Abstract
Background:
Deep learning can extract predictive and prognostic biomarkers from histopathology whole slide images, but its interpretability remains elusive.
Methods:
We develop and validate MoPaDi (Morphing histoPathology Diffusion), which generates counterfactual mechanistic explanations. MoPaDi uses diffusion autoencoders to manipulate pathology image patches and flip their biomarker status by changing the morphology. Importantly, MoPaDi includes multiple instance learning for weakly supervised problems. We validate our method on four datasets classifying tissue types, cancer types within different organs, center of slide origin, and a biomarker – microsatellite instability. Counterfactual transitions were evaluated through pathologists’ user studies and quantitative cell analysis.
Results:
MoPaDi achieves excellent image reconstruction quality (multiscale structural similarity index measure 0.966–0.992) and good classification performance (AUCs 0.76–0.98). In a blinded user study for tissue-type counterfactuals, counterfactual images were realistic (63.3–73.3% of original images identified correctly). For other tasks, pathologists identified meaningful morphological features from counterfactual images.
Conclusion:
MoPaDi generates realistic counterfactual explanations that reveal key morphological features driving deep learning model predictions in histopathology, improving interpretability.
Introduction
Deep learning (DL) is preferred for computer vision tasks in biomedical sciences requiring complex decision-making, prediction, or perception. In particular, in computational pathology, DL can predict diagnostic and prognostic biomarkers from hematoxylin and eosin (H&E) stained whole slide images (WSIs) derived from pathology slides1–4. DL performs well for many clinically relevant tasks, such as automating pathology workflows to make diagnoses or extract subtle visual information related to biomarkers5. However, the so-called ‘black box’ nature of DL can be problematic because it is often unclear which visual features the model bases its predictions on, limiting interpretability and trust in the classifier6. Established explainability methods, such as feature visualization7 and pixel attribution (e.g., saliency maps, including attention heatmaps)8, are limited and cannot guarantee that a model’s decision is correct6. Although widely used in the biomedical field, interpreting these methods is challenging, often requiring domain expertise, and can result in overestimating a model’s performance due to confirmation bias6,9,10.
An alternative approach, counterfactual explanations11,12, is available but has been underexplored in computational pathology. Counterfactual explanations answer the question: ‘how should an image look to be classified as class X’ (Fig. 1). Here, we develop and validate MoPaDi (Morphing histoPathology Diffusion), a new method to generate counterfactual explanations for histopathology images. Our objective is to make the decision-making process of ‘black-box’ artificial intelligence (AI) models more understandable and trustworthy.
Fig. 1 |. Overview of the experimental setup.
a, Counterfactual explanations answer the question: ‘how should an image look to be classified as class X’. b, Datasets used in this study and preprocessing steps. The colorectal and breast cancer datasets from the TCGA, consisting of digitized WSIs, were resized to 512 × 512 px at 0.5 MPP, tessellated, and filtered only to include pathologist-annotated tumor regions. The NCT-CRC-HE-100k dataset included 100,000 pre-color-normalized with Macenko’s method tiles across nine tissue classes. TCGA Pan-cancer dataset featured tiles from tumor regions of 32 solid cancer types. All datasets, except NCT-CRC-HE-100k, which had a separate test set CRC-VAL-HE-7K, were divided into training and hold-out test sets based on patient-level splits. c, The diffusion autoencoder is trained simultaneously as a feature extractor and a denoising diffusion implicit model (DDIM), which is conditioned on feature vectors and the original image encoded into noise. This approach allows the model to reconstruct either original or manipulated images. d, The most straightforward approach to determine how manipulations of feature vectors should be performed is to train a tile-based logistic regression classifier on feature vectors. This approach allows the manipulation of feature vectors towards the opposite class in a linear direction. e, For histopathological WSIs, labeling each tile as positive, e.g., for a molecular biomarker, is not entirely precise because only a few tiles might show representative features. Therefore, to address this problem, we implemented multiple instance learning (MIL) into MoPaDi, enabling predictions to be made on a patient level. f, Extracted feature vectors are shifted towards the opposite class by multiplication with varying amplitude and normalized class direction vector. Subsequently, a denormalized manipulated feature vector is used to condition DDIM, yielding a manipulated image. MLP, multilayer perceptron; MPP, micrometers per pixel.
Previous studies in other image analysis domains have shown that counterfactual explanations can help uncover biases and confounding variables, as well as provide model prediction explanations, yet these efforts are limited to radiology data or outdated generative AI models13–16. Therefore, we propose utilizing state-of-the-art diffusion models to generate counterfactual explanations. An alternative approach to diffusion models is generative adversarial networks (GANs), which have been explored in previous studies17–23. However, diffusion models are now considered the state-of-the-art technique, surpassing GANs in synthetic image quality and training stability24. Diffusion models work by gradually transforming images into noise and learning the reverse process, enabling the generation of novel images by sampling from the learned data distribution25. A key strength of diffusion models over GAN-based methods is their ability to modify real images while preserving details unrelated to the specific manipulation26. Furthermore, most computational pathology problems require a weakly supervised approach since labels are typically available only for WSIs consisting of many patches but not for every patch individually27. These tasks are usually addressed by multiple instance learning (MIL)28. Therefore, we extend the concept of counterfactual image generation to the weakly supervised scenario, in which we train an attention-based classifier on bags of tiles with a patient-level label, and then generate counterfactual explanations for the most predictive tiles. MoPaDi combines diffusion autoencoders26 with MIL to generate high-quality counterfactual images of histopathology patches, preserving original histopathology patch details and enabling pathologists to comprehensively evaluate a classifier’s learned morphologies.
The aim of MoPaDi is to show which morphological features in histopathology images drive a DL classifier’s predictions by creating counterfactual explanations. To validate our method, we first demonstrate that meaningful features were learned by the model by generating tissue-type counterfactual images. To our knowledge, diffusion-based explainability methods have not been applied to histopathology data for counterfactual image generation combined with MIL. Finally, we apply MoPaDi to explain DL model predictions for a broad range of clinically relevant tasks. The evaluation tasks include differentiating between WSI processing sites for batch effect analysis and microsatellite instability (MSI) status, a colorectal cancer biomarker predictable from H&E-stained WSIs3,29. Four datasets were utilized: two colorectal carcinomas (CRC) datasets, a breast cancer (BRCA) dataset, and a pan-cancer dataset comprising 32 cancer types. For the latter, we focused on hepatobiliary cancers (hepatocellular carcinoma [HCC], and cholangiocarcinoma [CCA]) and lung cancers (lung adenocarcinoma [LUAD], and lung squamous cell carcinoma [LUSC]) (Fig. 1b). Our method, validated across diverse datasets, reveals key morphological features driving classifiers predictions through automated, targeted manipulation of histopathological images toward counterfactual images, enabling understanding of morphological patterns learned by the DL model across cancer types and a biomarker.
Results
Diffusion Autoencoders Efficiently Encode Histopathology Images
The first step in evaluating a generative model is to assess the fidelity of the image embeddings. Therefore, we evaluated the performance of our custom-trained diffusion autoencoders in reconstructing histopathology images by comparing 1,000 original and reconstructed images using mean squared error (MSE) and multi-scale structural similarity index measure (MS-SSIM) values. We found that the models achieved high performance across different datasets, as indicated by low MSE and high MS-SSIM values (Table 1). A qualitative evaluation confirmed a high rate of near-perfect reconstructions, although some minor artifacts were sometimes found to be accentuated by the compression-decompression process (Fig. S1). To further assess the encoder’s ability to learn meaningful histopathological features, we trained additional denoising diffusion implicit models (DDIM) on feature vectors extracted by the feature extractor part of the diffusion autoencoder. This enabled the sampling of synthetic feature vectors that are subsequently used for novel image generation. These models produced high-quality synthetic images, indicated by low Fréchet Inception Distance (FID) between a held-out set of original images and 10,000 generated synthetic images (Fig. S2) for most datasets: 16.85 for TCGA CRC, 17.85 for TCGA BRCA, and 4.71 for TCGA Pan-cancer. In contrast, the CRC-VAL-HE-7K had a higher FID score of 36.95 (Table 1). Together, these data show that our custom-trained diffusion autoencoders effectively capture key image features and reconstruct histopathological patches across multiple datasets, demonstrating their potential as a suitable foundation for generating counterfactual explanations.
Table 1 |. Evaluation of diffusion autoencoders trained on different datasets.
The reconstruction quality of encoded images was evaluated using multi-scale structural similarity index measure (MS-SSIM) and mean square error (MSE), which perform pixel-by-pixel comparisons of 1,000 reconstructed images. The ability of the diffusion model’s latent space to capture meaningful information was further assessed by generating 10,000 synthetic histopathology images and computing the Fréchet Inception Distance (FID) score between the generated and held-out test set images to evaluate their quality.
| Model | MS-SSIM ↑ | MSE ↓ | FID score ↓ |
|---|---|---|---|
| NCT100k | 0.968 ± 0.036 | (0.4 ± 0.5) * 10−3 | 36.95 |
| TCGA Pan-cancer | 0.992 ± 0.001 | (0.8 ± 0.2) * 10−3 | 4.71 |
| TCGA CRC | 0.967 ± 0.060 | (8.1 ± 14.0) * 10−3 | 16.85 |
| TCGA BRCA | 0.966 ± 0.062 | (4.5 ± 11.3) * 10−3 | 17.85 |
Tissue Type Counterfactuals Exhibit Meaningful Patterns
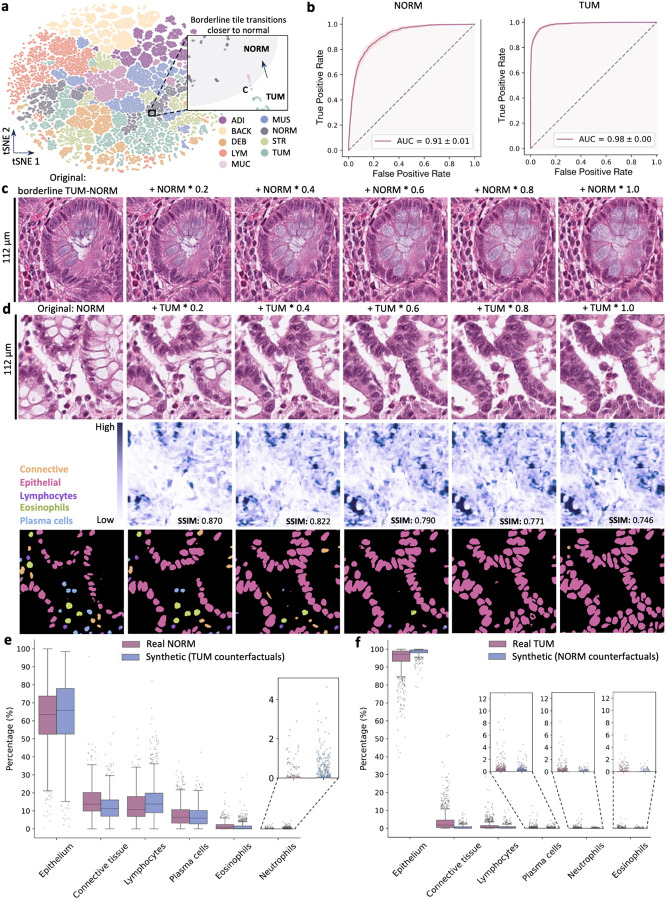
Next, we assessed MoPaDi’s capabilities to generate counterfactual images for different tissue types, i.e., to flip the class in a given image. We started by training a diffusion autoencoder alongside a feature extractor (Fig. 1c) on the NCT-CRC-HE-100k dataset, representing tissue classes in colorectal cancer (Fig. S3a). Features extracted from the training dataset formed distinct clusters upon dimensionality reduction, suggesting the model learned meaningful histopathological class features (Fig. 2a). We then hypothesized that a logistic regression classifier trained on the extracted features (Fig. 1d) could linearly separate two major classes, normal colon mucosa (NORM) and dysplastic epithelium (TUM) (Fig. 1d). We evaluated this in a separate dataset (CRC-VAL-HE-7K) and found a high classification performance, represented by a high area under the receiver operating characteristic curve (AUROC) of 0.91 ± 0.01 (NORM) and 0.98 ± 0.01 (TUM) (bootstrapped confidence intervals) (Fig. 2b). AUROCs for other classes ranged from 0.79 to 1.00 (Fig. S3b). This classifier guided the manipulation of feature vectors, which conditioned the diffusion-based decoder (Fig. 1f), enabling us to generate counterfactual images for patches from the CRC-VAL-HE-7K dataset with varying degrees of manipulation. Manipulated feature vectors shifted closer to the opposite class in the lower dimensional space (Fig. 2a zoomed-in plot), particularly when features are near the boundary between classes (as seen in the colon mucosa tile bordering dysplastic and healthy tissue in Fig. 2c).
Fig. 2 |. Results for the counterfactual image generation with the diffusion model for the CRC-VAL-HE-7K dataset.
a, The feature extractor’s latent space of the training set, reduced to two dimensions and colored according to the nine different classes in the dataset: adipose (ADI), background (BACK), debris (DEB), lymphocytes (LYM), mucus (MUC), smooth muscle (MUS), normal colon mucosa (NORM), cancer-associated stroma (STR), colorectal adenocarcinoma epithelium (TUM). The latter class contains tiles that exhibit cancerous tissue and dysplasia. For example, the features extracted from the tile in c, located on the borderline between the TUM (aqua) and NORM (gray) in the t-SNE plot, move towards the normal tissue cluster (pink data points in the zoomed-in area of the plot; gray area – arbitrary healthy mucosa zone) as they are increasingly manipulated in the latent space. b, Receiver operating characteristic (ROC) curves of the logistic regression classifier in the latent space for the linearly separable TUM and NORM classes show that both classes are predicted with areas under the curves (AUCs) greater than 0.9 (ROC curves for the other classes can be found in the extended data [Fig S3]). c, The dysplastic epithelium tile manipulated to resemble healthy colon mucosa, with increasing manipulation amplitudes indicated above each tile. d, An opposite example (healthy colon mucosa to dysplastic epithelium), included in a blinded user study and identified as a highly realistic counterfactual example. In the row below, pixel-level difference images between the original tile and the manipulated ones are displayed, where darker regions indicate greater changes in pixel values. Structural Similarity Index Measure (SSIM) values, which quantify the similarity between the original and manipulated tiles, are displayed on each tile (the higher, the better). Pixel-wise difference maps between the original and manipulated histopathology images reveal that the greatest differences during the manipulation process occur in regions where glands were present. In the next row, automatically segmented and classified nuclei in corresponding tiles are displayed. Pink, epithelial cells; orange, connective tissue cells; blue, plasma cells; deep purple, lymphocytes; aqua, neutrophils; green, eosinophils. e, The distribution of different cell types in real healthy colon mucosa tiles (NORM class, n tiles = 741) and synthetic (counterfactual images for TUM class, i.e., the most manipulated tiles, n = 1,233). Cell type percentages between groups were significantly different (p < 0.001, two-sided Mann-Whitney U test with correction for multiple comparisons using the Bonferroni correction method), except for plasma cells, indicating the synthetic nature of generated images. A full description of the statistical parameters including p values is provided in Table S2. f, The distribution of percentages of different cell types in real dysplastic epithelium tiles (TUM class, n tiles = 1,233) and synthetic images (counterfactual images for NORM class, n tiles = 741). Cell type percentages between groups were also significantly different (p < 0.001 for all cell types), except for neutrophils and eosinophils. The model tended to generate more epithelial cells in counterfactual images, as it potentially learned this is a prominent feature of the malignant epithelium class. A full description of the statistical parameters including p values is provided in Table S2.
To further evaluate the quality of counterfactual images, we performed a blinded evaluation study with two pathologists. They were asked to distinguish between original and synthetic images in 30 patch transitions to counterfactual images of the opposite class (example in Fig. 2d). When half of the 30 cases were flipped, correct identification rates ranged from 63.3% to 73.3%, indicating that MoPaDi generates highly realistic images. Occasional mistakes revealed the synthetic nature of some images, such as insufficient hyperchromasia or gland formation without a visible basement membrane, leading to unrealistically smooth boundaries between cells and adjacent stroma (Fig. S4). Overall, transitions were biologically plausible. For example, counterfactual images showed that the model learned the appearance of healthy, well-ordered goblet cells associated with the NORM class. The reverse counterfactual pair (NORM to TUM) displayed loss of goblet cells and connective tissue, along with the appearance of hyperchromatic nuclei (Fig. 2d). To further evaluate the quality of generated images in a quantitative way, we analyzed the distribution of six cell types in real and synthetic images (i.e., epithelial cells, connective tissue cells, lymphocytes, plasma cells, eosinophils, and neutrophils). To achieve this, we applied a DL-based nuclei segmentation and classification model. The results indicated that synthetic healthy colon mucosa images had cell type distributions similar to real images (Fig. 2e). Synthetic dysplastic epithelium tiles predominantly contained epithelial cells, with a few connective tissue cells and immune cells (Fig. 2f).
Collectively, these findings demonstrate that our model learned and could generate key histological features associated with normal colon mucosa and dysplastic epithelium despite occasional inaccuracies in specific cellular details.
Counterfactuals Enhance Interpretation of Linear Liver Cancer Predictions
We further validated our method by training a linear MoPaDi classifier (Fig. 1d) to distinguish two hepatobiliary cancer types, HCC and CCA, and generating related counterfactuals. A logistic regression model trained on features extracted from HCC and CCA cases in the TCGA Pan-cancer dataset achieved an AUROC of 0.90 ± 0.01 (95% CI) and an average precision (AP) of 0.57 ± 0.03 on the held-out test set (Fig. 3a). The lower AP is attributed to the imbalanced dataset, with only 10% of patients having CCA (Table S1). Averaging patch-level predictions to patient-level yielded an AUROC of 0.94 ± 0.10, indicating an effective distinction between hepatobiliary cancer types despite some patch-level errors. A qualitative analysis of the generated counterfactual images revealed that the model accurately learned representative morphological features for both classes. HCC patches manipulated to resemble CCA displayed a more glandular pattern, reduced eosinophilic cytoplasm, and lesser trabecular architecture (Fig. 3c). Meanwhile, CCA patches manipulated to resemble HCC displayed a more trabecular growth pattern with loss of glandular structure, eosinophilic cytoplasmic inclusions, possible Mallory bodies, and reduced stromal tissue (Fig. 3d). Increasing manipulation levels resulted in higher opposite-class predictions and decreased MS-SSIM values. These distinct morphological changes in counterfactual images demonstrate the model’s ability to capture hepatobiliary cancer characteristics while enhancing the classifier’s interpretability.
Fig. 3 |. Results for explainability of liver cancer types classifiers.
a, Performance of the logistic regression classifier in distinguishing hepatobiliary cancers (hepatocellular carcinoma [HCC], and cholangiocarcinoma [CCA]) b, 5-fold cross-validation results of the MIL-based classifier for the same task. c, Representative example of HCC tile transitioning to its counterfactual HCC image generated with a linear approach. Comparison to the MIL approach can be found in the extended data (Fig. S5). Difference maps display pixel-wise differences between the original and synthetic tile. d, Representative example of CCA tile transitioning to its counterfactual HCC image. AUC, area under the curve; AP, average precision; SSIM, structural similarity index measure.
MIL Enables WSI-level Cancer-type Classification Counterfactuals
Given the complexity of weakly supervised WSI tasks, where histological phenotypes are visible only in select regions, we replace the linear classifier in MoPaDi with MIL to assess its potential for generating counterfactual explanations for tissue properties which are only defined by slide-level annotations, not patch-level annotations. For this, we trained a diffusion autoencoder, which contains a feature extractor, and combined it with an attention-based classifier of extracted features, enabling targeted generation of counterfactual images that reveal key morphologic features associated with different classes. We investigated the efficacy of this approach in tumor classification problems: lung cancer (LUAD vs. LUSC), breast cancer (invasive lobular carcinoma [ILC] vs. invasive ductal carcinoma [IDC]), and the previously discussed HCC vs. CCA. Using HCC and CCA features from the feature extractor of our diffusion autoencoder, we train an attention-based MIL classifier. Integrating MIL led to predictive performance similar to the patch-based linear approach for this task, reaching a mean AUROC of 0.96 ± 0.01 (Fig. 3b) and an AP of 0.82 ± 0.07 (Fig. S5a) in the 5-fold cross-validation. Top tiles from correctly classified patients displayed distinctive features (Fig. S5b): original HCC tiles showed cells arranged in sheets or trabecular patterns with lipid droplets, lacking glandular structures and fibrous stroma, while original CCA tiles showed glandular structures and more fibrous stroma. Counterfactual images guided by the MIL classifier resembled those generated using the linear approach with patch-level logistic regression (Fig. S5c,d). Cell type analysis showed similar distributions between counterfactual and real images (Fig. S5e,f).
We further validated counterfactual image generation guided by the MIL classifier by training a LUSC-LUAD classifier, achieving a mean AUROC of 0.91 ± 0.01 (with an improvement of 0.10 over the linear method) and an AP of 0.89 ± 0.02 (Fig. S6a). Pathologists analyzed LUSC to LUAD counterfactual transitions (Fig. 4a), with one noting increased glandular differentiation in 4 out of 8 patients and the other in 5 out of 8 (two top tiles per patient), while both observed lower pleomorphism (6/8). Additional features identified included increased vacuolization (4/8), clearer cells (4/8), and decreased tumor solidity (3/8). However, some counterfactual tiles retained LUSC features atypical for LUAD, such as prominent dyskaryotic changes, nuclear molding, and residual squamous differentiation (Fig. S6d). LUAD to LUSC transitions showed decreased glandular architecture, increased solid growth patterns or tumor cell nests (4/8; 5/8), and increased cell density (2/8; 5/8). Other features included lower atypia (1/8; 2/8), intercellular bridges, and a keratin pearl (Fig. S6e). Taken together, this data demonstrates that MIL classifier-guided counterfactual image generation works effectively; thus, MoPaDi can be applied to WSI-level datasets.
Fig. 4 |. Results for explainability of breast and lung cancer type classifiers.
Classifiers’ performance metrics can be found in the extended data (Fig. S6). a, Representative examples of lung squamous cell carcinoma (LUSC) tile transitioning to its counterfactual lung adenocarcinoma (LUAD) image and vice versa. b, Representative examples of invasive lobular carcinoma (ILC) tile transitioning to its counterfactual invasive ductal carcinoma (IDC) image and vice versa. Difference maps display pixel-wise differences between the original and synthetic tile. SSIM, structural similarity index measure.
We then investigated whether meaningful morphological features could be identified in counterfactuals for IDC and ILC classification in the TCGA BRCA cohort. The trained classifier reached a mean AUROC of 0.76 ± 0.03 and AP of 0.43 ± 0.02 (Fig. S6c). In ILC to IDC counterfactual transitions (Fig. 4b top), the most consistently observed changes were increased hyperchromasia (5/8) and atypia (4/8). Other notable changes included a shift towards less single-cell arrangement without clear glandular pattern formation (2–3/8), larger tumor cells (3/8), and tumor cell arrangement in larger nests (2/8). Some top tiles contained only muscle with single tumor cells, making feature assessment not feasible but potentially representing the diffusely infiltrative nature of these neoplasms. Meanwhile, IDC to ILC counterfactual transitions (Fig. 4b bottom) lacked glandular architecture (7/8), showed less prominent nucleoli (8/8), and exhibited a single-cell arrangement of tumor cells (8/8). Although generated images appeared as realistic ILC representations, top tiles often lacked characteristic IDC features. Despite the classifier’s moderate performance and inconsistent features in top tiles, we identified distinct ILC and IDC morphological characteristics, indicating that the classifier learned meaningful distinctions between these cancer types.
Counterfactual Transitions Explain Batch Effects
Considering the impact of batch effects on histopathological analyses, we next focused on whether batch effects could be identified by generating counterfactual explanations for classifiers differentiating the center of origin of WSIs. First, we demonstrated that features extracted with a pretrained diffusion autoencoder, when visualized in a reduced-dimensional space, revealed clustering of tiles from WSIs obtained at the same center, i.e., the institution from which the sample was derived, aiming to highlight potential batch effects (Fig. 5a).
Fig. 5 |. Results of the counterfactual image generation to explain batch effects.
a, Dimensionality reduced space of TCGA BRCA training set’s features according to the center of WSI origin (number of centers = 18). Twenty other centers that contributed less data were only in the test set, while E2 (Roswell Park) was in both for experimentation. b, 5-fold cross-validation results of the MIL-based classifier to differentiate between WSI coming from the E2 center versus the rest. Even with a high class imbalance, the models reach a high AP value. c, Representative examples of counterfactual images for different centers. d, Pixel-wise difference images comparing the original image with the most manipulated one. The difference maps reveal that the areas of highest change predominantly occur in regions with dense cellular structures and at tissue interfaces. AUC, area under the curve; SSIM – structural similarity index measure.
We hypothesized that the originating institution of WSIs could be accurately predicted with a DL model. To test this, we trained a MIL classifier to distinguish between WSIs in the BRCA cohort from Roswell Park Comprehensive Cancer Center in Buffalo, NY (identifier E2 in TCGA) and those from other centers. The 5-fold cross-validation models achieved a mean AUROC of 0.98 ± 0.00 and an AP of 0.87 ± 0.04 (Fig. 5b), confirming previous studies which demonstrated that the original institution is encoded in patterns in histopathology images30.
We then generated counterfactual explanations for histology patches from other (non-E2) centers WSIs (Fig. 5c). Quantitative analysis of the transitions revealed an increase in epithelial cell counts while connective tissue cell numbers remained relatively constant (Fig. S7). Some counterfactual images revealed significant changes (p < 0.01 or p < 0.05) in nuclear size and mean intensity (Fig. S7). Qualitative analyses at the tissue level revealed a transition towards more intense eosin staining in the stroma, higher contrast between tissue components, the appearance of clusters of red blood cells, and the disappearance or shrinkage of adipocytes (Fig. 5c). Conversely, when E2 samples are manipulated to look less like E2 (Fig. S8b), epithelial and connective tissue cell counts had an inverse relation, i.e., epithelial cells decreased, while connective tissue cells increased or stayed in a similar range (Fig. S9). Counterfactual images also exhibited significant decreases (p < 0.001) in mean nuclear intensity for both cell types in all representative examples (Fig. S9). Together, these data confirm pronounced batch effects between institutions in TCGA and show that MoPaDi can uncover the morphological patterns associated with them.
Counterfactual Images Help Identify Features of a Molecular Biomarker
Having developed and refined MoPaDi, we finally assessed its capability to predict molecular biomarkers from pathology images and to explain morphological patterns. We explored the link between genotype and phenotype by investigating counterfactual explanations for MSI in the TCGA CRC cohort. A 5-fold cross-validation was performed with MIL to predict MSI-high (MSIH) versus non-MSIH-high (nonMSIH), achieving a mean AUROC of 0.73 ± 0.08 and a mean AP of 0.40 ± 0.09, with lower AP reflecting high-class imbalance (Fig. 6a). We then visualized top predictive tiles, which showed high staining variability (Fig 6b), and subsequently generated counterfactual transitions to explain the classifier’s predictions. These transitions revealed center-specific batch effects through clearly visible color changes (Fig 6c,d). Quantitative measurements supported these observations, showing significant (p < 0.001 or p < 0.01) changes in mean nuclear intensity for both epithelial and connective tissue cells (Fig. S10, S11).
Fig. 6 |. Results for the explainability of the classifier for the molecular biomarker in colorectal cancer.
a, 5-fold cross-validation results of the MIL-based classifier to differentiate between high microsatellite instability (MSIH) and microsatellite stable (nonMSIH). b, The most predictive tiles from randomly selected patients for both classes. c, Representative examples of MSIH tiles transitioning to its counterfactual nonMSIH image. Pixel-wise difference images compare the original image with the most manipulated one. d, Representative examples of nonMSIH tiles transitioning to its counterfactual MSIH image. Pixel-based difference maps highlight changes in nuclei, stroma, cell density and tissue architecture. AUC, area under the curve; SSIM – structural similarity index measure.
Pathologists identified that top predictive tiles from MSIH patients typically contained inflammatory cells, atypical epithelium lacking normal bowel crypt structure, debris, high-grade nuclear pleomorphism, and in some cases, medullary or mucinous morphology, and signet-ring cell (Fig. 6c and S12). Counterfactual transitions for MSIH to nonMSIH typically displayed a more glandular pattern (7/8), decreased mucin (4/8), and a less solid or sheet-like growth pattern when the original tile displayed a medullary growth (1/8). Quantitative analysis showed a marked increase in epithelial and connective tissue cell counts (Fig. S10). Regarding the nonMSIH to MSIH counterfactual transitions (Fig. 6d), pathologists identified the loss of glandular architecture as the most prominent feature (7/8). Other features included more solid growth patterns (5/8), increased mucinous appearance (3/8), and a more vacuolated appearance (4/8). Quantitative analysis revealed an increase in inflammatory cells, along with decreases in epithelial cell counts and nuclear eccentricity of both epithelial and connective tissue cells (Fig. S11).
These findings, from pathologists’ observations of architectural changes in counterfactual transitions to quantitative analyses of cellular composition, reveal features the DL classifier uses to distinguish MSIH tumors from nonMSIH, enhancing the interpretability of the prediction process.
Discussion
Throughout this study, we developed a diffusion model – MoPaDi – capable of generating meaningful counterfactual images and enhanced it with MIL to handle WSIs. MoPaDi demonstrates how diffusion models can transform computational pathology from a passive descriptive tool into an instrument for virtual experimentation. Unlike traditional DL approaches that simply map images to predictions, MoPaDi enables researchers to ask and answer “what if” questions about biological specimens through counterfactual image generation.
The effectiveness of this approach was extensively validated across multiple experimental scenarios, spanning multiple tumor types and providing quantitative and qualitative evidence for the plausibility of generated images by using several orthogonal metrics. Our diffusion autoencoder achieved excellent image reconstruction quality (MS-SSIM) and generated highly realistic counterfactual images that were often indistinguishable from real histological samples in blinded pathologist evaluations. Furthermore, we provide evidence that MoPaDi captures and recapitulates key biological processes. For example, the overall trend for cell type distributions in generated images of colorectal cancer aligns with established cancer and adjacent normal tissue microenvironment characteristics31. Tumor tiles showed high epithelial cell concentrations, especially in normal to tumor transitions, suggesting the model recognizes epithelial abundance as a key tumor characteristic (Fig. 2f), consistent with tumor biology32. Similarly, for distinguishing liver tumor types and lung tumor types, our model recapitulated key biological properties33–35. Finally, we extended our analysis to molecular biomarkers by investigating MSI status in colorectal cancer. While achieving moderate performance compared to state-of-the-art models3, MoPaDi revealed key morphological patterns associated with MSI status through counterfactual explanations. Even with a limited set of top predictive tiles, pathologists identified characteristic features such as signet-ring cells, mucinous, medullary patterns, inflammatory infiltration, and poor differentiation that are known to be associated with MSI in colorectal cancer36–40. These findings are relevant because they demonstrate that DL models can not only recognize patterns in pathology images but can also manipulate them in biologically meaningful ways. Unlike standard attention heatmaps typically used in computational histopathology, which highlight broad regions of interest and can lead to confirmation bias6, our approach identifies specific features the model uses for predictions.
As another application of MoPaDi, we demonstrate that it can pinpoint the underlying reason for batch effects. Batch effects are another important issue in computational pathology because DL models often learn variations in data collection techniques across different centers rather than focusing on target-specific morphological features. We and others have shown that WSIs from the specific center can be predicted with high accuracy30,41. Our qualitative and quantitative analysis of generated counterfactual images indicated that the classifier learns not only staining variations but also histologic differences that may stem from biological variations in treated patient populations at different centers, such as differing cohort compositions. These findings are consistent with literature showing that TCGA WSIs contain a multifactorial site-specific signature30. The advantage of MoPaDi is that these center-level differences can be visualized and quantified for each image, deepening our understanding of batch effects at a granular level.
Finally, MoPaDi is the first generative model that works in a MIL framework to generate counterfactual explanations for biomarkers which are only defined on the level of slide annotations. This capability is important because many important molecular and clinical features in computational pathology are only available at the whole-slide level, not at the patch level. By combining diffusion models with MIL, we enable the interpretation of weakly-supervised learning problems that are common in clinical practice.
Our approach has several important limitations. Results are currently limited to patch-level analysis and require careful validation by domain experts to ensure biological plausibility. Each dataset requires training of a custom diffusion autoencoder model, which is computationally intensive and time-consuming, ranging from several days to weeks on consumer hardware, depending on dataset complexity. Although the model typically generates realistic images, it sometimes fails to capture fine biological nuances – for instance, in colorectal cancer transitions, we observed cases where generated images lacked proper basement membrane structure or showed insufficient nuclear hyperchromatism. Additionally, while our cell type distributions generally align with known tumor biology, the model shows consistent biases in generating certain cell types over others. Future work could address these limitations by integrating pathology foundation models with diffusion-based counterfactual generation to improve both predictive performance and biological accuracy while maintaining the advantages of high-quality image synthesis and selective feature manipulation.
Looking forward, this work opens new possibilities for computational pathology. By enabling virtual experimentation, tools like MoPaDi could help researchers pre-screen hypotheses before conducting expensive and time-consuming wet lab experiments. This could be particularly valuable given increasing restrictions on animal testing and the challenge of translating animal model results to humans. Additionally, these methods could provide new insights into morphological features that correlate with outcomes, potentially revealing previously unknown biological mechanisms. As these methods continue to evolve, they may become an integral part of the cancer research toolkit, complementing traditional experimental approaches and accelerating the pace of discovery in cancer biology.
Methods
Ethics Statement
This study was carried out in accordance with the Declaration of Helsinki. We retrospectively analyzed anonymized patient samples using publicly available data from “The Cancer Genome Atlas” (TCGA, https://portal.gdc.cancer.gov) and Zenodo. The Ethics commission of the Medical Faculty of the Technical University Dresden provided guidelines for study procedures.
Datasets
This study explored multiple datasets (Fig. 1b), starting with the publicly accessible NCT-CRC-HE-100K dataset42. This dataset comprises 100,000 H&E-stained histopathological images derived from 86 formalin-fixed paraffin-embedded (FFPE) samples. These samples represent both human CRC and normal tissues. The dataset encompasses nine distinct tissue classes: adipose, background, debris, lymphocytes, mucus, smooth muscle, normal colon mucosa, cancer-associated stroma, colorectal adenocarcinoma epithelium (Fig. S3a). All classes were utilized to train the diffusion autoencoder. The images, each with a resolution of 224 × 224 pixels at 0.5 microns per pixel (MPP), were color-normalized with Macenko’s method43. The trained model was evaluated on 7,180 non-color-normalized image patches from the CRC-VAL-HE-7K dataset, published alongside the NCT-CRC-HE-100K, and serving as a non-overlapping dataset for testing purposes42. Further investigation extended to a second CRC dataset from The Cancer Genome Atlas Research Network (TCGA), comprising WSIs obtained from FFPE samples. TCGA BRCA cohort was utilized as well to explore counterfactual explanations for this cancer type. Finally, we used another publicly available dataset, further referred to as the TCGA Pan-cancer dataset, where varying resolution patches (256 × 256 pixels at MPP ranging from 0.5 to 1) were extracted from tumor regions from WSIs of 32 cancer types44.
Data Pre-processing
A 10–20% split of the data at the patient level, depending on the original cohort size, was performed before preprocessing to create the training and held-out test sets, with exact patient numbers given in Table S1. Testing data was used to evaluate the performance of the classifier and assess the quality of image reconstruction, generation, and manipulation. We ensured that many centers were only in the test set, except one bigger one for batch effects analysis in TCGA BRCA. Subsequently, for TCGA datasets, where raw WSIs were processed directly, the images were tessellated to obtain patches measuring 512 × 512 pixels at 0.5 MPP. WSIs that did not have the original MPP value in the metadata or no available tumor annotations were discarded. This resulted in 1,115 WSIs from 1046 patients and 588 WSIs from 582 patients for BRCA and CRC cohorts, respectively. Patches were derived from tissue regions determined by Canny edge detection. Then, for both cohorts, patches derived from non-tumorous regions were filtered out, retaining only those originating from tumor areas. This selection process was guided by tumor area annotations provided by pathology trainees supervised by an expert pathologist. Patches that overlapped by at least 60% with annotated tumor areas were included. During the diffusion model’s training, the channels of each image were scaled to achieve a mean of 0.5 and a standard deviation of 0.5, thereby standardizing the pixel value distribution throughout the dataset.
Diffusion Autoencoders
The MoPaDi autoencoder’s architecture is based on the official implementation of diffusion autoencoders26. The model consists of a conditional DDIM image decoder that is also conditioned on a feature vector obtained with a simultaneously trained feature extractor (Fig. 1c). The latter part learns to extract meaningful features that can be used to reconstruct an encoded image back to the original one by conditioning the autoencoding model. We trained four separate autoencoding models for different datasets. For datasets with smaller images (224 × 224 or 256 × 256 px), we used 8 Nvidia A100 40 GB graphics processing units (GPUs), while for datasets with bigger images (512 × 512 px) – 4 Nvidia A100 80 GB GPUs. The models were trained until the convergence, i.e., until the approximate FID score, computed on 5,000 images, was below 20 (~10 for datasets with smaller patches). The training duration varied from several days to weeks due to the different complexities of datasets. A global batch size of 24 for 512 × 512 px models and 64 for smaller models, divided evenly among the GPUs, and a learning rate of 10−4 were used. To evaluate the quality of the latent space learned by the autoencoding model and generate novel synthetic images, we additionally train a latent DDIM on the distribution of feature vectors obtained from the diffusion-based encoder.
Classifiers
To distinguish linearly separable classes, e.g., tissue types, we train a logistic regression classifier on 512-dimensional features extracted with a pretrained MoPaDi feature extractor (Fig. 1d). Before going into a classifier, features are first normalized so that its entire distribution has zero mean and unit variance. The model is trained with a binary cross-entropy loss. The training process involved 300,000 sample exposures. For linearly non-separable classes, we utilized the MIL approach, which involved attention-based classifiers consisting of layer normalization followed by pooling with a multi-head cross-attention layer and a self-attention block (Fig. 1f). The output is then fed into a classifier head comprising two fully connected layers with dropout and SiLU45 activation, ending with a final linear layer for class prediction. The classifiers were trained using a learning rate of 0.0001, Adam optimizer, early stopping, and cross-entropy loss with class weighting to address class imbalances. 5-fold cross-validation experiments were performed to assess the model’s variability depending on the train/validation data split. For counterfactual image generation, one final model was trained on the full training dataset without validation split. The number of epochs was selected according to cross-validation results and ranged from 50 to 200.
Counterfactual Image Generation
Counterfactual images are generated by manipulating extracted feature vectors with classifier guidance. The gradients for the counterfactual class serve as the manipulation directions and are added with varying amplitudes to the original feature vectors (Fig. 1f). Subsequently, manipulated feature vectors are used to condition the diffusion model together with the original image encoded into noise. We manipulate the images until the classifier strongly predicts them as the opposite class when features extracted from the rendered edited images are passed through. The classifier’s predictions are based on what it would predict if it only had seen this tile. For the MIL approach, we generate counterfactual images for top predictive tiles, with each tile being manipulated separately.
Evaluation Metrics
We used MSE between the pixels of the original image and the reconstructed one from the diffusion latent space and SM-SSIM46 to assess the quality of the autoencoder’s reconstruction capability for 1,000 images in each dataset. Structural similarity index47 was used to visually represent similar and dissimilar regions between the two images converted to grayscale for more straightforward results interpretation. Latent space’s ability to retain semantically meaningful information was also evaluated by sampling 10,000 synthetic images and computing the FID score to compare the distribution of generated images with the distribution of real images in the test set using clean-fid library48. A lower FID score indicates higher similarity.
To evaluate the performance of classifiers for each experiment, we computed AUROC for the held-out test set. Bootstrapping was employed as a resampling technique to estimate robustness and confidence intervals. Resampling was done with replacement 1,000 times. We constructed ROC curves for each resampled set and interpolated between them to obtain a set of smoothed curves. 95% confidence intervals were extracted from smoothed curves. The latent space and the direction of manipulation were visualized using t-SNE, enabling a representation of extracted 512-dimensional feature vectors in a reduced two-dimensional format. For the TCGA BRCA dataset, we used UMAP instead of t-SNE due to the large number of tiles, which made t-SNE computationally inefficient.
User Studies
For the first experiment (tissue type counterfactual explanations), a pathologist and a pathology trainee supervised by a board-certified pathologist reviewed a total of 30 transitions (15 normal colon mucosa to dysplastic epithelium and 15 vice versa) in a blinded fashion, meaning they were not informed which side contained the original image, and in half of the cases, the transitions were flipped to prevent bias. The doctors were asked to identify the original image and features that are changing during the transition to a counterfactual synthetic image. Computing the number of correctly identified original images allowed us to estimate the diffusion model’s ability to generate realistic and not easily distinguishable from real histopathology images. For further experiments, i.e., cancer types or a biomarker, the classes of both sides (original and counterfactual) were indicated, and the users were asked to identify only histopathological features on both sides. They were given 16 examples for each classifier (8 examples per class), each containing two top predictive tiles from the same patient who was predicted correctly by the model with high confidence.
Quantitative Assessment of Morphological Changes
Pretrained nuclei segmentation and cell type classification models were applied to original and counterfactual images to quantify changes in certain cell types and morphological characteristics. DeepCMorph models were used for this purpose49. These models were trained to classify nuclei of six cell types: epithelial, connective tissue, lymphocytes, plasma cells, neutrophils, and eosinophils. Varying resolution 224 × 224 px patches from H&E WSIs coming from many different datasets were used to train DeepCMorph, including NCT-CRC-HE-100K and TCGA pan-cancer datasets. However, the models were primarily trained to classify cancer and tissue types, with the aim of enhancing performance by enabling the model to learn biological features such as cell types. Therefore, we were able to utilize the intermediate segmentation masks for our datasets.
To obtain instance segmentation masks only of nuclei of epithelial cells, we performed water-shed-based splitting of touching objects in a semantic segmentation mask of these cells, additional binary erosion operation to split remaining touching nuclei, and subsequently connected component labeling followed by a binary dilation to get back to the original nuclei size. We removed labels with an area lower than 30 pixels as these typically were artifacts. As for the instance segmentation masks of connective tissue cell nuclei, we skipped these preprocessing steps because nuclei are more elongated and sparser. These steps enabled automatic counting of cells in original and counterfactual images. Finally, quantitative shape and intensity-based measurements, i.e., area, mean intensity, and eccentricity, were extracted using the scikit-image library. We additionally quantify tissue architectural organization by computing the spatial entropy of cellular distributions. This involves creating a 2D histogram of cell centroids, normalizing it to a probability distribution, and calculating Shannon’s entropy. This measurement was expected to capture tissue differentiation patterns, but its strong correlation with cell count suggests the need for more refined metrics to characterize architectural organization in histopathology patches. Lastly, we perform statistical hypothesis testing to compare measurements of original and counterfactual images. Since generated images are not independent and the measurements are not normally distributed, we perform a two-sided permutation test with 10,000 resamples. For each cell type (epithelial or connective), the null hypothesis assumes no difference in measurements between original and counterfactual images. To evaluate this, the measurements for original and counterfactual images were shuffled randomly to create a null distribution of the test statistic under the null hypothesis. The p-value was then calculated as the proportion of permuted test statistics that are as extreme or more extreme than the observed test statistic. Cell type counts, measured as pixel counts in segmentation masks, were also compared between original and counterfactual images of the opposite class (i.e., manipulated to look like the compared images class). For each cell type, the Mann-Whitney U test was used to assess differences in median percentages between the two groups. To account for multiple comparisons across cell types, Bonferroni correction was applied to adjust the p-values.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the GWK’s support for funding this project by providing computing time through the Center for Information Services and HPC (ZIH) at TU Dresden. We also acknowledge the TCGA Research Network (https://www.cancer.gov/tcga), which generated the data on which the results shown in this manuscript are partly based.
Funding
NGR received funding from the Manfred-Stolte-Stiftung and the Bavarian Cancer Research Center (BZKF). SF is supported by the German Federal Ministry of Education and Research (SWAG, 01KD2215C), the German Cancer Aid (DECADE, 70115166 and TargHet, 70115995) and the German Research Foundation (504101714). DT is supported by the German Federal Ministry of Education (TRANSFORM LIVER, 031L0312A; SWAG, 01KD2215B), Deutsche Forschungsgemeinschaft (DFG) (TR 1700/7-1), and the European Union (Horizon Europe, ODELIA, GA 101057091). JNK is supported by the German Cancer Aid (DECADE, 70115166), the German Federal Ministry of Education and Research (PEARL, 01KD2104C; CAMINO, 01EO2101; SWAG, 01KD2215A; TRANSFORM LIVER, 031L0312A; TANGERINE, 01KT2302 through ERA-NET Transcan; Come2Data, 16DKZ2044A; DEEP-HCC, 031L0315A), the German Academic Exchange Service (SECAI, 57616814), the German Federal Joint Committee (TransplantKI, 01VSF21048) the European Union’s Horizon Europe and innovation programme (ODELIA, 101057091; GENIAL, 101096312), the European Research Council (ERC; NADIR, 101114631), the National Institutes of Health (EPICO, R01 CA263318) and the National Institute for Health and Care Research (NIHR, NIHR203331) Leeds Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This work was funded by the European Union. Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
Competing Interests
NGR received compensation for travel expenses from nanoString (Bruker Company). SF has received honoraria from MSD and BMS. DT received honoraria for lectures by Bayer, GE, and Philips and holds shares in StratifAI GmbH, Germany, and in Synagen GmbH, Germany. AP has received grant/research funding from AbbVie, Kura Oncology, and Merck and is on the advisory board for AbbVie, Elevar, Ayala, Prelude, and ThermoFisher. JNK declares consulting services for Bioptimus, France; Owkin, France; DoMore Diagnostics, Norway; Panakeia, UK; AstraZeneca, UK; Mindpeak, Germany; and MultiplexDx, Slovakia. Furthermore, he holds shares in StratifAI GmbH, Germany, Synagen GmbH, Germany, and has received a research grant by GSK, and has received honoraria by AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Janssen, Merck, MSD, BMS, Roche, Pfizer, and Fresenius. The remaining authors have no competing interests to declare.
Abbreviations
- AI
Artificial Intelligence
- AP
Average precision
- AUC
Area under the curve
- AUROC
Area under the receiver operating characteristic
- BRCA
Breast invasive carcinoma
- CCA
Cholangiocarcinoma
- CRC
Colorectal carcinoma
- DDIM
Denoising diffusion implicit models
- DL
Deep learning
- FFPE
Formalin-fixed paraffin-embedded
- GAN
Generative adversarial network
- H&E
Hematoxylin and eosin
- GPU
Graphics processing unit
- HCC
Hepatocellular carcinoma
- IDC
Invasive ductal carcinoma
- ILC
Invasive lobular carcinoma
- HCC
Hepatocellular carcinoma
- TCGA
The Cancer Genome Atlas
- CCA
Cholangiocarcinoma
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- MIL
Multiple instance learning
- MPP
Microns per pixel
- MSE
Mean square error
- MS-SSIM
Multi-scale structural similarity index measure
- MSI
Microsatellite instability
- MSIH
High microsatellite instability
- NORM
Normal colon mucosa
- TCGA
The Cancer Genome Atlas
- TUM
Colorectal adenocarcinoma epithelium
- FID
Fréchet inception distance
- WSI
Whole slide image
Funding Statement
NGR received funding from the Manfred-Stolte-Stiftung and the Bavarian Cancer Research Center (BZKF). SF is supported by the German Federal Ministry of Education and Research (SWAG, 01KD2215C), the German Cancer Aid (DECADE, 70115166 and TargHet, 70115995) and the German Research Foundation (504101714). DT is supported by the German Federal Ministry of Education (TRANSFORM LIVER, 031L0312A; SWAG, 01KD2215B), Deutsche Forschungsgemeinschaft (DFG) (TR 1700/7-1), and the European Union (Horizon Europe, ODELIA, GA 101057091). JNK is supported by the German Cancer Aid (DECADE, 70115166), the German Federal Ministry of Education and Research (PEARL, 01KD2104C; CAMINO, 01EO2101; SWAG, 01KD2215A; TRANSFORM LIVER, 031L0312A; TANGERINE, 01KT2302 through ERA-NET Transcan; Come2Data, 16DKZ2044A; DEEP-HCC, 031L0315A), the German Academic Exchange Service (SECAI, 57616814), the German Federal Joint Committee (TransplantKI, 01VSF21048) the European Union’s Horizon Europe and innovation programme (ODELIA, 101057091; GENIAL, 101096312), the European Research Council (ERC; NADIR, 101114631), the National Institutes of Health (EPICO, R01 CA263318) and the National Institute for Health and Care Research (NIHR, NIHR203331) Leeds Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This work was funded by the European Union. Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
Data and Code Availability
MoPaDi code and instructions to download trained models are publicly available on GitHub at https://github.com/KatherLab/mopadi. The official implementation of diffusion autoencoders for natural images can be found on GitHub at https://github.com/phizaz/diffae. Digital histopathology WSIs from TCGA, together with the clinical data used in this study, are available at https://portal.gdc.cancer.gov and https://cbioportal.org. NCT-CRC-HE-100K/CRC-VAL-HE-7K42 and TCGA Pan-cancer44 datasets are publicly available at https://zenodo.org/records/1214456 and https://zenodo.org/records/5889558.
References
- 1.Kather J. N. et al. Predicting survival from colorectal cancer histology slides using deep learning: A retrospective multicenter study. PLoS Med. 16, e1002730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng Q. et al. Artificial intelligence-based pathology as a biomarker of sensitivity to atezolizumab-bevacizumab in patients with hepatocellular carcinoma: a multicentre retrospective study. Lancet Oncol. 24, 1411–1422 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Wagner S. J. et al. Transformer-based biomarker prediction from colorectal cancer histology: A large-scale multicentric study. Cancer Cell 41, 1650–1661.e4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderaro J., Žigutytė L., Truhn D., Jaffe A. & Kather J. N. Artificial intelligence in liver cancer - new tools for research and patient management. Nat. Rev. Gastroenterol. Hepatol. 21, 585–599 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Unger M. & Kather J. N. Deep learning in cancer genomics and histopathology. Genome Med. 16, 44 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghassemi M., Oakden-Rayner L. & Beam A. L. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit. Health 3, e745–e750 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Huff D. T., Weisman A. J. & Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys. Med. Biol. 66, 04TR01 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim B. et al. Interpretability Beyond Feature Attribution: Quantitative Testing with Concept Activation Vectors (TCAV). in Proceedings of the 35th International Conference on Machine Learning (eds. Dy J. & Krause A.) vol. 80 2668–2677 (PMLR, 10--15 Jul 2018). [Google Scholar]
- 9.Graziani M., Lompech T., Müller H., & Andrearczyk V. Evaluation and comparison of CNN visual explanations for histopathology. in Proceedings of the AAAI Conference on Artificial Intelligence Workshops (XAI-AAAI-21), Virtual Event 8–9. [Google Scholar]
- 10.Amorim J. P. et al. Interpreting Deep Machine Learning Models: An Easy Guide for Oncologists. IEEE Rev. Biomed. Eng. 16, 192–207 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Guidotti R. Counterfactual explanations and how to find them: literature review and benchmarking. Data Min. Knowl. Discov. (2022) doi: 10.1007/s10618-022-00831-6. [DOI] [Google Scholar]
- 12.Goyal Y. et al. Counterfactual Visual Explanations. in Proceedings of Machine Learning Research 2376–2384 (2019). [Google Scholar]
- 13.De Sousa Ribeiro F., Xia T., Monteiro M., Pawlowski N. & Glocker B. High Fidelity Image Counterfactuals with Probabilistic Causal Models. arXiv [cs.LG] (2023). [Google Scholar]
- 14.Sanchez P. et al. Causal machine learning for healthcare and precision medicine. R Soc Open Sci 9, 220638 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez P., Kascenas A., Liu X., O’Neil A. Q. & Tsaftaris S. A. What is Healthy? Generative Counterfactual Diffusion for Lesion Localization. in Deep Generative Models 34–44 (Springer Nature; Switzerland, 2022). doi: 10.1007/978-3-031-18576-2_4. [DOI] [Google Scholar]
- 16.Han T. et al. Reconstruction of patient-specific confounders in AI-based radiologic image interpretation using generative pretraining. Cell Reports Medicine 5, (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolezal J. M. et al. Deep learning generates synthetic cancer histology for explainability and education. NPJ Precis Oncol 7, 49 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naglah A., Khalifa F., El-Baz A. & Gondim D. Conditional GANs based system for fibrosis detection and quantification in Hematoxylin and Eosin whole slide images. Med. Image Anal. 81, 102537 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Wölflein G., Um I. H., Harrison D. J. & Arandjelović O. HoechstGAN: Virtual lymphocyte staining using generative adversarial networks. arXiv [cs.CV] 4997–5007 (2022). [Google Scholar]
- 20.Howard F. M. et al. Generative adversarial networks accurately reconstruct pan-cancer histology from pathologic, genomic, and radiographic latent features. bioRxivorg (2024) doi: 10.1101/2024.03.22.586306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeGrave A. J., Cai Z. R., Janizek J. D., Daneshjou R. & Lee S.-I. Auditing the inference processes of medical-image classifiers by leveraging generative AI and the expertise of physicians. Nat Biomed Eng (2023) doi: 10.1038/s41551-023-01160-9. [DOI] [PubMed] [Google Scholar]
- 22.Singla S., Eslami M., Pollack B., Wallace S. & Batmanghelich K. Explaining the black-box smoothly-A counterfactual approach. Med. Image Anal. 84, 102721 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang O. et al. Using generative AI to investigate medical imagery models and datasets. EBioMedicine 102, 105075 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhariwal P. & Nichol A. Diffusion models beat gans on image synthesis. Adv. Neural Inf. Process. Syst. 34, 8780–8794 (2021). [Google Scholar]
- 25.Song J., Meng C. & Ermon S. Denoising Diffusion Implicit Models. arXiv [cs.LG] (2020). [Google Scholar]
- 26.Preechakul K., Chatthee N., Wizadwongsa S. & Suwajanakorn S. Diffusion autoencoders: Toward a meaningful and decodable representation. Proc. IEEE Comput. Soc. Conf. Comput. Vis. Pattern Recognit. 10609–10619 (2021) doi: 10.1109/CVPR52688.2022.01036. [DOI] [Google Scholar]
- 27.Augustine T. N. Weakly-supervised deep learning models in computational pathology. EBioMedicine 81, 104117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadermayr M. & Tschuchnig M. Multiple instance learning for digital pathology: A review of the state-of-the-art, limitations & future potential. Comput. Med. Imaging Graph. 112, 102337 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Kather J. N. et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 25, 1054–1056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard F. M. et al. The impact of site-specific digital histology signatures on deep learning model accuracy and bias. Nat. Commun. 12, 4423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y. et al. An atlas of genetic effects on cellular composition of the tumor microenvironment. Nat. Immunol. 25, 1959–1975 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Hamilton S. R. Carcinoma of the colon and rectum. Pathology and genetics of tumors of digestive system (2000). [Google Scholar]
- 33.Travis W. D. Pathology of lung cancer. Clin. Chest Med. 32, 669–692 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Schlageter M., Terracciano L. M., D’Angelo S. & Sorrentino P. Histopathology of hepatocellular carcinoma. World J. Gastroenterol. 20, 15955–15964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vijgen S., Terris B. & Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 6, 22–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins M. A. et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 133, 48–56 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shia J. et al. Morphological characterization of colorectal cancers in The Cancer Genome Atlas reveals distinct morphology-molecular associations: clinical and biological implications. Mod. Pathol. 30, 599–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puccini A. et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene 41, 3455–3460 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reitsam N. G. et al. Concurrent loss of MLH1, PMS2 and MSH6 immunoexpression in digestive system cancers indicating a widespread dysregulation in DNA repair processes. Front. Oncol. 12, 1019798 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remo A. et al. Morphology and molecular features of rare colorectal carcinoma histotypes. Cancers (Basel) 11, 1036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehkharghanian T. et al. Biased data, biased AI: deep networks predict the acquisition site of TCGA images. Diagn. Pathol. 18, 67 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kather J. N., Halama N., Marx A. 100,000 histological images of human colorectal cancer and healthy tissue. 10.5281/zenodo.1214456 (2018). [DOI] [Google Scholar]
- 43.Macenko M. et al. A method for normalizing histology slides for quantitative analysis. in 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro 1107–1110 (IEEE, 2009). doi: 10.1109/ISBI.2009.5193250. [DOI] [Google Scholar]
- 44.Komura D. & Ishikawa S. Histology images from uniform tumor regions in TCGA Whole Slide Images. Cell Rep. 38, 110424 (2021). [Google Scholar]
- 45.Elfwing S., Uchibe E. & Doya K. Sigmoid-weighted linear units for neural network function approximation in reinforcement learning. Neural Netw. 107, 3–11 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Simoncelli E. P. & Bovik A. C. Multiscale structural similarity for image quality assessment. in The Thrity-Seventh Asilomar Conference on Signals, Systems & Computers, 2003 vol. 2 1398–1402 Vol.2 (IEEE, 2003). [Google Scholar]
- 47.Wang Z., Bovik A. C., Sheikh H. R. & Simoncelli E. P. Image quality assessment: from error visibility to structural similarity. IEEE Trans. Image Process. 13, 600–612 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Parmar G., Zhang R. & Zhu J.-Y. On aliased resizing and surprising subtleties in GAN evaluation. Proc. IEEE Comput. Soc. Conf. Comput. Vis. Pattern Recognit. 11400–11410 (2022) doi: 10.1109/CVPR52688.2022.01112. [DOI] [Google Scholar]
- 49.Ignatov A., Yates J. & Boeva V. Histopathological image classification with cell morphology aware deep neural networks. (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MoPaDi code and instructions to download trained models are publicly available on GitHub at https://github.com/KatherLab/mopadi. The official implementation of diffusion autoencoders for natural images can be found on GitHub at https://github.com/phizaz/diffae. Digital histopathology WSIs from TCGA, together with the clinical data used in this study, are available at https://portal.gdc.cancer.gov and https://cbioportal.org. NCT-CRC-HE-100K/CRC-VAL-HE-7K42 and TCGA Pan-cancer44 datasets are publicly available at https://zenodo.org/records/1214456 and https://zenodo.org/records/5889558.