channel current while progesterone had no effect on the peak Ca2+ channel current (7% at 10 microM). The low-threshold Ca2+ (T-type) current, evoked from -100 to -30 mV, was unaffected by MA. 3. Intracellular dialysis with MA had no effect on the Ca2+ channel current. Concomitant extracellular perfusion of MA showed normal inhibitory activity, suggesting that the MA binding site can only be accessed extracellularly. 4. The high-threshold Ca2+ channel current in VMN neurones was found to consist of four pharmacologically distinguishable components: an N-type current, an L-type current, a P-type current, and a residual current. MA had no effect on the N-, L- and P-type Ca2+ channel currents, but inhibited the residual current. 5. In neurones isolated from cholera toxin-treated animals, the MA-induced inhibition of the Ca2+ channel current was significantly diminished, suggesting a G-protein alpha S-subunit involvement. 6. Treatment with antisense phosphothio-oligodeoxynucleotides to the G alpha S-subunit (antisense-G alpha S) significantly reduced the MA-induced inhibition of the Ca2+ channel current. Treatment with either sense-G alpha S or antisense-G alpha 11 had no effect, confirming a G alpha S-subunit involvement. 7. These results suggest that appetite enhancement induced by MA in cachectic patients may in part be due to a novel central nervous system action, that is, inhibition of a fraction of the whole-cell Ca2+ channel current to attenuate the firing of VMN neurones that may be involved in satiety mechanisms.
Full text
PDF




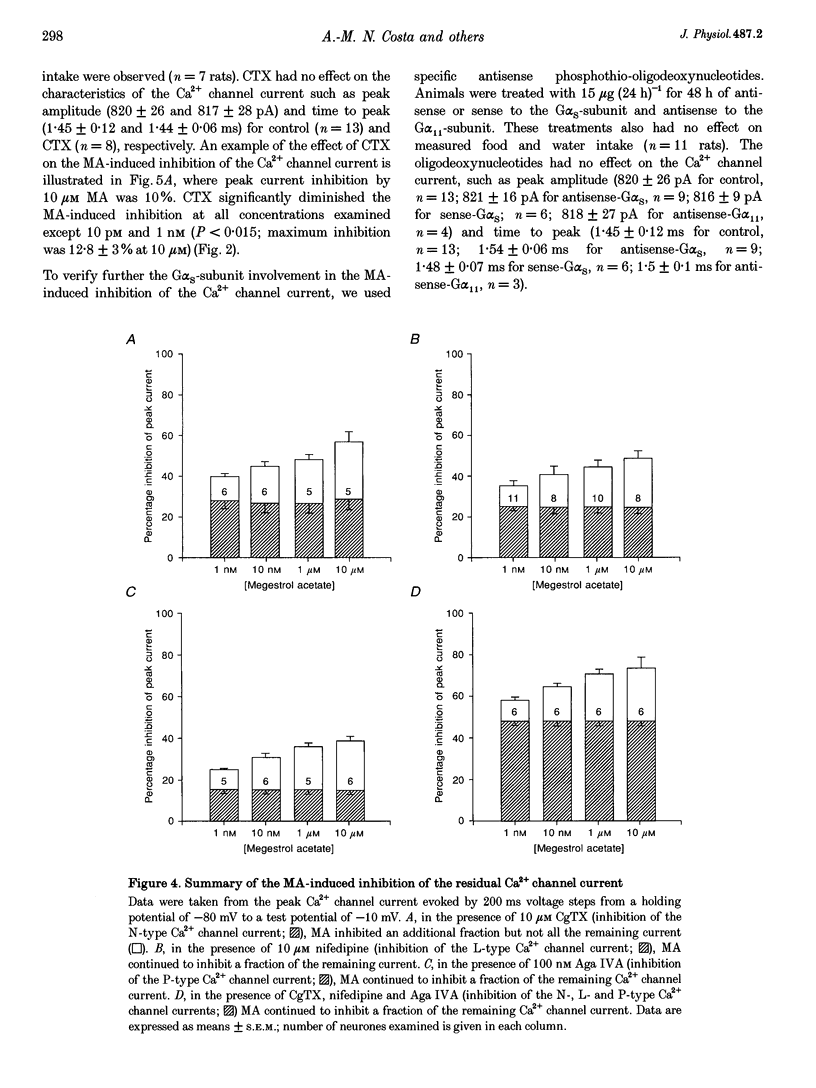



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisner J., Tchekmedyian N. S., Moody M., Tait N. High-dose megestrol acetate for the treatment of advanced breast cancer: dose and toxicities. Semin Hematol. 1987 Apr;24(2 Suppl 1):48–55. [PubMed] [Google Scholar]
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Bertrand D., Valera S., Bertrand S., Ballivet M., Rungger D. Steroids inhibit nicotinic acetylcholine receptors. Neuroreport. 1991 May;2(5):277–280. doi: 10.1097/00001756-199105000-00016. [DOI] [PubMed] [Google Scholar]
- Bielajew C., Stenger J., Schindler D. Factors that contribute to the reduced weight gain following chronic ventromedial hypothalamic stimulation. Behav Brain Res. 1994 Jun 30;62(2):143–148. doi: 10.1016/0166-4328(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bruera E. Current pharmacological management of anorexia in cancer patients. Oncology (Williston Park) 1992 Jan;6(1):125-30; discussion 132, 137. [PubMed] [Google Scholar]
- Carbone E., Swandulla D. Neuronal calcium channels: kinetics, blockade and modulation. Prog Biophys Mol Biol. 1989;54(1):31–58. doi: 10.1016/0079-6107(89)90008-4. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Dostmann W. R., Taylor S. S., Genieser H. G., Jastorff B., Døskeland S. O., Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3',5'-cyclic phosphorothioates. J Biol Chem. 1990 Jun 25;265(18):10484–10491. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Eliot L. S., Johnston D. Multiple components of calcium current in acutely dissociated dentate gyrus granule neurons. J Neurophysiol. 1994 Aug;72(2):762–777. doi: 10.1152/jn.1994.72.2.762. [DOI] [PubMed] [Google Scholar]
- Frey U., Huang Y. Y., Kandel E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993 Jun 11;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Graham K. K., Mikolich D. J., Fisher A. E., Posner M. R., Dudley M. N. Pharmacologic evaluation of megestrol acetate oral suspension in cachectic AIDS patients. J Acquir Immune Defic Syndr. 1994 Jun;7(6):580–586. [PubMed] [Google Scholar]
- Gregory E. J., Cohen S. C., Oines D. W., Mims C. H. Megestrol acetate therapy for advanced breast cancer. J Clin Oncol. 1985 Feb;3(2):155–160. doi: 10.1200/JCO.1985.3.2.155. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Parnes H., Gordon G. B., Shantz L. M., O'Donnell K. A., Aisner J. Megestrol acetate-induced differentiation of 3T3-L1 adipocytes in vitro. Semin Oncol. 1988 Apr;15(2 Suppl 1):76–78. [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992 Aug;9(2):187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Loprinzi C. L., Goldberg R. M., Burnham N. L. Cancer-associated anorexia and cachexia. Implications for drug therapy. Drugs. 1992 Apr;43(4):499–506. doi: 10.2165/00003495-199243040-00006. [DOI] [PubMed] [Google Scholar]
- Loprinzi C. L., Schaid D. J., Dose A. M., Burnham N. L., Jensen M. D. Body-composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993 Jan;11(1):152–154. doi: 10.1200/JCO.1993.11.1.152. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Morgan L. R. Megestrol acetate v tamoxifen in advanced breast cancer in postmenopausal patients. Semin Oncol. 1985 Mar;12(1 Suppl 1):43–47. [PubMed] [Google Scholar]
- Nelson K. A., Walsh D., Sheehan F. A. The cancer anorexia-cachexia syndrome. J Clin Oncol. 1994 Jan;12(1):213–225. doi: 10.1200/JCO.1994.12.1.213. [DOI] [PubMed] [Google Scholar]
- Orr J. W., Keranen L. M., Newton A. C. Reversible exposure of the pseudosubstrate domain of protein kinase C by phosphatidylserine and diacylglycerol. J Biol Chem. 1992 Aug 5;267(22):15263–15266. [PubMed] [Google Scholar]
- Plata-Salamán C. R. Regulation of hunger and satiety in man. Dig Dis. 1991;9(5):253–268. doi: 10.1159/000171310. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Richmond G., Clemens L. Ventromedial hypothalamic lesions and cholinergic control of female sexual behavior. Physiol Behav. 1988;42(2):179–182. doi: 10.1016/0031-9384(88)90295-8. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Waterhouse B. D., Woodward D. J. Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull. 1987 Jun;18(6):739–747. doi: 10.1016/0361-9230(87)90209-7. [DOI] [PubMed] [Google Scholar]
- Tisdale M. J. Cancer cachexia. Anticancer Drugs. 1993 Apr;4(2):115–125. doi: 10.1097/00001813-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994 Sep;14(9):5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Y., Watkins D. C., Malbon C. C. Antisense oligodeoxynucleotides to GS protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992 Jul 23;358(6384):334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- Wu F. S., Gibbs T. T., Farb D. H. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990 May;37(5):597–602. [PubMed] [Google Scholar]
- Zhu Y., Ikeda S. R. VIP inhibits N-type Ca2+ channels of sympathetic neurons via a pertussis toxin-insensitive but cholera toxin-sensitive pathway. Neuron. 1994 Sep;13(3):657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Danks P., Spence K. T. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J Neurosci. 1994 Apr;14(4):1963–1977. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Plata-Salamán C. R., Buckley N. J., Danks P. Muscarine modulation by a G-protein alpha-subunit of delayed rectifier K+ current in rat ventromedial hypothalamic neurones. J Physiol. 1994 Jan 1;474(1):21–26. doi: 10.1113/jphysiol.1994.sp019998. [DOI] [PMC free article] [PubMed] [Google Scholar]



