Abstract
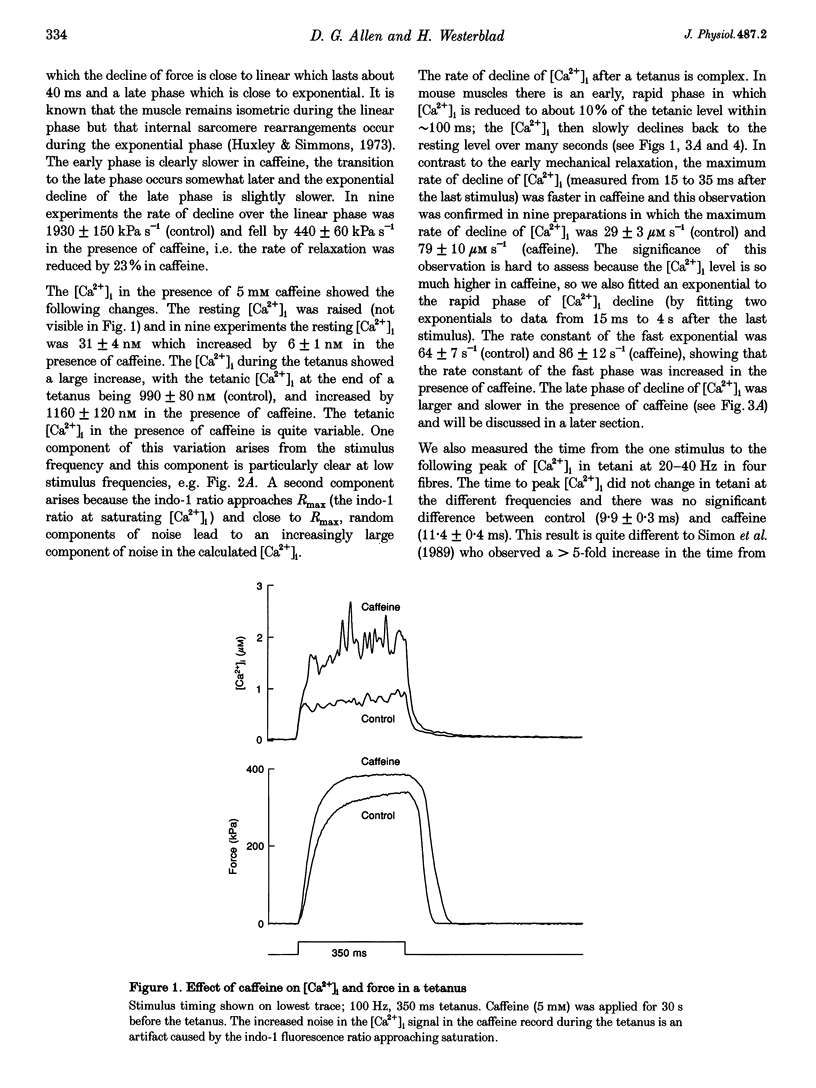
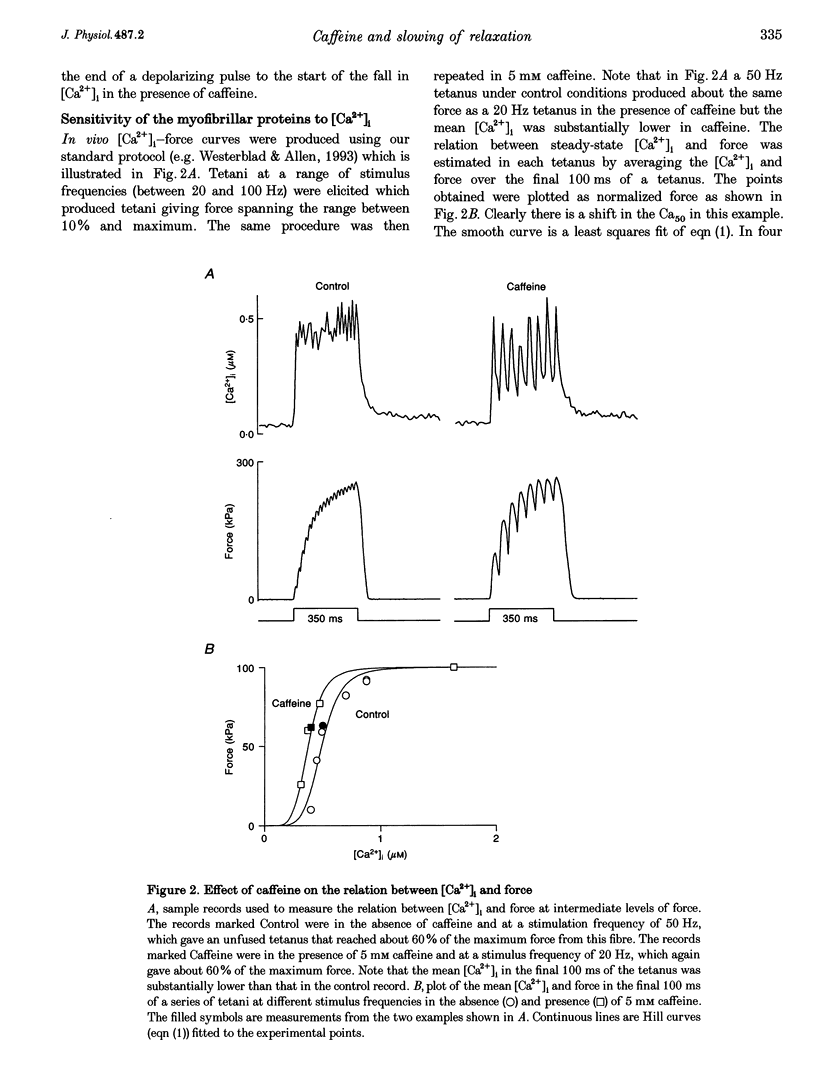
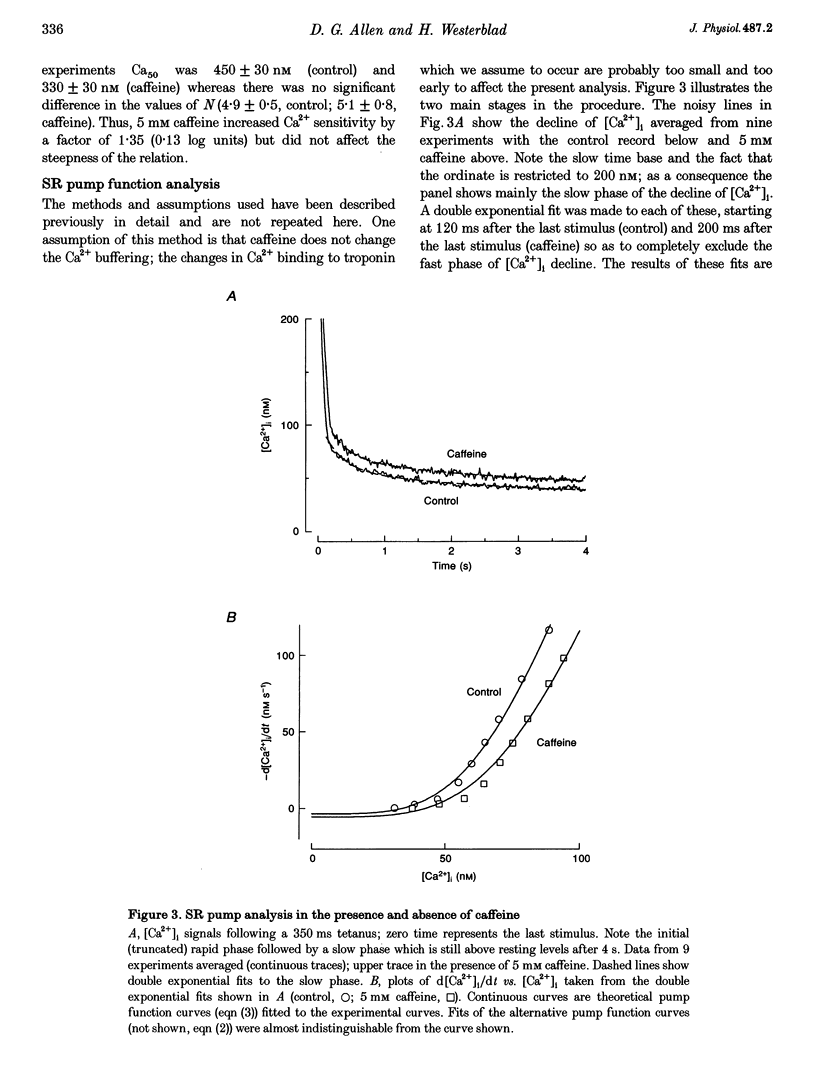
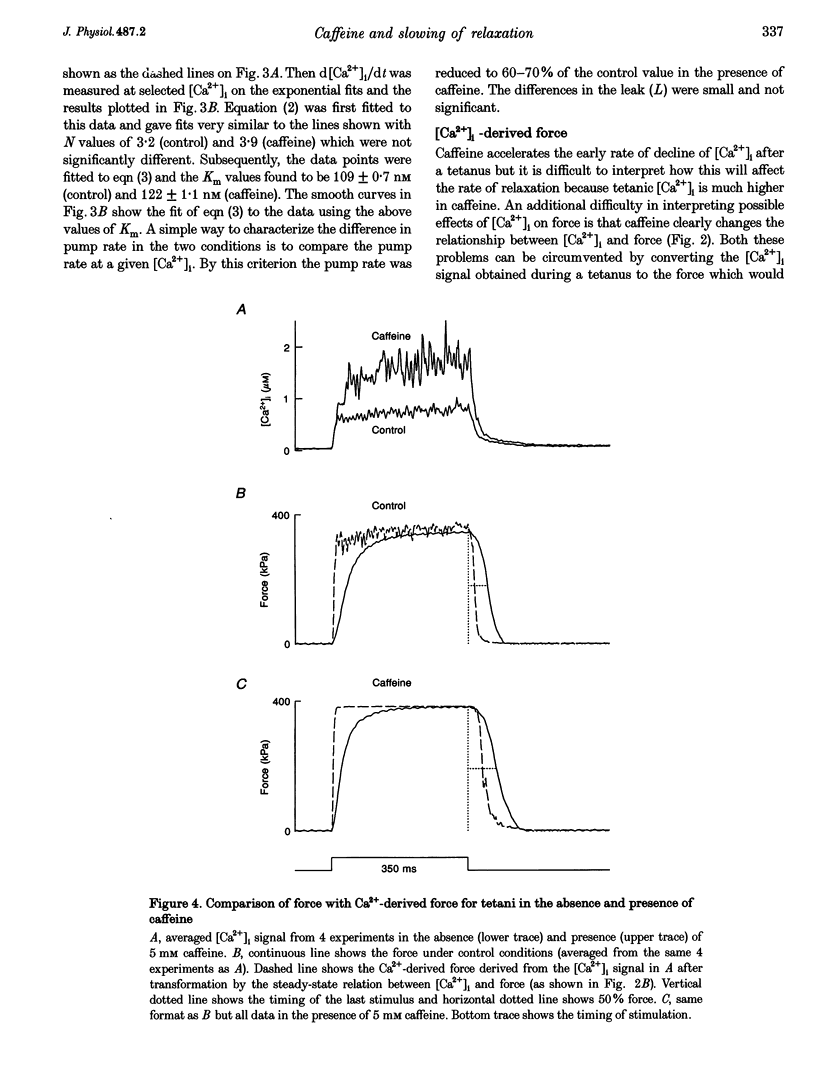
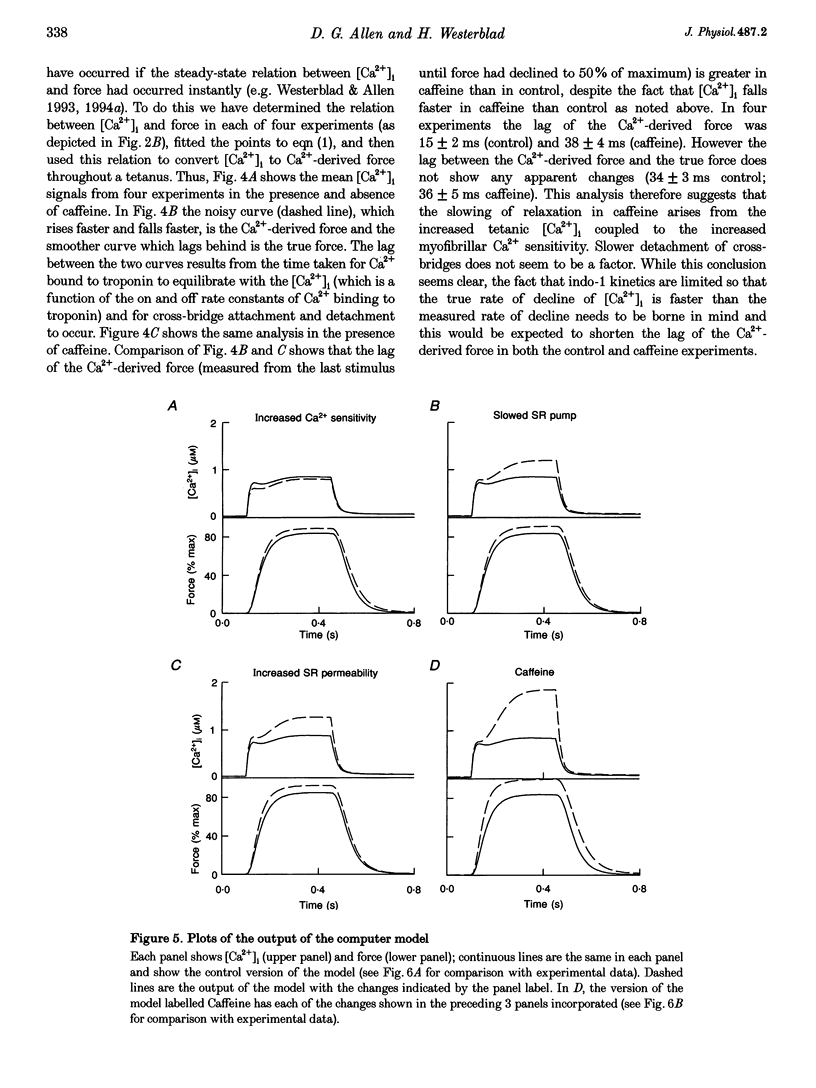
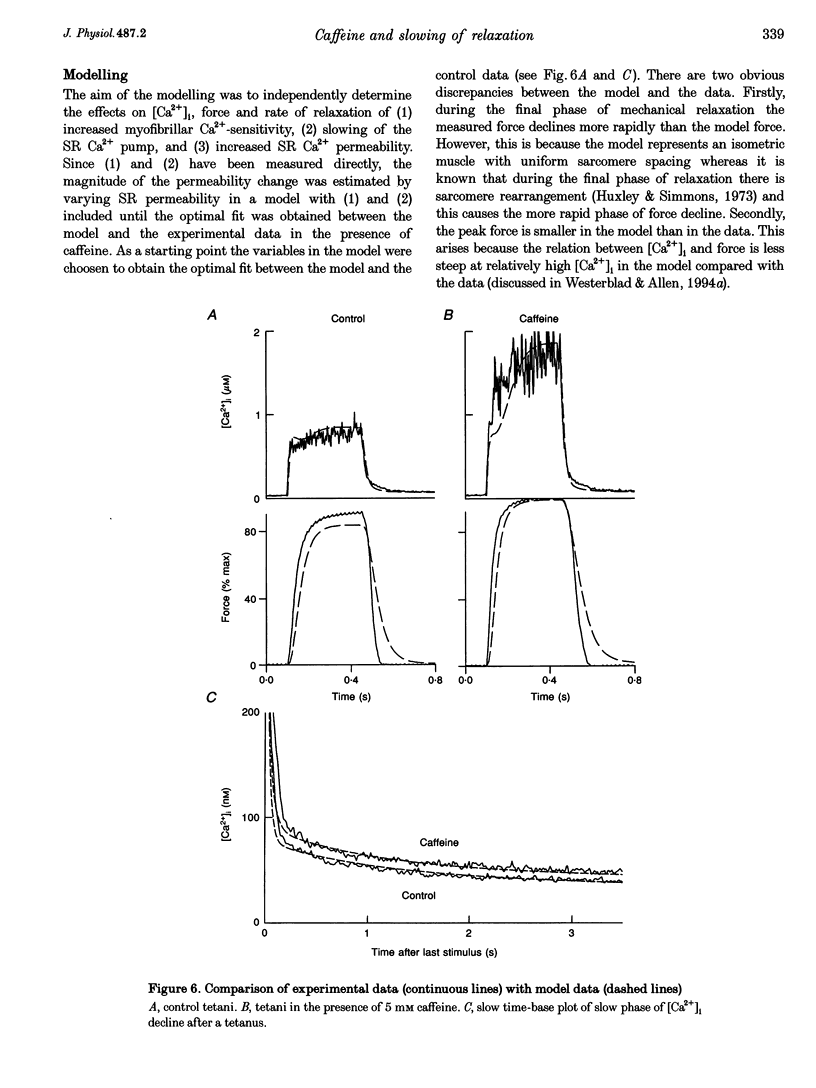
1. Intracellular calcium concentration ([Ca2+]i) and force were measured from isolated single fibres of mouse skeletal muscle. The effects of 5 mM caffeine on muscle fibres at rest and during short tetani were examined. 2. Caffeine increased tetanic tension and slowed the rate of relaxation. [Ca2+]i was increased in the presence of caffeine both in the resting muscle and during tetani. The time course of decline of [Ca2+]i after a tetanus is complex with a large, early, rapid phase followed by a smaller and slower phase. Caffeine accelerated the early phase but slowed the later phase. 3. The sensitivity of the myofibrillar proteins to Ca2+ measured in the intact fibre was increased in the presence of caffeine, confirming earlier findings on skinned muscle fibres. 4. Analysis of the late phase of the decline of [Ca2+]i after a tetanus provides information about the properties of the sarcoplasmic reticulum (SR) Ca2+ pump. Caffeine slowed the pump to 60-70% of the control value at a given [Ca2+]i but had no effect on the Ca2+ leak from the SR. 5. Analysis of relaxation made use of the Ca(2+)-derived force in which the [Ca2+]i during relaxation was converted to the Ca(2+)-derived force by means of the steady-state relation between [Ca2+]i and force. The Ca(2+)-derived force fell more slowly in the presence of caffeine but the lag between Ca(2+)-derived force and measured force was unaffected. Thus, the slowed relaxation was caused by changes in Ca2+ handling and not by slowed cross-bridge kinetics. 6. A model of the Ca2+ movements and force production of muscle was used to examine independently the effects of increased Ca2+ sensitivity, slowing of the SR Ca2+ pump and increased SR Ca2+ permeability. The effects of caffeine on [Ca2+]i, tetanic force and relaxation could be explained by a combination of these three effects.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M. W., Neering I. R. Actions of caffeine on fast- and slow-twitch muscles of the rat. J Physiol. 1989 Sep;416:435–454. doi: 10.1113/jphysiol.1989.sp017770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F. Inhibition of sarcotubular calcium transport by caffeine: species and temperature dependence. Biochim Biophys Acta. 1969 Apr 8;172(3):566–570. doi: 10.1016/0005-2728(69)90152-2. [DOI] [PubMed] [Google Scholar]
- Garcia J., Schneider M. F. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. J Physiol. 1993 Apr;463:709–728. doi: 10.1113/jphysiol.1993.sp019618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Kanaya H., Takauji M., Nagai T. Properties of caffeine- and potassium-contractures in fatigued frog single twitch muscle fibers. Jpn J Physiol. 1983;33(6):945–954. doi: 10.2170/jjphysiol.33.945. [DOI] [PubMed] [Google Scholar]
- Klein M. G., Kovacs L., Simon B. J., Schneider M. F. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991 Sep;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Kurihara S. Effects of caffeine on intracellular calcium concentrations in frog skeletal muscle fibres. J Physiol. 1987 Feb;383:269–283. doi: 10.1113/jphysiol.1987.sp016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Harkins A. B., Baylor S. M. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993 Jun;64(6):1934–1960. doi: 10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991 Mar;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987 Sep;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagala M. K. Effect of length and caffeine on isometric tetanus relaxation of frog sartorius muscles. Biochim Biophys Acta. 1980 Jun 10;591(1):177–186. doi: 10.1016/0005-2728(80)90231-5. [DOI] [PubMed] [Google Scholar]
- Palmer S., Kentish J. C. The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J Physiol. 1994 Oct 1;480(Pt 1):45–60. doi: 10.1113/jphysiol.1994.sp020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Simon B. J., Klein M. G., Schneider M. F. Caffeine slows turn-off of calcium release in voltage clamped skeletal muscle fibers. Biophys J. 1989 Apr;55(4):793–797. doi: 10.1016/S0006-3495(89)82878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. J Physiol. 1994 Oct 1;480(Pt 1):31–43. doi: 10.1113/jphysiol.1994.sp020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994 Jan 15;474(2):291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]



