Abstract
Viruses are key drivers of microbial diversity, nutrient cycling, and co-evolution in ecosystems, yet their study is hindered due to challenges in culturing. Traditional gene-centric methods, which focus on a few hallmark genes like for capsids, miss much of the viral genome, leaving key viral proteins and functions undiscovered. Here, we introduce two powerful annotation-free metrics, V-score and VL-score, designed to quantify the “virus-likeness” of protein families and genomes and create an open-access searchable database, ‘V-Score-Search’. By applying V- and VL-scores to public databases (KEGG, Pfam, and eggNOG), we link 38−77% of protein families with viruses, a 9−16x increase over current estimates. These metrics outperform existing approaches, enabling precise detection of viral genomes, prophages, and host-derived auxiliary viral genes (AVGs) from fragmented sequences, and significantly improving genome binning. Remarkably, we identify up to 17x more AVGs, dominated by non-metabolic proteins of unknown function. This innovation unlocks new insights into virus signatures and host interactions, with wide-ranging implications from genomics to biotechnology.
Viruses are indispensable components of the biosphere. By their sheer abundance in microbiomes and ecosystems and their high genetic diversity1, viruses have the ability to regulate populations2, facilitate nutrient cycling3, promote genetic diversity4, and drive co-evolutionary dynamics5. In spite of their importance, viruses are difficult to culture in the laboratory necessitating advances in computational approaches to study uncultured viruses. Understanding viral genomes and proteins is crucial for grasping their diversity and understanding their roles in ecosystems. This knowledge helps unravel the complexity of life and advances biotechnological applications like vaccines and phage therapy.
Traditionally, virus-specific genes, including hallmark genes such as for capsid proteins, have been considered the definitive signatures of viral genomes and used for identifying and characterizing viral genomes6–8. However, hallmark genes account for a small portion of viral genomes9. Genome or metagenome fragments often do not contain hallmark genes, making it difficult to identify and classify viruses using traditional gene-centric approaches. As a result, many viral genomes remain unidentified, leading to a significant loss of information and a growing recognition of the need to overcome these limitations in viral discovery and protein annotation.
Annotating viral genes and predicting their functions provide clues about the nature of viral sequences and protein families. We reasoned that analyzing entire viral genomes, even when fragmented, with functional annotations could break convention and yield innovative viral signatures. Here we introduce the concepts of V-scores and VL-scores that are quantitative metrics to serve as a virus-like signature for differentiating between viral and non-viral protein families and genomes. We demonstrate specific use cases of V-scores and VL-scores in virus identification, prophage discovery, annotation of host-derived and metabolic proteins on viral genomes, and virus genome binning. Finally, to facilitate adoption of our approach, we created a publicly available database of V-scores and VL-scores associated with every protein cluster or family in five widely used public databases (https://anantharamanlab.github.io/V-Score-Search/) including Prokaryotic Virus Remote Homologous Groups (PHROG), Virus Orthologous Groups (VOG), Kyoto Encyclopedia of Genes and Genomes (KEGG), Protein Families Database (Pfam), and evolutionary genealogy of genes: Non-supervised Orthologous Groups (eggNOG). We propose that V-scores and VL-scores will serve as a metric to define the likelihood of protein families being detected in viruses and enable diverse applications associated with viral genomics, ecology, and evolution.
RESULTS
Assessment of protein families for virus-like proteins
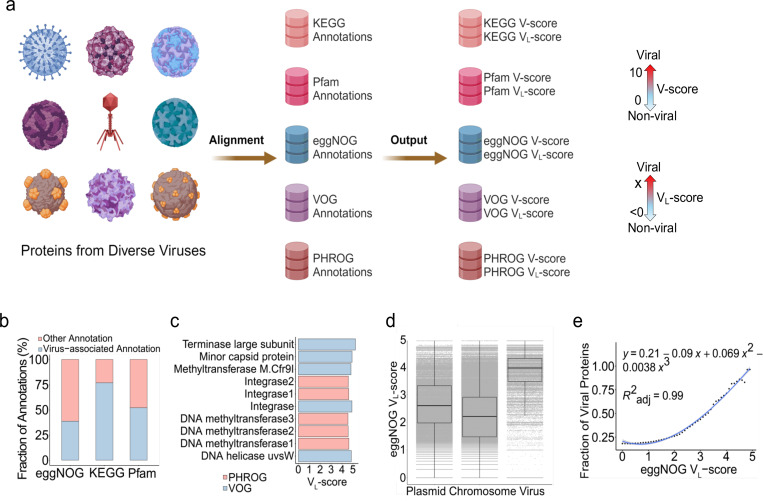
We used 18,435,589 viral proteins sourced from diverse viruses to construct associations between viruses and protein families (Fig. 1a). Each protein family (i.e., clusters of similar proteins represented under a single annotation in databases, which includes proteins of unknown function) was assigned a V-score and a VL-score, representing metrics of virus association when the protein family had significant hits to viral proteins (see details in Methods and in Supplementary Tables S1-5). We identified cutoffs of V-score = 0.01 and VL-score = 0 to define viral proteins with high certainty. High V-scores and VL-scores indicated a strong association with viral proteins, whereas low scores suggested a weaker association. Protein families associated with viral proteins constituted approximately 76.9%, 52.1%, and 38.7% of the total protein families in KEGG (20,005), Pfam (10,835), and eggNOG (135,509), respectively (Fig. 1b). In contrast, current estimates of viral protein entries in KEGG, Pfam, and eggNOG are limited, representing a very small fraction (<10%) (Supplementary Fig. S1). Our analysis substantially increases the number of protein families in public databases associated with viruses and significantly improves the overall representation of viral proteins in these databases. This increase in viral representation will facilitate better understanding of viral roles in ecosystems, their interactions with hosts, and their evolutionary dynamics.
Fig. 1 |. Concepts of V-score and VL-score.
a, Workflow of V-score and VL-score generation. Nine representatives of viral taxa are shown here for the diverse viruses used in the study. A scale for VL-scores and VL-scores is displayed by two-sided arrows going from 0 to 10 and <0 to X, respectively, suggesting low scores indicate non-viral and high-scores indicate viral. b, Frequency of virus-associated annotations with V-score ≥ 0.01 and/or VL-score ≥ 0. c, Top five annotations associated with viruses based on VL-scores. d, Distribution of eggNOG VL-score across proteins from prokaryotic chromosomes (n = 7,561,596), plasmids (n = 437,241) and prokaryotic viruses (n = 83,664). The horizontal line that splits the box represents the median, upper and lower sides of the box represent upper and lower quartiles, whiskers are 1.5 times the interquartile ranges and data points beyond whiskers are considered potential outliers. e, Relationship between the fraction of viral proteins used in (d) and eggNOG VL-score. The generation of the fraction of viral proteins from the comparison between plasmids, chromosomes, and viruses is illustrated in Supplementary Fig. S10.
Next, we hypothesized that the associative nature of V-scores and VL-scores could also reflect gene frequencies in viral communities. Towards this, we used the PHROG and VOG protein families that provide valuable resources for characterizing viral proteins. We determined that the range of V-scores and VL-scores were associated with patterns of gene frequencies with high scores indicating frequent distributions and low scores indicating infrequent distributions. For example, according to PHROG and VOG VL-scores, methyltransferase-coding genes were frequently distributed in viral communities (Fig. 1c), which was also evidenced by the high VL-scores for these protein families in KEGG, Pfam, and eggNOG (e.g., KEGG VL-score = 4.8 and Pfam VL-score = 4.7). This approach will allow for the identification of new viral hallmark proteins and other proteins commonly encountered on viruses but whose function is currently not known. In contrast, protein families with very low V-scores and VL-scores, e.g., host-derived proteins, metabolic proteins, and hypothetical proteins with V-scores of 0.01, indicated the presence of viral proteins that are rare in communities and may confer specialized functions more likely to be involved in niche-specific interactions10.
Interestingly, VL-scores of eggNOG protein families revealed the likelihood of viral origin of different protein families. VL-scores revealed a significant difference between viral and non-viral proteins when comparing viral proteins to those found in plasmids and prokaryotic chromosomes (Fig. 1d). The proportion of viral proteins in a protein family increased with higher eggNOG VL-scores, demonstrating a clear relationship between scores and the probability of viral origin (Fig. 1e). High VL-scores (>4) indicated that the protein families are likely virus-specific, while low VL-scores (<2.2) suggest non-viral origin (Fig. 1e). This finding offers a promising approach for the differentiation between viral and non-viral proteins, extending beyond simple gene presence or absence and incorporating quantitative assessment. Such metrics could be particularly useful in cases where traditional methods struggle, such as in distinguishing viral genes embedded within plasmids11 or identifying viral elements within bacterial genomes12, 13. Additionally, these quantitative metrics for protein families can also be applied for the differentiation of viral and non-viral genome sequences using combined VL-scores or V-scores across different proteins.
Generation of AV-scores and AVL-scores for viral differentiation and prediction
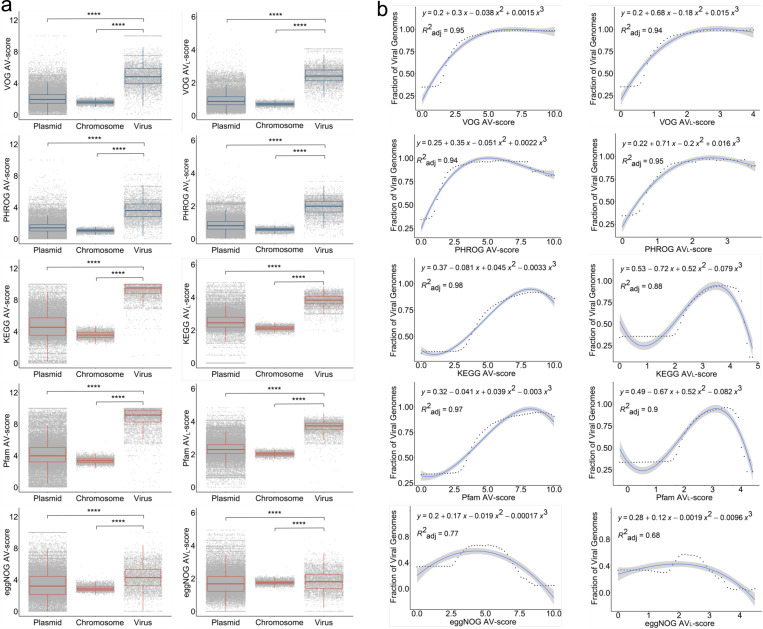
To build upon our understanding of V-scores and VL-scores from the protein to genome-scales, we posited that the association and frequency of V-scores and VL-scores may confer features on viral genomes that distinguish them from other organisms. To test on this, we investigated a whole-genome catalog of 5,800 viral, 50,523 plasmid, and 4,813 prokaryotic genomes and developed the concepts of average V-score (AV-score) and average VL-score (AVL-score) (See methods for details) (Fig. 2a). We proposed that AV- and AVL-scores represented the average scores of protein families across an entire genome and would thus be representative of the overall virus-like character of a given genome. We determined that prokaryotic viruses had significantly higher medians of AV-scores (3.602−9.515) and AVL-scores (1.802−3.830) compared to plasmids and prokaryotic chromosomes regardless of annotation databases (p-value < 10−5). Interestingly, viral genome fragments (1−15kb) extracted from whole genomes also displayed significantly higher medians (see examples of KEGG and Pfam AV-scores and AVL-scores in Supplementary Fig. S2 and S3, respectively). The higher median scores for viral genomes suggest that this metric could capture features unique to viruses, making it highly effective for identifying viral genomes in mixed communities such as metagenomes of viruses, plasmids, and chromosomes. To validate this, we conducted polynomial regression analyses on the fraction of viral genomes within mixed metagenomes containing viruses, plasmids, and chromosomes at various cutoffs of AV-scores and AVL-scores for both whole genomes and genome fragments (Supplementary Tables S6-9). At the whole-genome level, the fraction of viral genomes increased with higher AV-score and AVL - scores (for VOG) (Fig. 2b). Similarly, at the fragment level, the fraction of viral genomes increased with higher AV-score cutoffs for KEGG and Pfam (Supplementary Fig. S4 and S5). From regression analyses (Fig. 2b), whole genomes with AV-scores/ AVL -scores exceeding the corresponding cutoffs (e.g., a VOG AV-score of 2, which surpasses the VOG AV-score cutoff of 1.93) were predicted to be viral with a 70% probability (likely viral) or a 90% probability (most likely viral) (see detailed cutoffs in Supplementary Table S10). For genome fragments, only the AV-scores of VOG, PHROG, KEGG, and Pfam were able to generate cutoffs predictive of viral genomes with a 70% or 90% probability (Supplementary Fig. S4−7). Given that cutoffs may vary with fragment size, different cutoffs were established for corresponding sizes (Supplementary Table S10). Overall, the concepts of AV-scores and AVL-scores offer novel insights into genome signatures, traditionally defined by k-mer frequency14 or single-copy signature genes15. The cutoffs for AV-scores and AVL-scores, used to differentiate between viral and non-viral genomes, may prove valuable for viral identification in metagenomic studies. Overall, these metrics address limitations of conventional gene-centric and alignment-dependent methods8, 16–18.
Fig. 2 |. Concepts of Average V-score (AV-score), Average VL-score (AVL-score), and cutoffs of AV-score and AVL-score.
a, Distribution of AV-score and AVL-score of prokaryotic chromosomes (n = 4,813) and the genomes of plasmids (n = 50,523) and prokaryotic viruses (n = 5,800). The blue boxes denote the AV-scores and AVL-scores of VOG and PHROG. The red boxes denote the AV-scores and AVL-scores of KEGG, Pfam, and eggNOG. The horizontal line that splits the box is the median, the upper and lower sides of the box are upper and lower quartiles, whiskers are 1.5 times the interquartile ranges and data points beyond whiskers are considered potential outliers. An ANOVA test was used to show differences between three means are significant (p < 2.2 × 10−16). **** denotes p < 10-4. b, Relationship between the fraction of viral genomes used in (a) and the AV-scores and AVL-scores. In this study, we define the fraction of viral genomes as the probability that a given genome sequence is viral. The dots on the dotted line represent the actual values of the fraction of viral genome sequences, while the blue lines indicate the predicted values. The process for generating the fraction of viral genome sequences is identical to the method used for generating the fraction of viral proteins, as illustrated in Supplementary Fig. S10.
Maximizing identification of viral genomes
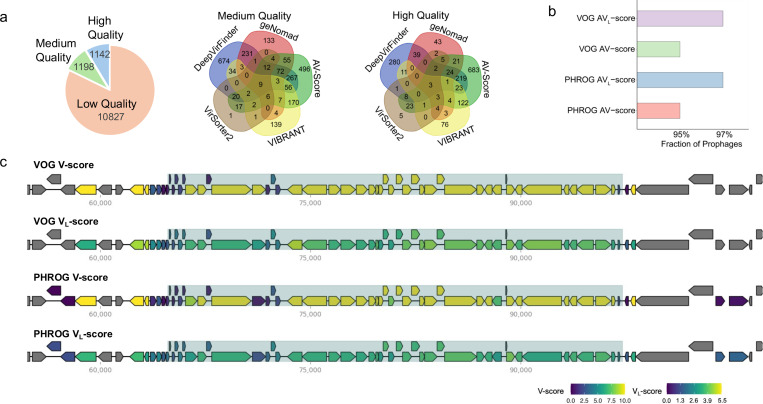
To evaluate the potential of AV-scores and AVL-scores for applications in metagenomics, we analyzed a dataset of 39 host-associated metagenomes. By applying AV-score cutoffs (with a 70% probability of being viral) for genome fragments of varying sizes, derived from KEGG, Pfam, VOG, or PHROG, we identified 13,167 viral sequences of low, medium, and high quality (Fig. 3a). Of these, 2,064 sequences overlapped with those identified using geNomad which is a virus identifier dependent on virus-specific markers8 (Supplementary Fig. S8a). Notably, for medium- and high-quality sequences, the AV-score-based approach outperformed geNomad, identifying more than 1,000 high-quality viral sequences—approximately seven times more than geNomad identified (Supplementary Fig. S8b).
Fig. 3 |. Application of V-scores, VL-scores, AV-scores, and AVL-scores for viral identification in genomes and metagenomes.
a, Number of sequences identified with AV-scores and AVL-scores and four commonly used software. For medium- and high-quality sequences, as assessed by CheckV, the overlap between the five approaches was illustrated using Venn diagrams, showing the number of shared and specific sequences identified by different methods. b, Fraction of prophages in a database that have AV-scores and AVL-scores above corresponding cutoffs for viral-like determination. c, Distribution of V-scores and VL-scores for genes within a verified Escherichia coli prophage and its adjacent host sequences. Prophage regions are shaded for emphasis.
Additionally, the AV-score-based method surpassed other conventional tools, including machine learning-dependent DeepVirFinder19, VIBRANT17, a hybrid approach incorporating machine learning and protein similarity, and VirSorter218, in identifying high-quality sequences (Fig. 3a). Moreover, compared to previous studies on sponge-associated microbiomes20, 21, we identified 129 viral sequences of medium or higher quality—more than 15 times the number of viral genomes (7 sequences) previously predicted using VirSorter27. Most of the high-quality viral genomes identified by the AV-score approach are specific to AV-score, indicating that this method can uncover viral genomes that other tools may not recognize (Fig. 3a). These findings suggest that the usage of AV-scores and AVL-scores can detect many viral sequences that traditional, viral-specific gene-dependent methods may overlook. Overall, the application of AV-scores and AVL-scores as metrics for genome differentiation offers a novel and powerful tool for identifying viral genomes in metagenomic studies.
We further tested the potential of this approach for prophage identification and assessment. The results showed that over 95% of sequences in a prophage database used by a popular prophage identification tool, PHASTER22 (65,668 prophages), had AV-scores and AVL-scores above our suggested cutoffs for whole genomes (70% probability, based on VOG and PHROG scores) (Fig. 3b). Additionally, clear boundaries between a verified Escherichia coli prophage and its adjacent host sequences were delineated by relatively low V-scores and VL-scores using VOG and PHROG (Fig. 3c). Furthermore, the higher AV-scores observed for VOG, PHROG, Pfam, KEGG, and eggNOG families in prophages (see Supplementary Fig. S9) strongly support the idea that AV-scores and/or AVL-scores are useful in identifying prophage boundaries when combined with sliding window approaches (e.g., a 10 kb sliding window23). In addition to AV-scores and AVL-scores, VL-scores may also be valuable for determining boundaries, as a gene with an eggNOG VL-score greater than 4 has over a 70% probability of being viral (Fig. 1e). Accurately predicting prophage boundaries has long been a challenge24, 25, possibly due to the presence of auxiliary metabolic genes (AMGs) in phages26, 27 or the ability of phages to be transposable and encode serine-integrases rather than tyrosine integrases24. Given their ability to distinguish viral from non-viral genes and sequences, AV-scores, AVL-scores, and VL-scores may offer highly precise methods for boundary recognition.
Advancing the identification of auxiliary genes in viral genomes
Despite recent efforts, the vast majority of viral proteins (>80%) have no known function which has hindered our understanding of the roles of viruses in ecosystems and microbiomes. V-scores and VL-scores as quantitative metrics display a property of measuring the frequency of individual protein families among viral genomes in public databases. Leveraging this property through the development of hidden Markov models for protein families, we assessed their effectiveness in identifying AVGs, including AMGs on viral genomes. AVGs are virus-encoded genes of prokaryotic origin that are not essential for viral propagation processes such as genome replication, lysis, or capsid assembly, while AMGs are auxiliary genes that are associated with metabolic roles28. Such genes likely provide a fitness benefit to the virus encoding them28–30. Identifying AVGs is a particularly difficult problem compounded by host-associated contamination and the host-derived nature of these genes. Given their importance due to the increasing recognition of auxiliary genes involved in human and environmental microbiomes30–34, we investigated whether V-scores and VL-scores could effectively identify auxiliary genes.
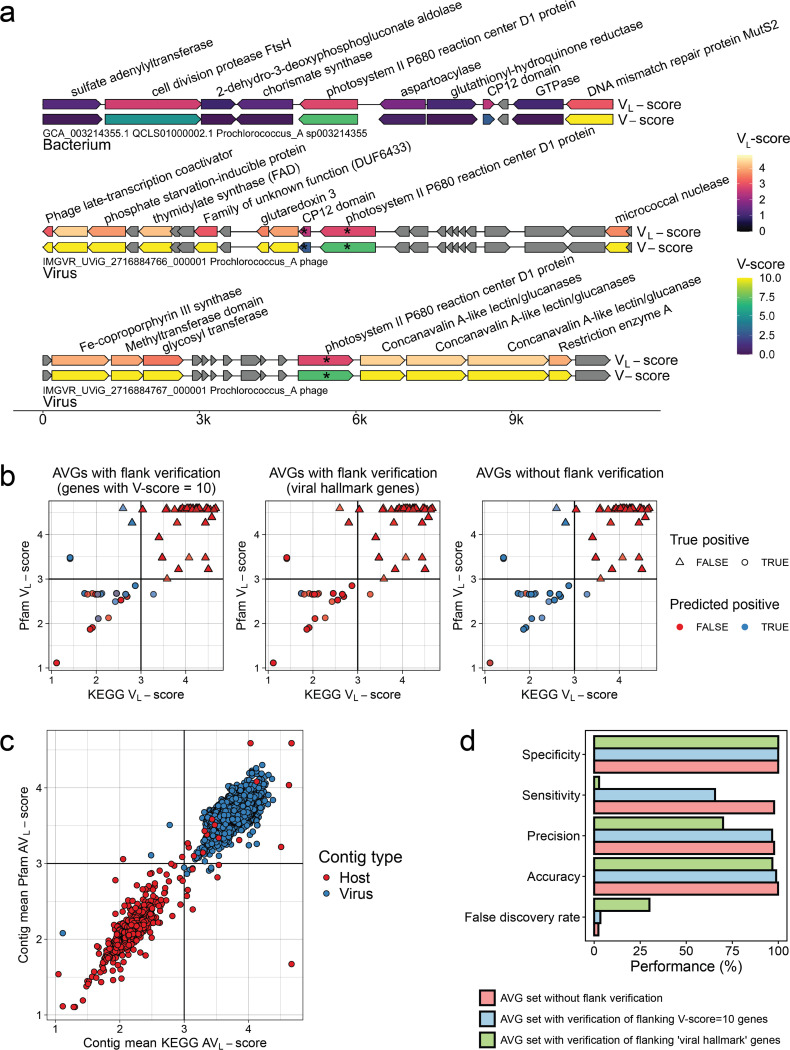
To test this hypothesis, we evaluated the ability of V-scores, VL-scores, AV-scores, and AVL-scores to identify 17 experimentally verified AMGs. We first distinguished AMGs from host-encoded metabolic genes and non-auxiliary genes by using V-scores and VL-scores (Fig. 4a and 4b). We then averaged the VL-scores of all KEGG or Pfam protein families across entire scaffolds, establishing a scaffold Pfam/KEGG AVL-score of 3 as optimal for differentiating viral from host scaffolds (Fig. 4c). Our workflow effectively detected AMGs (Fig. 4d). We achieved a sensitivity of 97.71% and a false positivity rate of 2.29% using a database of biochemically characterized AMGs (experimentally verified) for benchmarking (see details in Supplementary Table S11). Community standards for analyzing AMGs recommend verifying that a virally encoded AMG is flanked both upstream and downstream by hallmark genes35, 36. This check ensures that metabolic genes identified from proviral sequences are not in regions of host contamination, however, this standard hinders AMG recall for non-proviruses. The requirement for verification significantly reduced sensitivity to 66% (when verified with genes having V-scores of 10) and to 2.67% (when verified with hallmark genes), while also increasing the false discovery rate to 30% when using hallmark gene verification (Fig. 4d, Supplementary Tables S11, S12). The ability of V-scores and VL-scores to confidently identify viral proteins circumvents the need to identify hallmark proteins. Therefore V-scores offer a novel methodology for verifying that AMGs encoded by proviruses are not the result of host contamination.
Fig. 4 |. Application of V-scores, VL-scores, and AVL-scores to auxiliary gene (AVG) detection.
a, V-scores and VL-scores reveal AMGs in viral genomes and distinguish AMGs from host-encoded metabolic genes. Genes with an asterisk (*) were predicted as AMGs using the described workflow (see Methods). b, Establishing optimal Pfam VL-score / KEGG VL-score combinations to distinguish viral auxiliary vs. non-auxiliary genes. Points represent individual genes in our database of viral and host genomes that had both Pfam5 and KEGG6 annotations matching to either the database of the 17 AMGs or 10 non-AMG protein families. Genes marked as potentially auxiliary have a maximum KEGG and Pfam VL-scores of 3, as indicated by the vertical and horizontal lines. c, Establishing the optimal Pfam/KEGG AVL-score of query scaffolds to distinguish viral vs. host genomes. Points represent individual genes, plotted by the AVL-score of all Pfam or KEGG annotations encoded by the gene’s origin scaffold. Vertical and horizontal lines represent the chosen scaffold AVL-score used to distinguish viral from host scaffolds (> 3: virus, < 3: host). Points are colored by the actual scaffold type of the gene’s origin (host or virus). d, Performance of the proposed AMG identification workflow. Performance was evaluated based on the confusion matrices in Supplementary Table S12.
Leveraging this advantage, we were able to predict a significantly larger number of auxiliary genes from 5,116 high-quality viral genomes, providing deeper insights into viral functions. Our workflow (with verified flanking genes with V-score=10) identified a total of 27,442 viral genes likely to be auxiliary and the workflow without verification predicted 34,015 auxiliary genes (4.85% of all viral genes in our test dataset and 16.50% of all annotated viral genes) (Supplementary Table S13). Notably, non-metabolic AVGs comprise a substantial majority, accounting for 89%, while auxiliary metabolic genes represent a small subset, making up only 11% (Fig. 5a). The identified AVGs included genes encoding various metabolic enzymes, antibiotic resistance proteins, transporters, DNA/RNA replication proteins, transposases/recombinases, nucleases/endonucleases, and uncharacterized/hypothetical proteins. These AVGs serve diverse functions including metabolism, genetic information processing, environmental information processing, and cellular processes (Fig. 5b; Supplementary Table S13). Some of the genes have been considered auxiliary, for example, the genes encoding D-3-phosphoglycerate dehydrogenase for carbon metabolism26, S-adenosylmethionine decarboxylase for amino acid metabolism37, and alpha-L-fucosidase for glycan degradation38. Notably, our study predicted numerous auxiliary genes that were typically overlooked in previous studies of auxiliary genes. For instance, over 700 viral auxiliary genes related to toxin-antitoxin systems were identified. These systems, which are typically used by hosts as a defense mechanism against viral infections39, 40, may be employed by viruses to enhance their ability to infect host organisms39, 41, 42, contributing to viral evolution in the ongoing virus-host arms race. Additionally, the presence of many genes with unknown functions suggests that there are still numerous unexplored roles for viruses, likely with important ecosystem or microbiome contexts.
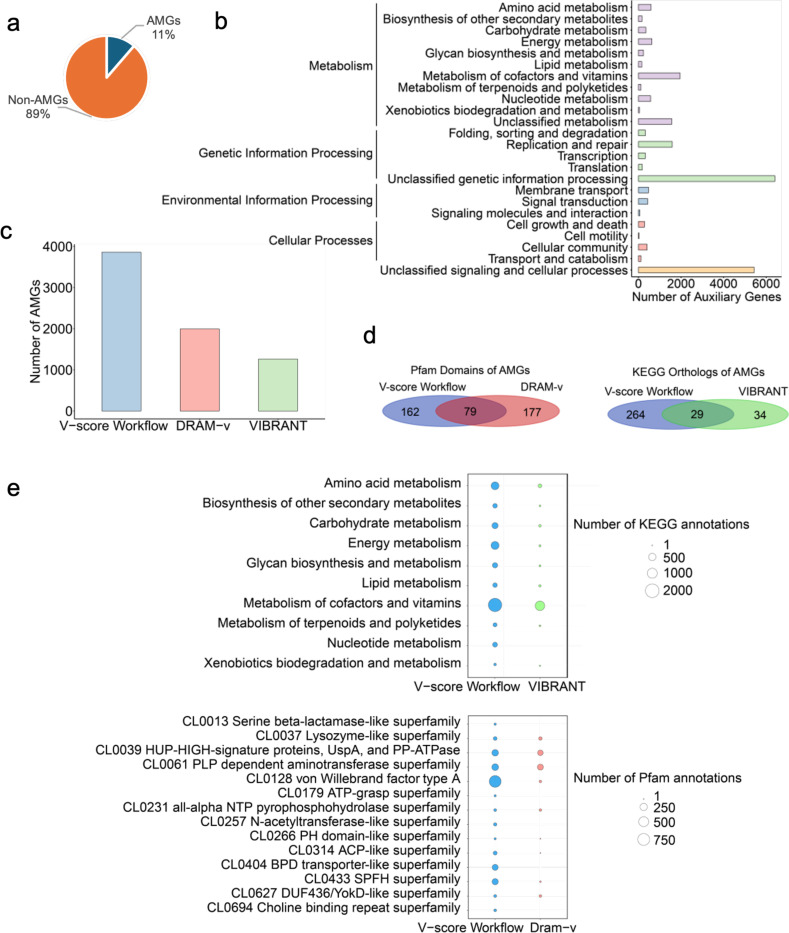
Fig. 5 |. Auxiliary genes identified in the study and comparison with existing methods.
a, auxiliary gene composition. AMG: auxiliary metabolic genes. b, Potential functions of auxiliary genes with annotations detected using the V-score workflow. Purple bars represent categories within Metabolism, green bars denote Genetic Information Processing, blue bars indicate Environmental Information Processing, pink bars correspond to Cellular Processes, and orange bars represent unclassified signaling and cellular processes. c, Number of AMGs identified by the V-score workflow compared to other existing methods, including DRAM-v and VIBRANT. d, Overlap and unique Pfam domains or KEGG orthologs of AMGs identified by the V-score workflow, DRAM-v, and VIBRANT. e, Comparison of the number of KEGG or Pfam annotations of AMGs identified using the V-score workflow, DRAM-v, and VIBRANT. Please note that VIBRANT exclusively outputs results that contain KEGG annotations, while DRAM-v mainly generated Pfam annotations for AMGs identified in the study.
In comparison to other existing approaches, our workflow significantly outperformed widely used approaches including VIBRANT17 and DRAM-v35, as demonstrated by the identification of AMGs. When applied to the same set of viral genomes, our V-score workflow identified 3,859 AMGs (Fig. 5c; Supplementary Table S13), while VIBRANT and DRAM-v identified only 1,261 and 1,993 AMGs, respectively (Fig. 5c; Supplementary Tables S14 and S15). Notably, only a small fraction of Pfam domains or KEGG orthologs of AMGs were commonly identified by three approaches (Fig. 5d), with most AMGs being unique to each method. This suggests that our V-score workflow reveals novel functions that are often overlooked by existing AMG detection tools. Some unique metabolic enzymes uncovered by our method include the serine beta-lactamase-like superfamily (Pfam clan accession: CL0013), ATP-grasp superfamily, N-acetyltransferase-like superfamily, and Choline binding repeat superfamily (Fig. 5e). Furthermore, our workflow outperformed VIBRANT, as shown by the higher number of AMGs identified across all KEGG categories (Fig. 5d). Collectively, these findings demonstrate that the V-score-based approach can detect a greater number of potential AVGs with high precision.
Signatures of population differentiation and enhancing genome binning strategies
Characterizing new viral species in complex systems is crucial for understanding how microbial interactions impact the spread of diseases and their development and impact on health43. AV-scores and AVL-scores capture the association and frequency of viral genomes, as well as their differentiation from other genomes. Leveraging these signatures, we assessed whether AV-score and AVL-score analyses could effectively recover viral metagenome-assembled genomes (vMAGs) from a mixed metagenome. Prior to this assessment, we evaluated the ability of AV-scores and AVL-scores to cluster population genomes, to verify their relevance and effectiveness in the context of genome binning. We analyzed a dataset of 11 viral species that were available in the NCBI RefSeq database. We found that the similar viral species had very similar AV-scores or AVL-scores, while different species exhibited distinct scores (Fig. 6a). This highlights the reliability and accuracy of these metrics for viral genome classification and identification of novel species. For instance, changes in the gut phage population have been repeatedly linked to various gastrointestinal diseases44–46. The application of AV-scores or AVL-scores into gut phage population studies would provide opportunity to differentiate viral populations in complex host-associated systems and contribute to uncover certain disease-related viral species.
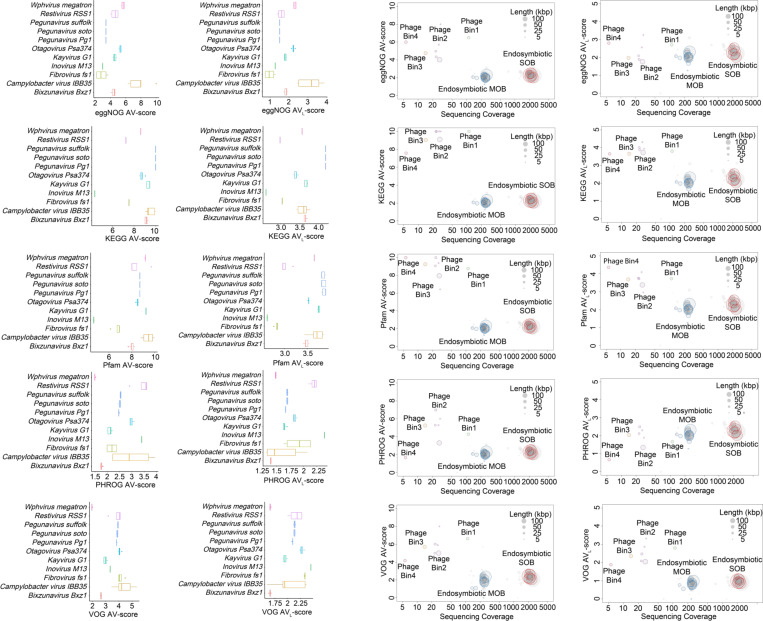
Fig. 6 |. Application of AV-scores, and AVL-scores to metagenomic binning.
a, Viral population differentiation with AV-scores and AVL-scores. Viral species include Bixzunavirus Bxz1 (n = 13), Campylobacter virus IBB35 (n = 5), Fibrovirus fs1 (n = 4), Inovirus M13 (n = 8), Kayvirus G1 (n = 15), Otagovirus Psa374 (n = 7), Pegunavirus Pg1 (n = 6), Pegunavirus soto (n = 5), Pegunavirus Suffolk (n = 6), Restivirus RSS1 (n = 4), and Wphvirus megatron (n = 4). The horizontal line that splits the box is the median, the upper and lower sides of the box are upper and lower quartiles, whiskers are 1.5 times the interquartile ranges and data points beyond whiskers are considered potential outliers. b, Genome binning with AV-scores, AVL-scores, and sequencing coverage for a snail-associated metagenome. SOB: sulfur-oxidizing bacteria; MOB: methane-oxidizing bacteria.
AV-scores and AVL-scores facilitate species clustering and even strain-level differentiation, as demonstrated by the distinct separation of viral populations based on AV-scores and AVL-scores of VOG and PHROG (Fig. 6a). AV-scores and AVL-scores can therefore be effective metrics for differentiating microbial and viral species or strains and facilitating genome binning in metagenomic studies. We next tested a host-associated metagenome. The analysis of a deep-sea snail microbiome using AV-scores, AVL-scores, and sequencing coverage demonstrated the effectiveness of these metrics in genome binning of microbes and viruses (Fig. 6b). We observed clear clustering of four phage genome bins and two bacterial chromosome bins, which was consistent with a prior study47, thereby highlighting the capability of these metrics to differentiate between viral and bacterial genomes accurately. This approach could complement current tools, such as vRhyme48, and enhance the construction of vMAGs that more accurately represent the true composition of viruses within a sample. Significantly, this approach would reduce the overestimation of viral diversity that can result from the assumption that a single genome fragment represents an uncultivated viral genome (UViG) or a viral population49, 50.
DISCUSSION
In conclusion, V-scores, VL-scores, AV-scores, and AVL-scores represent powerful quantitative metrics that describe the virus-like nature and origin of protein families and genomes. These metrics can serve as the foundation of new tools to advance viral genomics, ecology, and evolutionary analyses. By enabling open and public distribution of these scores ((https://anantharamanlab.github.io/V-Score-Search/), we propose that they will propagate broadly in microbiology. Our approach allows for citation of these scores using databases identifiers like for KEGG, Pfam etc or using protein annotations. For example, a picornavirus capsid protein (PF00073) has a V-score of 10 implying a strong virus association while a Hepatitis C virus capsid protein (PF01543) has a V-score of 1 implying a weaker virus association, presumably because its proteins domains are not specific to capsids.
The versatility of these scores allows for their incorporation into diverse genomics tools such as for genome binning, genome completion, virus identification in complex datasets, and identification of AMGs. These scores can enhance genome binning strategies by providing an additional layer of resolution in separating viral from non-viral sequences. This capability is especially valuable in metagenomic studies, where the accurate classification of sequences is critical for understanding the composition and dynamics of microbial communities. By integrating metrics like AV-scores and AVL-scores, researchers could develop more refined tools for viral identification, potentially leading to the discovery of novel viral genomes and a deeper understanding of virus-host interactions. The broader implication of this approach is that it allows for more nuanced and data-driven differentiation between viral and non-viral entities at both the gene and genome levels. This could revolutionize how we identify and characterize viruses in complex biological systems, offering new insights into viral evolution, diversity, and function. The quantitative nature of the metrics also opens up possibilities for automating and scaling viral genome study across large datasets, for example the completeness assessment of linear viral genome in cases where identifiable terminal repeats are absent6, making it an invaluable resource in the field of viral (meta)genomics.
METHODS AND MATERIALS
Viral protein database construction
Viral protein sequences were downloaded from public databases (accessed January 2024), including the National Center for Biotechnology Information (NCBI) RefSeq database, the Virus Orthologous Groups (VOG) database (version 221, https://fileshare.csb.univie.ac.at/vog/), the Prokaryotic Virus Remote Homologous Groups (PHROG) database51, and the IMG/VR Viral Resources v4.152. Protein sequences from IMG/VR Viral Resources were filtered and we only retained high-quality and medium-quality viral sequences that were assessed by CheckV v1.0.153. To dereplicate proteins, MMseqs2 linclust version 13.4511154 was used with an identity cutoff of 95% (custom parameters: --min-seq-id 0.95 --cluster-mode 2 --cov-mode 1 -c 1.0), and generated non-redundant 18,435,589 protein sequences.
Annotation profile database selection
To construct a wide range of associations between annotation profiles and viral proteins, a diverse collection of profile databases was selected. The profile databases included Kyoto Encyclopedia of Genes and Genomes (KEGG) KOfam (version 2024–01-01)55 that is a customized Hidden Markov Models (HMMs) profile collection of KEGG Orthologs, Pfam-A (release 36.0)56 database of a large collection of diverse protein families, and eggNOG (version 5.0)57 that is a database of non-supervised orthologs created from a large number of various organisms. Two additional curated viral ortholog collections are the VOG (release 221, vogdb.org) and PHROG both of which were constructed based on remote homology.
V-score and VL-score generation
The V-score and VL-score for each annotation profile in the KEGG, Pfam, eggNOG, PHROG, and VOG databases was determined based on the number of significant hits (E-value ≤ 10−5) identified by hmmsearch (HMMER 3.4)58 and MMseqs2. For V-score, the resulting number was divided by 100, with a maximum limit set at 10 after division. For VL-score, the resulting number was scaled down using the common logarithm (base 10) without a maximum limit. In the case of annotations containing viral keywords including “virus”, “viral”, “phage”, “portal”, “terminase”, “spike”, “capsid”, “sheath”, “tail”, “coat”, “virion”, “lysin”, “holin”, “base plate”, “lysozyme”, “head”, “structural”, or “Viral protein families”, protein families/annotations were assigned adjusted V-score of 1 and VL-score of 2 if the original V-score was less than 1 and VL-score less than 2. Each annotation profile is given a V-score and a VL-score, serving as metrics for virus association. It is important to note that the V-scores do not consider virus specificity or association with non-viruses and have been manually adjusted to prioritize viral hallmark genes.
Databases of chromosomes, plasmids, and viral genomes for AV-score and AVL-score generation
Databases of prokaryotic chromosomes, plasmid sequences, and prokaryotic viral genomes were constructed for the generation of AV-score and AVL-score. Prokaryotic genomes (release 214) were downloaded from the Genome Taxonomy Database (GTDB; gtdb.ecogenomic.org)59, 60. We assessed the quality of each genome with a quality score (score = completeness − 5 × contamination − 0.05 × no. scaffolds)8, genomes of each GTDB family with the highest quality score were selected as family representatives to reduce computational load and taxonomic bias. As a result, 4,304 bacterial and 509 archaeal genomes were selected to be used in the following analyses. Then, provirus and provirus-like sequence regions were identified with VirSorter2 version 2.2.4 and VIBRANT version 1.2.1 and removed from the selected prokaryotic genomes. Additionally, plasmid sequences (sequence headers containing the word “plasmid”) were removed from the selected prokaryotic genomes. For plasmids and prokaryotic viruses, 50,523 plasmid sequences were downloaded from the PLSDB database version 2023_11_2361 and viral genomes were downloaded from the NCBI RefSeq database62 (retrieved in January 2024). To retrieve prokaryotic viral genomes, the GenBank dabase division PHG was used to filter bacterial and archaeal viruses in the RefSeq database. Finally, 5,800 genomes of prokaryotic viruses were retained.
Generation of AV-score and AVL-score
Databases of prokaryotic chromosomes, plasmids, and prokaryotic viruses constructed above were used to calculate the AV-score and AVL-score for each genome. Each whole genome of prokaryotic viruses, plasmids, and chromosomes were randomly split into non-overlapping, non-redundant genome fragments at length from 1 to 15 kb to simulate metagenome-assembled sequences. Proteins of each whole genome and split genome fragment were predicted using Prodigal V2.6.3 (parameters: -m -p meta)63. Hmmsearch58 (HMMER 3.4, parameter: -E 10−5) was used to match the proteins of prokaryotic viruses, plasmids, prokaryotes to the HMM profiles of KEGG, VOG, and Pfam. EggNOG-mapper version 2.1.12 (parameters: -m mmseqs --evalue 10−5)64 was used to annotate the proteins with the eggNOG database. MMseqs2 (parameter: E-value ≤ 10−5) was employed to search the predicted proteins against the PHROG database. Only the hit with the highest score was kept. Post this, V-score and VL-score of KEGG, VOG, eggNOG, Pfam, and PHROG were assigned to each protein. For comparison between viruses, plasmids, and chromosomes, AV-score and AVL-score were calculated for each whole genome and genome fragment. The AV-score and AVL-score of KEGG, Pfam, and eggNOG were expressed as:
AV-score = (Sum of V-score of Proteins with Significant Hits) / (Number of Proteins with Significant Hits);
AVL-score = (Sum of VL-score of Proteins with Significant Hits) / (Number of Proteins with Significant Hits).
The AV-score and AVL-score of PHROG and VOG were calculated as:
AV-score= (Sum of V-score of Proteins with Significant Hits) / (Total Number of Proteins Encoded in a Genome);
AVL-score = (Sum of VL-score of Proteins with Significant Hits) / (Total Number of Proteins Encoded in a Genome).
Generation of cutoffs of VL-score, AV-score, and AVL-score for viral-like protein/genome determination
To predict the probability of a protein or a genome sequence being viral, the cutoff (see the definition of cutoff in Supplementary Fig. S10) of the VL-score, AV-score, and AVL-score generated above was examined to determine the probability. The cutoff of the AV-score was set from 0 to 10 with steps of 0.2. The cutoff of the VL-score/AVL-score was set from 0 to 5 with step 0.1. The probability of a protein/genome being viral was represented by the fraction of normalized viral proteins/genomes (Nv) compared with normalized plasmids (Np) and chromosomes (Nc) at each cutoff. The fraction at each cutoff was expressed as:
For proteins:
Fraction = Nv / (Nv+Np+Nc)
Nv = (Number of viral proteins with scores above cutoff) / (Total number of viral proteins)
Np = (Number of plasmid proteins with scores above cutoff) / (Total number of plasmid proteins)
Nc = (Number of chromosome proteins with scores above cutoff) / (Total number of chromosome proteins)
For genome sequences:
Fraction = Nv/(Nv+Np+Nc)
Nv = (Number of viral sequences with scores above cutoff) / (Total number of viral sequences)
Np = (Number of plasmid sequences with scores above cutoff) / (Total number of plasmid sequences)
Nc = (Number of chromosome sequences with scores above cutoff) / (Total number of chromosome sequences)
Polynomial regression with the smoothing method “lm” was used to predict the best-fit curve that matches the pattern of the cutoff and probability. The cutoffs for the probability of 70% and 90% were predicted according to estimated polynomial regression equations. If a protein or genome sequence has a score above the cutoff for the probability of 70%, this protein or sequence was determined as a “likely” viral-like protein or sequence. If a protein or genome sequence has an AV-score above the cutoff for the probability of 90%, this protein or sequence was determined as a “most likely” viral-like sequence.
Applying cutoffs to the identification of viral sequences
Metagenomes from host-associated microbiomes were analyzed as a use case to demonstrate the application of viral genome identification. Raw Illumina reads of one snail-associated metagenome47, three sponge-associated metagenomes20, 21, three human-associated metagenomes65, and 32 coral-associated metagenomes66 were retrieved from NCBI (BioProject accessions: PRJNA612619 for snail, PRJNA552185 for sponge, PRJNA763232 for human, PRJNA574146 for coral). The downloaded reads were then trimmed using Trimmomatic67 (version 0.36) with custom settings (ILLUMINACLIP: TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:40). Trimmed reads from the sponge-, human-, and snail-associated microbiomes were assembled with MEGAHIT68 version 1.2.9 using default parameters, while reads from coral-associated microbiomes were assembled using SPAdes69 version 3.11.1 with custom settings (--meta, k-mer sizes varied from 51 to 91, with a 10-mer step size). The assembled metagenomes were then functionally annotated using VOG, PHROG, KEGG, and Pfam via Hmmsearch (HMMER 3.4, parameter: -E 10–5) and MMseqs2 (E-value ⩽ 10–5). AV-scores for VOG, PHROG, KEGG, and Pfam were subsequently calculated for each sequence. Predicted viral genomes were identified based on the following criteria: (1) sequences with at least one AV-score (from VOG, PHROG, KEGG, or Pfam) exceeding the corresponding cutoffs for each fragment size (e.g., a PHROG AV-score > 4.24 or a VOG AV-score > 4.91 for a 2.5 kb scaffold; detailed cutoffs by fragment size are provided in Supplementary Table S10). For sequences larger than 15 kb, cutoffs for 14−15 kb fragments were used. (2) Sequences meeting criterion (1) were further filtered for completeness >0%, as assessed by CheckV53 v1.0.13. In parallel, geNomad8 v1.7.411, VirSorter218 v2.2.3, VIBRANT17 v1.2.0, and DeepVirFinder19 v1.0, (score ≥ 0.75, p < 0.05), were used to identify viral sequences from the host-associated metagenomes, allowing for a comparison between the V-score-based and specific gene- or hallmark- or machine learning-based viral identification methods. For consistency, viral sequences identified by geNomad, VirSorter2, VIBRANT, and DeepVirFinder were also required to have completeness >0%, as assessed by CheckV v1.0.13.
Applying cutoffs to the assessment of proviral sequences
Cutoffs of AV-scores and AVL-scores of whole genomes in Supplementary Table S10 were used for the assessment on proviral sequences by estimating the consistency of our method with a custom prophage database. The custom prophage database developed by Arndt et al.22 were downloaded from PHASTER (https://phaster.ca/databases). Then prophage sequences in the database were functionally annotated with VOG and PHROG using Hmmsearch (HMMER 3.4, parameter: -E 10−5) and MMseqs2 (E-value ≤10−5), followed by the calculation of the AV-scores and the AVL-scores of VOG and PHROG for each prophage. Any prophage sequences with an AV-score or AVL-score above their corresponding cutoff were considered consistent with the prophage database.
To show a potential application in prophage boundary identification, one experimentally verified provirus, Enterobacteria phage P8870, and its host were selected and downloaded from NCBI (Escherichia coli GenBank: GCA_001005685.1). Proteins of prophage and host genomes were predicted using Prodigal V2.6.3 (parameters: -m -p meta)63. Hmmsearch58 (HMMER 3.4, parameter: -E 10−5) was used to match the proteins of prophages and hosts to the HMM profiles of VOG. MMseqs2 with a custom parameter (E-value ≤ 10−5) was used to search prophage and host proteins against the PHROG database. Only the best hit to each protein was retained. Then V-score and VL-score of VOG and PHROG were assigned to each protein, followed by calculating AV-score and AVL-score for each prophage and adjacent host sequence. The gene feature plots of prophages were generated and visualized with DNA Features Viewer71.
Database construction for benchmarking on AMGs identification
We assembled a database of 17 KEGG and Pfam HMM profiles (VL-scores < 3 for KEGG annotations or VL-scores < 3 for Pfam annotations) representing AMGs experimentally demonstrated to affect host metabolism72–76 (Supplementary Table S16) and a database of 10 selected HMMs that represent non-AMGs (Supplementary Table S17). From IMG/VR v452, we compiled a database of 5,116 high-quality50 viral genomes (Supplementary Table S18) containing the 17 experimentally verified AMGs, the 10 non-AMGs, and genomes with neither to obtain a representative sample. We ensured each viral genome had a known host genus, and compiled a database of 180 host genomes (containing homologs of the 17 experimentally verified AMGs) representing the known host genera (Table S13). We used GeNomad8 v1.7.4 to predict viral scaffolds in the 180 host genomes and removed viral scaffolds binned in host genome assemblies (Supplementary Table S19).
Open reading frames in all virus and host genomes were identified and translated using pyrodigal-gv8, 63 v0.3.1 (github.com/althonos/pyrodigal-gv). Translated proteins were aligned to Pfam-A56 v36.0 HMMs and KEGG77 KO HMMs using pyhmmer58, 78 v0.10.10 hmmsearch58 with a maximum e-value of 1e-05. For proteins aligning to multiple HMM profiles within the same database, the highest scoring alignment was reported. Each protein with a Pfam or KEGG functional annotation was assigned its corresponding Pfam or KEGG VL-score and V-score.
Workflow for AMGs identification
Using the database of 17 KEGG and Pfam HMM profiles, we identified potential AMGs by searching for each protein with Pfam VL-score < 3 or KEGG VL-score < 3 and with Pfam and KEGG V-scores < 10. We distinguished AMGs from host-encoded metabolic genes by averaging the VL-scores of all KEGG or Pfam annotations in entire scaffolds, establishing a minimum scaffold Pfam/KEGG AVL-score of 3 as optimal for differentiating viral from host scaffolds. Thus, for a gene flagged as a potential AMG using our predefined VL-score and V-score cutoffs, we also required that the scaffold encoding the gene have an AVL-score > 3 for Pfam/KEGG annotations and AV-score > 4.81 for KEGG annotations or AV-score > 4.39 for Pfam annotations.
It is recommended by community standards for AMG analysis that a potential AMG should be validated by ensuring it is flanked on both the upstream and downstream sides by hallmark genes35, 36. However, given the poor annotation rate of virus proteins, this also impacts the identification of AMGs. Here, we conducted our flanking verification approach by running our AMG identification workflow using viral hallmark genes to verify flanking regions of potential AMGs. We defined viral hallmark genes in our KEGG and Pfam HMM databases as previously described79; any HMM profile with an annotation/description containing any of the following keywords: virion structure (truncated from structure to account for matches to the terms “structure” or “structural”), capsid, portal, tail, and terminase. A list of KEGG and Pfam HMMs defined as viral hallmark genes this way are provided in Supplementary Table S20. In parallel, we verified that AMGs identified with our workflow were flanked on both sides by at least one gene with a V-score of 10 within 10 kb of the AMG, recognizing that viral genes with unknown functions may still be characteristically viral. The verification approach may not be necessary when analyzing complete or cultured viral genomes, so we report results with and without flank verification.
Assessment on performance of the workflow for AMGs identification
To assess the performance of our workflow, we established true positives and negatives for AMGs in our test genome dataset. A gene encoded by a viral scaffold with an annotation in the experimentally verified AMG database was considered a true positive, while any host-encoded gene in the experimentally verified AMG database was considered a true negative. Genes encoded on viral scaffolds with annotations matching any of 10 selected HMMs that represent non-AMGs were also considered true negatives. Any other gene, encoded on a known host or viral genome, that was not annotated with the experimentally verified AMG database or non-AMG database was not considered a true positive or negative.
In addition to the true positives and negatives, we predicted positives and negatives. To ensure that we did not analyze viral genes in host genomes, all genes encoded on host scaffolds predicted as viral were removed before we predicted the positives and negatives of our AMG identification workflow. Predicted positives were any gene, encoded on a known host or viral scaffold, that met the following criteria: (1) the gene has a Pfam VL-score < 3 or a KEGG VL-score < 3, (2) the gene has a Pfam V-score < 10 or a KEGG V-score < 10, (3) the gene is encoded on a scaffold with a Pfam AVL-score > 3 or a KEGG AVL-score > 3, (4) the gene is encoded on a scaffold with a Pfam AV-score > 4.39 or a KEGG AV-score > 4.81, (5) the gene is flanked to the left and right by at least one other gene with a V-score of 10 within a 10 kb distance (only applies to results reporting prediction “with flank verification”). Any gene with an annotation belonging to the AMG database or the non-AMG database that did not meet these criteria was considered a predicted negative. Genes without annotations to the non-AMG or the AMG database were not predicted as positives or negatives. The counts of true positives, true negatives, predicted positives, and predicted negatives were used to construct the confusion matrices in Supplementary Table S12.
Identification of auxiliary genes using our workflow and other existing approaches
We assembled a dataset of 5,116 high-quality viral genomes from IMG/VR v452 (Supplementary Table S18). All viral genes were evaluated for potential auxiliary functions using the AMG identification workflow, both with and without flank verification. Genes annotated under KEGG’s “sulfur relay system” or “metabolic pathways” category, excluding those related to nucleotide metabolism or sulfonate transport system substrate-binding proteins, were considered potential AMGs. Additionally, auxiliary genes with KEGG and PFAM annotations were cross-referenced against a viral AMG database35, which includes experimentally verified AMGs from previous studies26, 37, 72–76, 80, 81. PFAM and KEGG accessions associated with AMGs were retrieved, and ORFs containing these accessions were retained and integrated into the AMG dataset. To compare our approach with other existing tools to identify AMGs, we ran VIBRANT17 with the “annoVIBRANT” implementation (github.com/AnantharamanLab/annoVIBRANT) and DRAM-v35 on the same set of high-quality viral genomes. For DRAM-v only the AMGs with a score of 1 were retained, which indicates the presence of at least one hallmark gene on both sides, suggesting the gene is likely viral.
Visualization of VL-scores, and V-scores of phage and host genomes containing psbA
We visualized the genomic context of one predicted AMG, the photosystem II P680 reaction center D1 protein (psbA KO K02703), in viral and host genomes. We identified one Prochlorococcus host genome (GenBank GCA_003214355.1) and two viral genomes (IMGVR_UViG_2716884766_000001 and IMGVR_UViG_2716884767_000001) encoding psbA (Supplementary Table S18) predicted by IMG/VR to be Prochlorococcus phages. We plotted genes within localized regions of these genomes using the R package gggenomes82 v1.0.0 using annotations, VL-scores, and V-scores obtained as described above.
Viral species differentiation based on AV-score and AVL-score
Reference prokaryotic viruses were used for assessment on viral population differentiation based on AV-score and AVL-score. Lineage of the reference viruses was downloaded from virushostdb (https://www.genome.jp/virushostdb). According to the lineage information of each viral RefSeq genome, 11 species of reference prokaryotic viruses were selected (each species with ≥ 4 genomes). Viral species include Bixzunavirus Bxz1, Campylobacter virus IBB35, Fibrovirus fs1, Inovirus M13, Kayvirus G1, Otagovirus Psa374, Pegunavirus Pg1, Pegunavirus soto, Pegunavirus Suffolk, Restivirus RSS1, and Wphvirus megatron. Viral genomes were annotated with databases of VOG, PHROG, KEGG, Pfam, and eggNOG using Hmmsearch (HMMER 3.4, parameter: -E 10−5), MMseqs2 (parameter: E-value ≤ 10−5), or EggNOG-mapper version 2.1.12 (parameters: -m mmseqs --evalue 10−5). In the following, the AV-score and AVL-score of each genome were calculated. Detailed information of NCBI RefSeq accessions and AV-score and AVL-score of viral genomes was provided in Supplementary Table S21.
Metagenome binning with AV-score and AVL-score
The metagenome of deep-sea snail (Gigantopelta aegis) microbiome47 was analyzed as a use case to show an application in genome binning. Raw Illumina reads of the snail G. aegis metagenome were retrieved from NCBI (BioProject accession: PRJNA612619). Then the downloaded reads were trimmed by Trimmomatic (version 0.36)67 with custom setting (ILLUMINACLIP: TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:40). Scaffolds of the genomes of two bacterial endosymbionts and four phages infecting the endosymbionts were mapped to the trimmed reads with Bowtie2 version 2.3.483 and SAMtools version 1.684 to calculate sequencing coverage. Additionally, the microbial genomes were functionally annotated with VOG, PHROG, KEGG, Pfam, and eggNOG with Hmmsearch (HMMER 3.4, parameter: -E 10−5), MMseqs2 (E-value ≤10−5), or EggNOG-mapper version 2.1.12 (parameters: -m mmseqs --evalue 10−5), followed by the calculation of AV-score and AVL-score for each scaffold in a genome. Finally, we manually binned bacterial and phage scaffolds (length ≥5 kb) following a previously described approach85 on the basis of AV-score and AVL-score, sequencing depth, phage hallmark genes, and bacterial conserved single-copy genes.
Supplementary Material
Acknowledgments
We thank members of the Anantharaman Laboratory for discussions and feedback on this manuscript.
Funding
This research was supported by the National Science Foundation under grant number DBI2047598 and National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM143024 to KA. JCK was supported by an NSF Graduate Research Fellowship.
Footnotes
Conflict of interest
The authors declare no competing interests.
References
- 1.Suttle C.A. Viruses in the sea. Nature 437, 356–361 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Suttle C.A. Marine viruses—major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Rohwer F. & Thurber R.V. Viruses manipulate the marine environment. Nature 459, 207–212 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Forterre P. & Prangishvili D. The origin of viruses. Res. Microbiol. 160, 466–472 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Morris D.H. et al. Predictive modeling of influenza shows the promise of applied evolutionary biology. Trends Microbiol. 26, 102–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieft K. & Anantharaman K. Virus genomics: what is being overlooked? Curr. Opin. Virol. 53, 101200 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux S., Enault F., Hurwitz B.L. & Sullivan M.B. VirSorter: mining viral signal from microbial genomic data. PeerJ 3, e985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo A.P. et al. Identification of mobile genetic elements with geNomad. Nat. Biotechnol. 42, 1303–1312 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koonin E.V., Dolja V.V. & Krupovic M. The logic of virus evolution. Cell Host Microbe 30, 917–929 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Wiles T.J. et al. A phyletically rare gene promotes the niche-specific fitness of an E. coli pathogen during bacteremia. PLoS Pathog. 9, e1003175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeifer E. & Rocha E.P.C. Phage-plasmids promote recombination and emergence of phages and plasmids. Nat. Commun. 15, 1545 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupovic M., Prangishvili D., Hendrix R.W. & Bamford D.H. Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere. MMBR 75, 610–635 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieft K. & Anantharaman K. Deciphering active prophages from metagenomes. mSystems 7, e0008422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pride D.T., Meinersmann R.J., Wassenaar T.M. & Blaser M.J. Evolutionary implications of microbial genome tetranucleotide frequency biases. Genome Res. 13, 145–158 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen D.M. et al. Orthologous gene clusters and taxon signature genes for viruses of prokaryotes. J. Bacteriol. 195, 941–950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J., Ahlgren N.A., Lu Y.Y., Fuhrman J.A. & Sun F. VirFinder: a novel k-mer based tool for identifying viral sequences from assembled metagenomic data. Microbiome 5, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieft K., Zhou Z. & Anantharaman K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome 8, 1–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J. et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome 9, 37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J. et al. Identifying viruses from metagenomic data using deep learning. Quant. Biol. 8, 64–77 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou K. et al. Potential interactions between clade SUP05 sulfur-oxidizing bacteria and phages in hydrothermal vent sponges. Appl. Environ. Microbiol. 85, e00992–00919 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou K., Qian P.Y., Zhang T., Xu Y. & Zhang R. Unique phage-bacterium interplay in sponge holobionts from the southern Okinawa Trough hydrothermal vent. Environ. Microbiol. Rep. 13, 675–683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arndt D. et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44, W16–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis-Cunha J.L., Bartholomeu D.C., Manson A.L., Earl A.M. & Cerqueira G.C. ProphET, prophage estimation tool: A stand-alone prophage sequence prediction tool with self-updating reference database. PloS one 14, e0223364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier C.H. et al. DEPhT: a novel approach for efficient prophage discovery and precise extraction. Nucleic Acids Res. 50, e75 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang K. et al. Prophage Tracer: precisely tracing prophages in prokaryotic genomes using overlapping split-read alignment. Nucleic Acids Res. 49, e128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux S. et al. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature 537, 689–693 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Kieft K. et al. Ecology of inorganic sulfur auxiliary metabolism in widespread bacteriophages. Nat. Commun. 12, 3503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Reilly D.R. in The baculoviruses 267–300 (Springer, 1997). [Google Scholar]
- 29.Heyerhoff B., Engelen B. & Bunse C. Auxiliary metabolic gene functions in pelagic and benthic viruses of the Baltic Sea. Front. Microbiol. 13, 863620 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X.Q. et al. Viral community-wide auxiliary metabolic genes differ by lifestyles, habitats, and hosts. Microbiome 10, 190 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian F. et al. Prokaryotic-virus-encoded auxiliary metabolic genes throughout the global oceans. Microbiome 12, 159 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham E.B. et al. A global atlas of soil viruses reveals unexplored biodiversity and potential biogeochemical impacts. Nat. Microbiol. 9, 1873–1883 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayfach S. et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 6, 960–970 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieft K. et al. Virus-associated organosulfur metabolism in human and environmental systems. Cell Rep. 36, 109471 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Shaffer M. et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 48, 8883–8900 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratama A.A. et al. Expanding standards in viromics: in silico evaluation of dsDNA viral genome identification, classification, and auxiliary metabolic gene curation. PeerJ 9, e11447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan M.B., Coleman M.L., Weigele P., Rohwer F. & Chisholm S.W. Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations. PLoS Biol. 3, 790–806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emerson J.B. et al. Host-linked soil viral ecology along a permafrost thaw gradient. Nat. Microbiol. 3, 870–880 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeRoux M. & Laub M.T. Toxin-antitoxin systems as phage defense elements. Annu. Rev. Microbiol. 76, 21–43 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Koonin E.V. Antitoxins within toxins: a new theme in bacterial antivirus defense. Proc. Natl. Acad. Sci. U.S.A. 120, e2311001120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srikant S., Guegler C.K. & Laub M.T. The evolution of a counter-defense mechanism in a virus constrains its host range. eLife 11, e79549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guegler C.K. et al. A phage-encoded RNA-binding protein inhibits the antiviral activity of a toxin–antitoxin system. Nucleic Acids Res. 52, 1298–1312 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fay E.J. et al. Natural rodent model of viral transmission reveals biological features of virus population dynamics. J. Exp. Med. 219, e20211220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norman J.M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manrique P. et al. Healthy human gut phageome. Proc. Natl. Acad. Sci. U.S.A. 113, 10400–10405 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Draper L.A. et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 6, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou K., Xu Y., Zhang R. & Qian P.Y. Arms race in a cell: genomic, transcriptomic, and proteomic insights into intracellular phage-bacteria interplay in deep-sea snail holobionts. Microbiome 9, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kieft K., Adams A., Salamzade R., Kalan L. & Anantharaman K. vRhyme enables binning of viral genomes from metagenomes. Nucleic Acids Res. 50, e83–e83 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregory A.C. et al. Marine DNA viral macro-and microdiversity from pole to pole. Cell 177, 1109–1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roux S. et al. Minimum information about an uncultivated virus genome (MIUViG). Nat. Biotechnol. 37, 29–37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terzian P. et al. PHROG: families of prokaryotic virus proteins clustered using remote homology. NAR Genomics Bioinf. 3, lqab067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camargo A.P. et al. IMG/VR v4: an expanded database of uncultivated virus genomes within a framework of extensive functional, taxonomic, and ecological metadata. Nucleic Acids Res. 51, D733–D743 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nayfach S. et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 39, 578–585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinegger M. & Soding J. Clustering huge protein sequence sets in linear time. Nat. Commun. 9, 2542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aramaki T. et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mistry J. et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 49, D412–D419 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huerta-Cepas J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eddy S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinke C. et al. A standardized archaeal taxonomy for the Genome Taxonomy Database. Nat. Microbiol. 6, 946–959 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Parks D.H. et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 36, 996–1004 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Schmartz G.P. et al. PLSDB: advancing a comprehensive database of bacterial plasmids. Nucleic Acids Res. 50, D273–D278 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Leary N.A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–745 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyatt D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 11, 1–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantalapiedra C.P., Hernandez-Plaza A., Letunic I., Bork P. & Huerta-Cepas J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swaney M.H. & Kalan L.R. Living in your skin: microbes, molecules, and mechanisms. Infect. Immun. 89, 10–1128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vohsen S.A. et al. Deep-sea corals provide new insight into the ecology, evolution, and the role of plastids in widespread apicomplexan symbionts of anthozoans. Microbiome 8, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolger A.M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D., Liu C.M., Luo R., Sadakane K. & Lam T.W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Bankevich A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen M. et al. Inducible prophage mutant of Escherichia coli can lyse new host and the key sites of receptor recognition identification. Front. Microbiol. 8, 147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zulkower V. & Rosser S. DNA Features Viewer: a sequence annotation formatting and plotting library for Python. Bioinformatics 36, 4350–4352 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Lindell D., Jaffe J.D., Johnson Z.I., Church G.M. & Chisholm S.W. Photosynthesis genes in marine viruses yield proteins during host infection. Nature 438, 86–89 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Clokie M.R.J. et al. Transcription of a ‘photosynthetic’ T4-type phage during infection of a marine cyanobacterium. Environ. Microbiol. 8, 827–835 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Lindell D. et al. Transfer of photosynthesis genes to and from Prochlorococcus viruses. Proc. Natl. Acad. Sci. U.S.A. 101, 11013–11018 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mann N.H., Cook A., Millard A., Bailey S. & Clokie M. Marine ecosystems: bacterial photosynthesis genes in a virus. Nature 424, 741–741 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Thompson L.R. et al. Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism. Proc. Natl. Acad. Sci. U.S.A. 108, E757–E764 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanehisa M., Sato Y., Kawashima M., Furumichi M. & Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larralde M. & Zeller G. PyHMMER: a Python library binding to HMMER for efficient sequence analysis. Bioinformatics 39, btad214 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roux S. et al. Ecology and evolution of viruses infecting uncultivated SUP05 bacteria as revealed by single-cell- and meta-genomics. eLife 3, e03125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng Q.L. & Chisholm S.W. Marine viruses exploit their host’s two-component regulatory system in response to resource limitation. Curr. Biol. 22, 124–128 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Hurwitz B.L., Brum J.R. & Sullivan M.B. Depth-stratified functional and taxonomic niche specialization in the ‘core’ and ‘flexible’ Pacific Ocean Virome. ISME J. 9, 472–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hackl T., Ankenbrand M. & B.v.A. gggenomes: a grammar of graphics for comparative genomics. R package version 1.0.0 9 (2024). [Google Scholar]
- 83.Langmead B. & Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albertsen M. et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 31, 533–538 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.