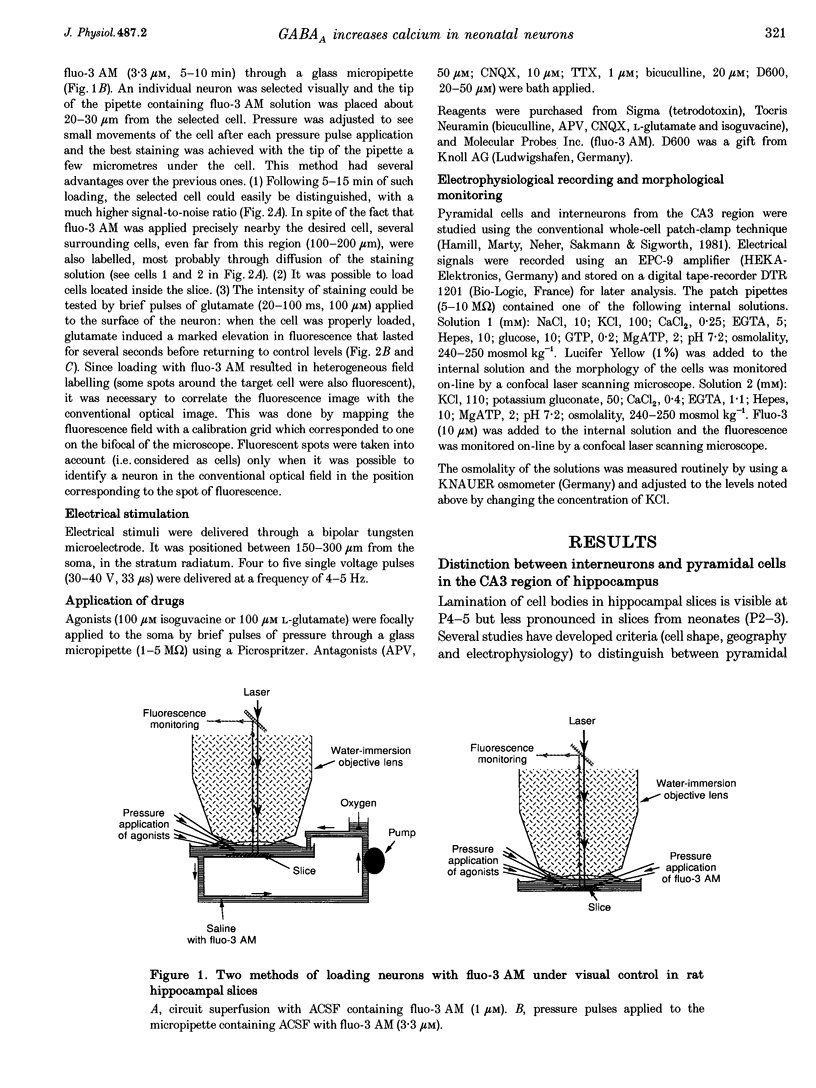
Abstract
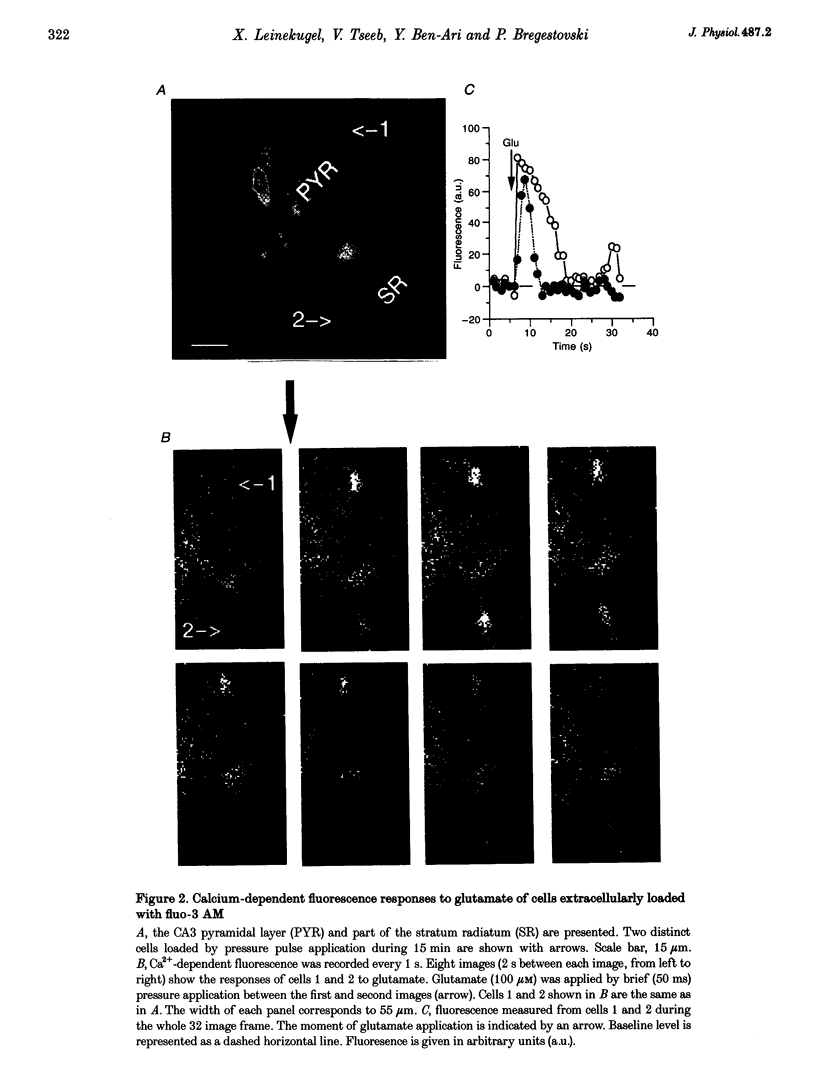
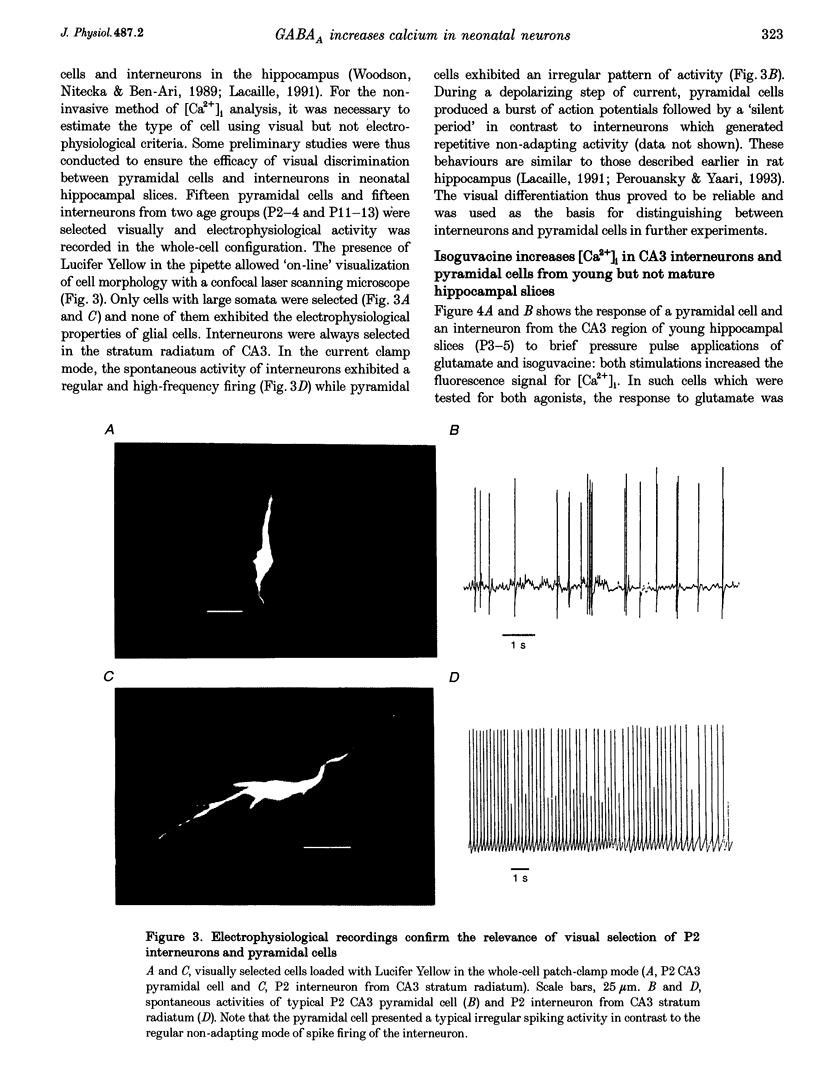
1. Changes in intracellular Ca2+ concentration ([Ca2+]i) induced by activation of GABAA receptors (synaptic stimulation or application of the GABAA agonist isoguvacine) were studied on pyramidal cells and interneurons from hippocampal slices of rats from two age groups (postnatal days (P) 2-5 and P12-13) using the fluorescent dye fluo-3 and a confocal laser scanning microscope. Cells were loaded with the dye either intracellularly, using patch pipettes containing fluo-3 in the internal solution, or extracellularly, using pressure pulses applied to an extracellular pipette containing the permeant dye fluo-3 AM. 2. Interneurons and pyramidal cells from P2-5 slices loaded with fluo-3 AM responded by an increase in [Ca2+]i to isoguvacine and to glutamate, in contrast to cells from P12-13 slices which responded to glutamate but not to isoguvacine. 3. The isoguvacine-induced rise in [Ca2+]i was reversibly blocked by bath application of the GABAA receptor antagonist bicuculline (20 microM), suggesting the specific involvement of GABAA receptors. The sodium channel blocker tetrodotoxin (TTX, 1 microM in the bath) did not prevent the isoguvacine-induced rise in [Ca2+]i. 4. The isoguvacine-induced rise in [Ca2+]i was reversibly blocked by bath application of the calcium channel blocker D600 (50 microM) suggesting the involvement of voltage-dependent Ca2+ channels. 5. Electrical stimulation of afferent fibres induced a transient increase in [Ca2+]i in neonatal pyramidal cells and interneurons (P5) loaded non-invasively with fluo-3 AM. This elevation of [Ca2+]i was reversibly blocked by bicuculline (20 microM) but not by APV (50 microM) and CNQX (10 microM). 6. During simultaneous electrophysiological recording in the current-clamp mode and [Ca2+]i monitoring from P5 pyramidal cells, electrical stimulation of afferent fibres, in the presence of APV (50 microM) and CNQX (10 microM), caused synaptic depolarization accompanied by a few action potentials and a transient increase in [Ca2+]i. In voltage clamp (-70 mV) however, there was no increase in [Ca2+]i following synaptic stimulation, showing that it is depolarization dependent. 7. Using a non-invasive method of [Ca2+]i monitoring, we demonstrate here that in neonatal (P2-5) hippocampus, GABA is an excitatory neurotransmitter which can cause an elevation of [Ca2+]i in interneurons and pyramidal cells via activation of voltage-dependent Ca2+ channels. This action may underlie the trophic role of GABA in hippocampal development.
Full text
PDF

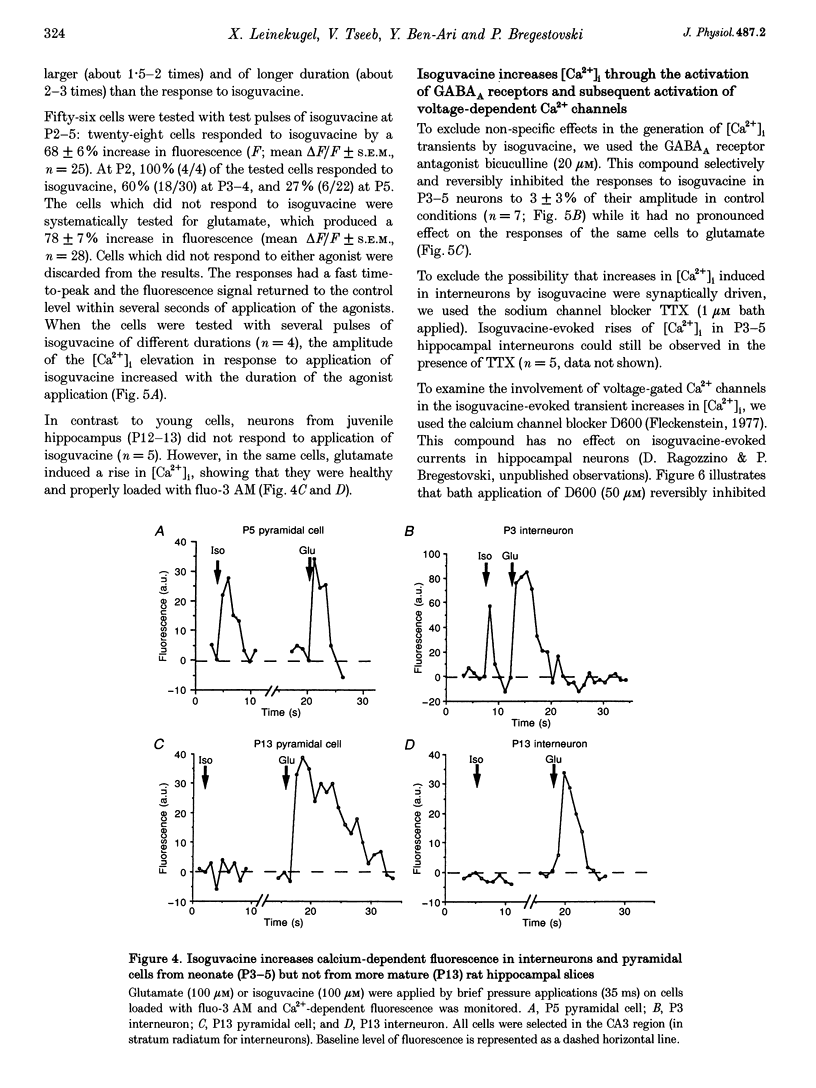
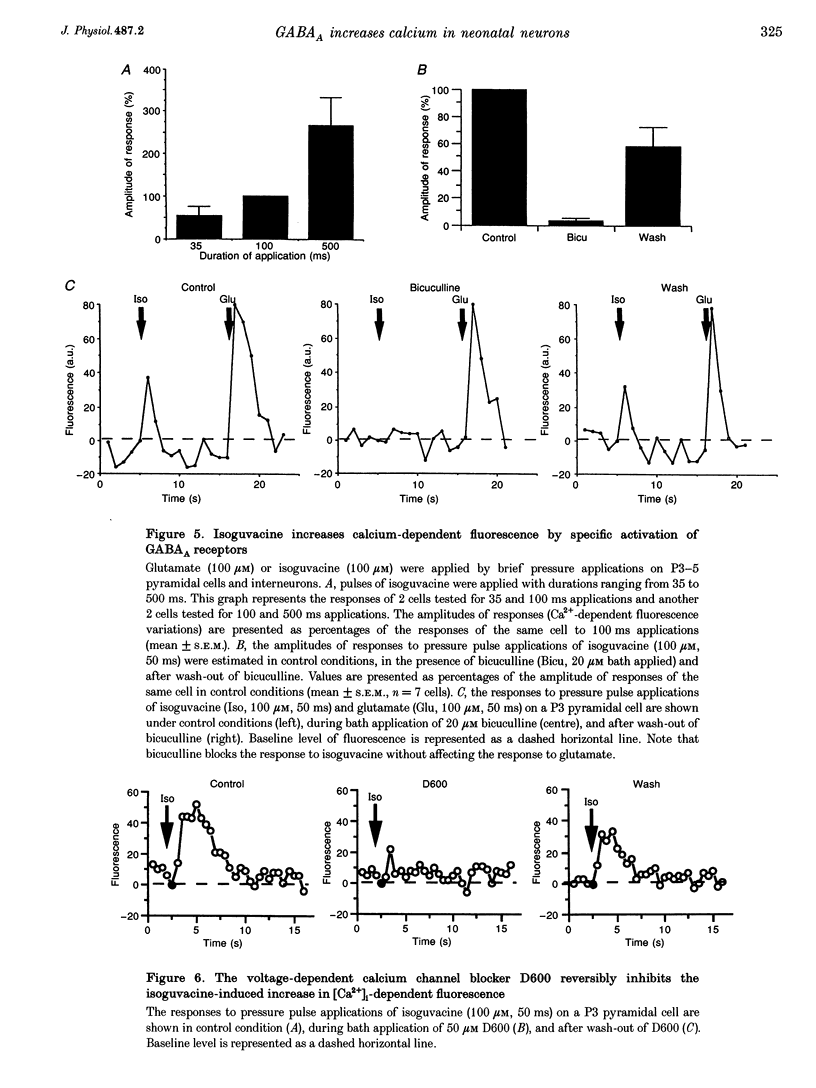
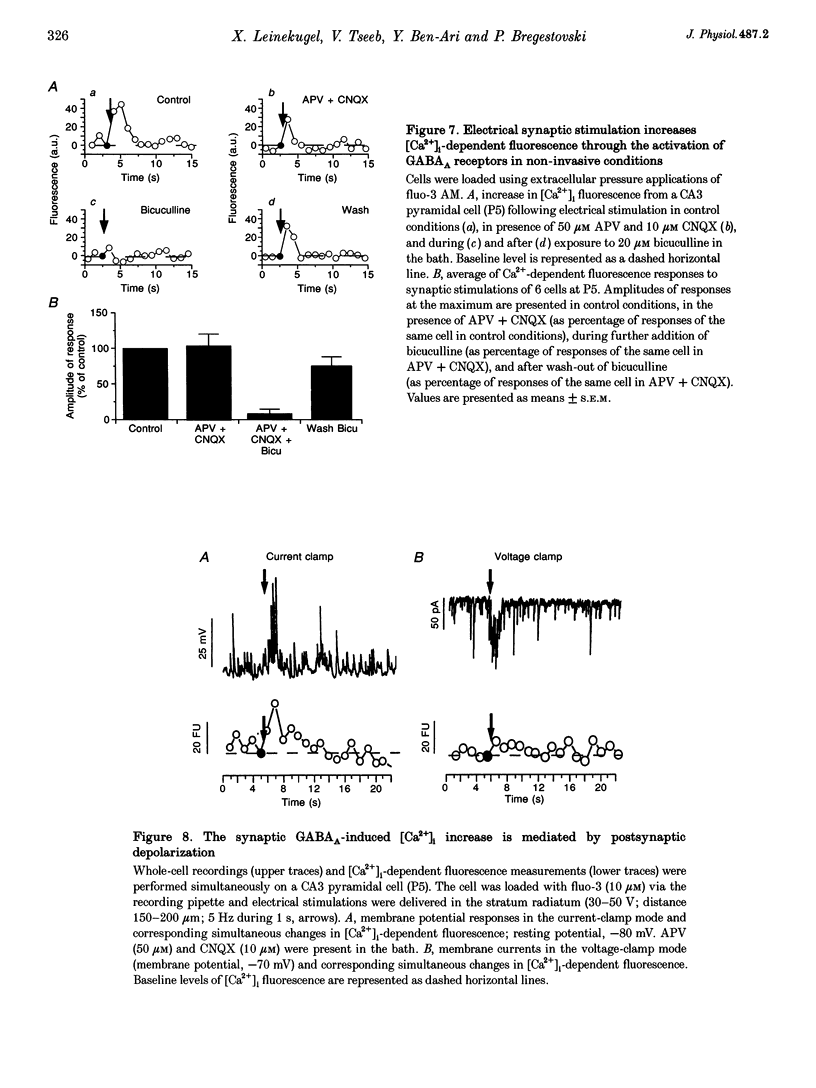



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Dent J. A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981 Jan 1;195(1):51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Barbin G., Pollard H., Gaïarsa J. L., Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993 Apr 2;152(1-2):150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980 Mar 1;190(1):87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Belhage B., Hansen G. H., Schousboe A., Meier E. GABA agonist promoted formation of low affinity GABA receptors on cerebellar granule cells is restricted to early development. Int J Dev Neurosci. 1988;6(2):125–128. doi: 10.1016/0736-5748(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Enna S. J. Neurochemical aspects of the ontogenesis of GABAnergic neurons in the rat brain. Brain Res. 1976 Jul 23;111(1):119–133. doi: 10.1016/0006-8993(76)91053-2. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Hales T. G., Sanderson M. J., Charles A. C. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology. 1994 Mar;59(3):297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen G. H., Belhage B., Schousboe A., Meier E. Gamma-aminobutyric acid agonist-induced alterations in the ultrastructure of cultured cerebellar granule cells is restricted to early development. J Neurochem. 1988 Jul;51(1):243–245. doi: 10.1111/j.1471-4159.1988.tb04862.x. [DOI] [PubMed] [Google Scholar]
- Horváth G., Acs Z., Mergl Z., Nagy I., Makara G. B. gamma-Aminobutyric acid-induced elevation of intracellular calcium concentration in pituitary cells of neonatal rats. Neuroendocrinology. 1993 Jun;57(6):1028–1034. doi: 10.1159/000126467. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y., Sciancalepore M., Stratta F., Martina M., Cherubini E. Developmental changes in spontaneous GABAA-mediated synaptic events in rat hippocampal CA3 neurons. Eur J Neurosci. 1994 May 1;6(5):805–813. doi: 10.1111/j.1460-9568.1994.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Kater S. B., Mattson M. P., Cohan C., Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988 Jul;11(7):315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff Frank, Kettenmann Helmut. GABA Triggers a [Ca2+]i Increase in Murine Precursor Cells of the Oligodendrocyte Lineage. Eur J Neurosci. 1992 Oct;4(11):1049–1058. doi: 10.1111/j.1460-9568.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C. Postsynaptic potentials mediated by excitatory and inhibitory amino acids in interneurons of stratum pyramidale of the CA1 region of rat hippocampal slices in vitro. J Neurophysiol. 1991 Nov;66(5):1441–1454. doi: 10.1152/jn.1991.66.5.1441. [DOI] [PubMed] [Google Scholar]
- Leidenheimer N. J., Browning M. D., Harris R. A. GABAA receptor phosphorylation: multiple sites, actions and artifacts. Trends Pharmacol Sci. 1991 Mar;12(3):84–87. doi: 10.1016/0165-6147(91)90509-q. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Kater S. B. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989 Jul;12(7):265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988 Apr-Jun;472(2):179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Miyakawa H., Ross W. N., Jaffe D., Callaway J. C., Lasser-Ross N., Lisman J. E., Johnston D. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992 Dec;9(6):1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Eriksson P. S., Rönnbäck L., Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993 Jun;54(3):605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- Perouansky M., Yaari Y. Kinetic properties of NMDA receptor-mediated synaptic currents in rat hippocampal pyramidal cells versus interneurones. J Physiol. 1993 Jun;465:223–244. doi: 10.1113/jphysiol.1993.sp019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorný J., Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res Bull. 1981 Aug;7(2):121–130. doi: 10.1016/0361-9230(81)90076-9. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994 May 1;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K., Wolf G. High-affinity glutamine uptake of the rat hippocampus during postnatal development: a quantitative autoradiographic study. Neuroscience. 1990;34(1):49–55. doi: 10.1016/0306-4522(90)90303-l. [DOI] [PubMed] [Google Scholar]
- Rozenberg F., Robain O., Jardin L., Ben-Ari Y. Distribution of GABAergic neurons in late fetal and early postnatal rat hippocampus. Brain Res Dev Brain Res. 1989 Dec 1;50(2):177–187. doi: 10.1016/0165-3806(89)90193-4. [DOI] [PubMed] [Google Scholar]
- Seay-Lowe S. L., Claiborne B. J. Morphology of intracellularly labeled interneurons in the dentate gyrus of the immature rat. J Comp Neurol. 1992 Oct 1;324(1):23–36. doi: 10.1002/cne.903240104. [DOI] [PubMed] [Google Scholar]
- Segal M. GABA induces a unique rise of [Ca]i in cultured rat hippocampal neurons. Hippocampus. 1993 Apr;3(2):229–238. doi: 10.1002/hipo.450030214. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Soriano E., Cobas A., Fairén A. Asynchronism in the neurogenesis of GABAergic and non-GABAergic neurons in the mouse hippocampus. Brain Res. 1986 Nov;395(1):88–92. doi: 10.1016/s0006-8993(86)80012-9. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Capogna M., Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993 Jun;16(6):222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Wang J., Reichling D. B., Kyrozis A., MacDermott A. B. Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur J Neurosci. 1994 Aug 1;6(8):1275–1280. doi: 10.1111/j.1460-9568.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Woodson W., Nitecka L., Ben-Ari Y. Organization of the GABAergic system in the rat hippocampal formation: a quantitative immunocytochemical study. J Comp Neurol. 1989 Feb 8;280(2):254–271. doi: 10.1002/cne.902800207. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Fukuda Y. Calcium channels and GABA receptors in the early embryonic chick retina. J Neurobiol. 1993 Dec;24(12):1600–1614. doi: 10.1002/neu.480241205. [DOI] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zhang L., Spigelman I., Carlen P. L. Development of GABA-mediated, chloride-dependent inhibition in CA1 pyramidal neurones of immature rat hippocampal slices. J Physiol. 1991 Dec;444:25–49. doi: 10.1113/jphysiol.1991.sp018864. [DOI] [PMC free article] [PubMed] [Google Scholar]