Abstract
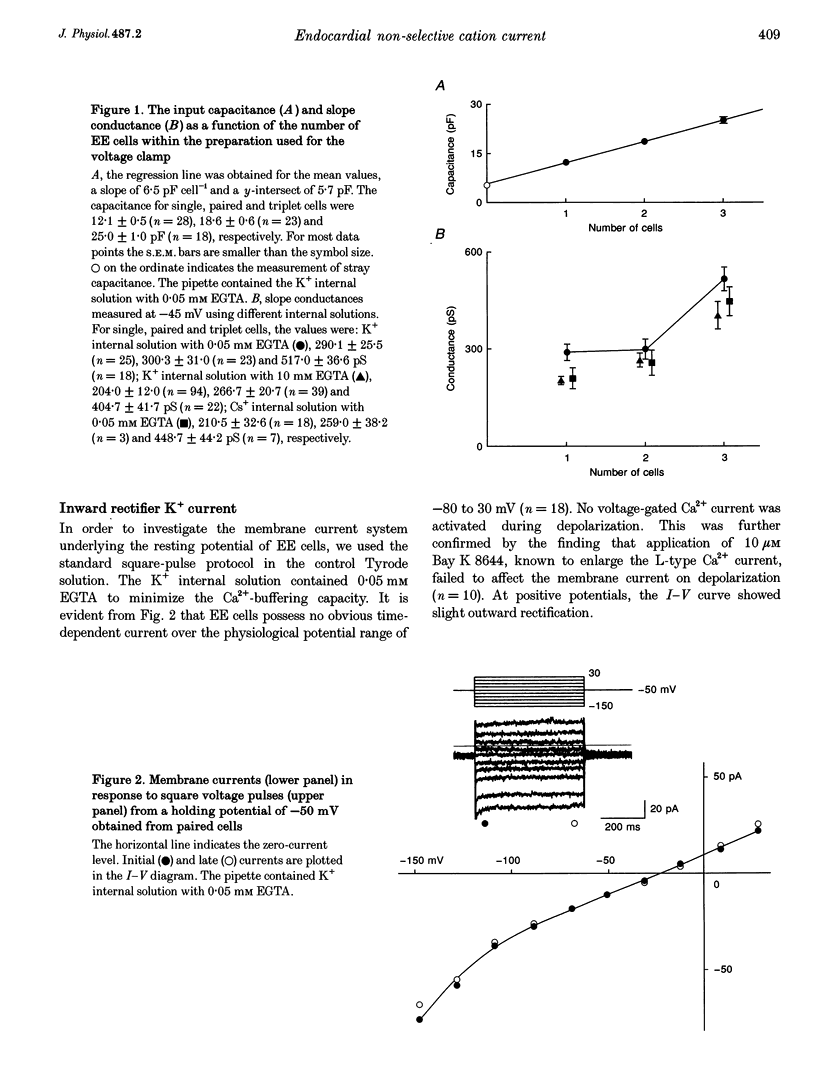
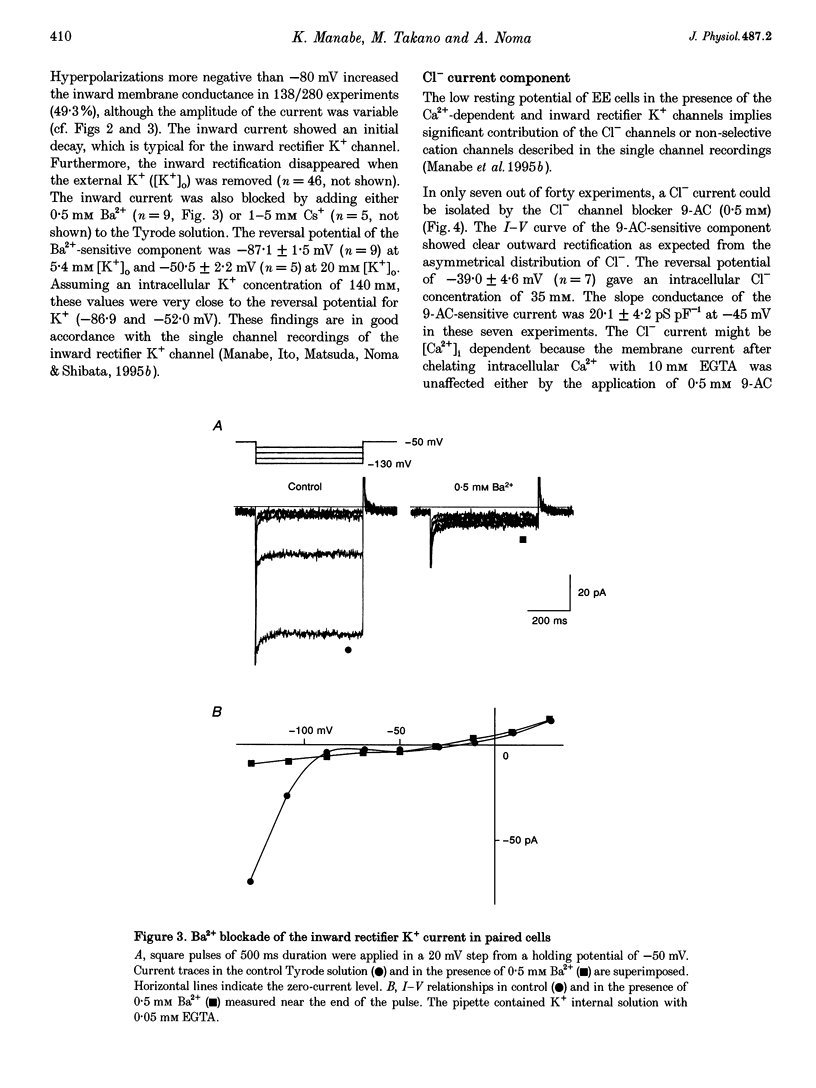
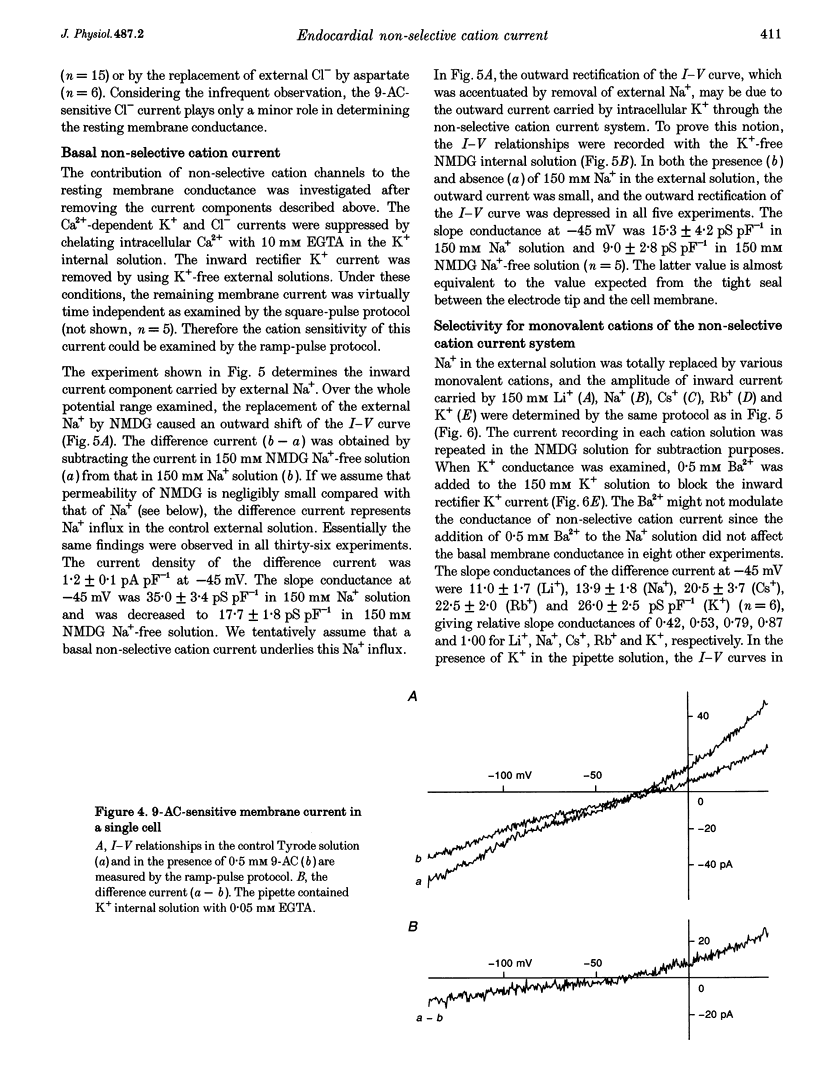
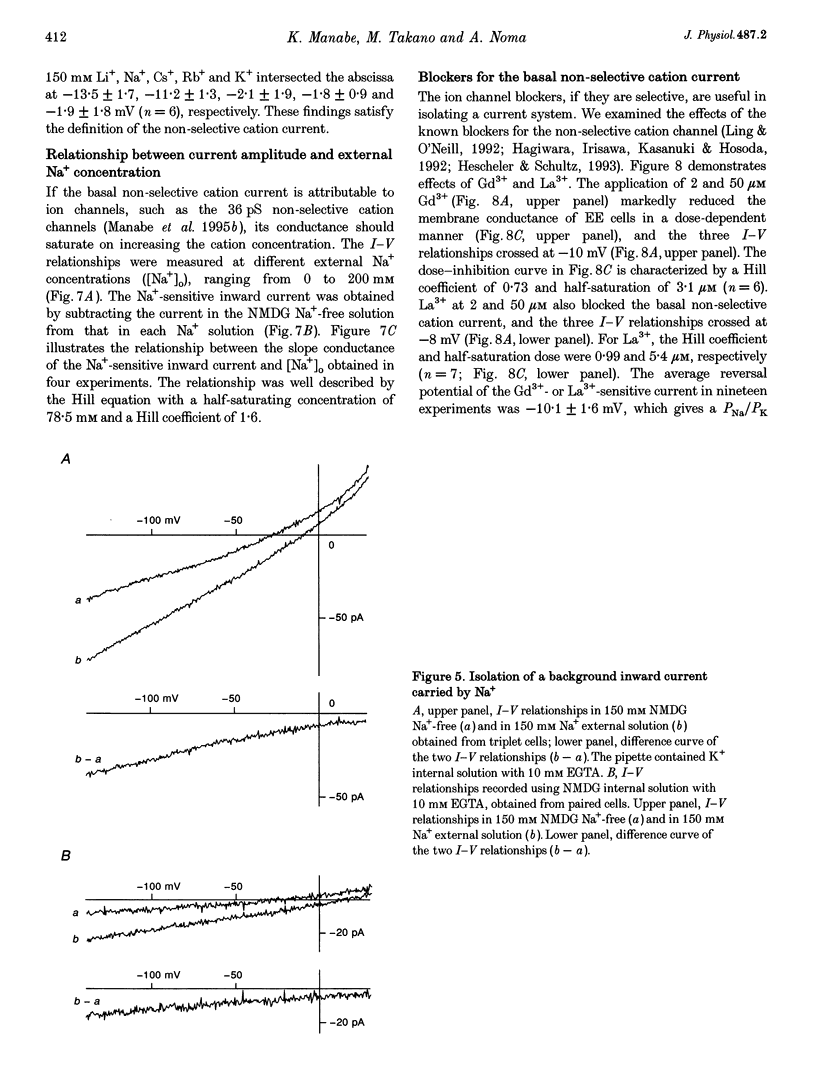
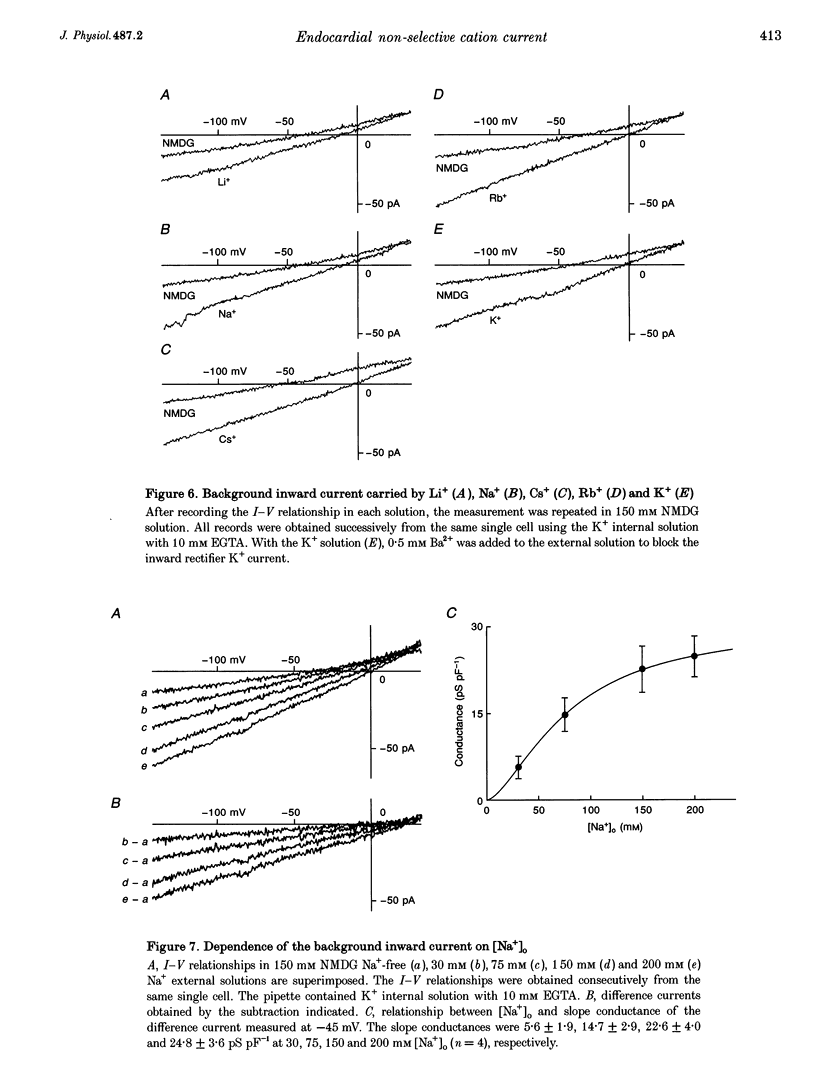
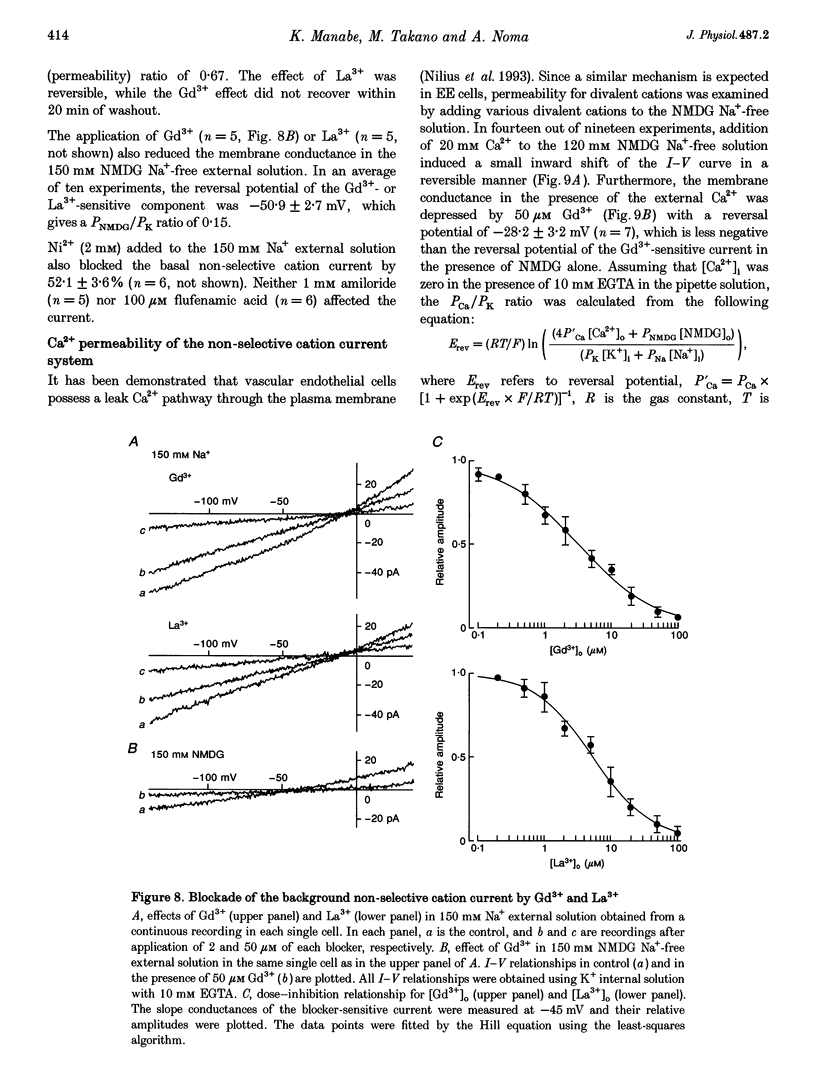
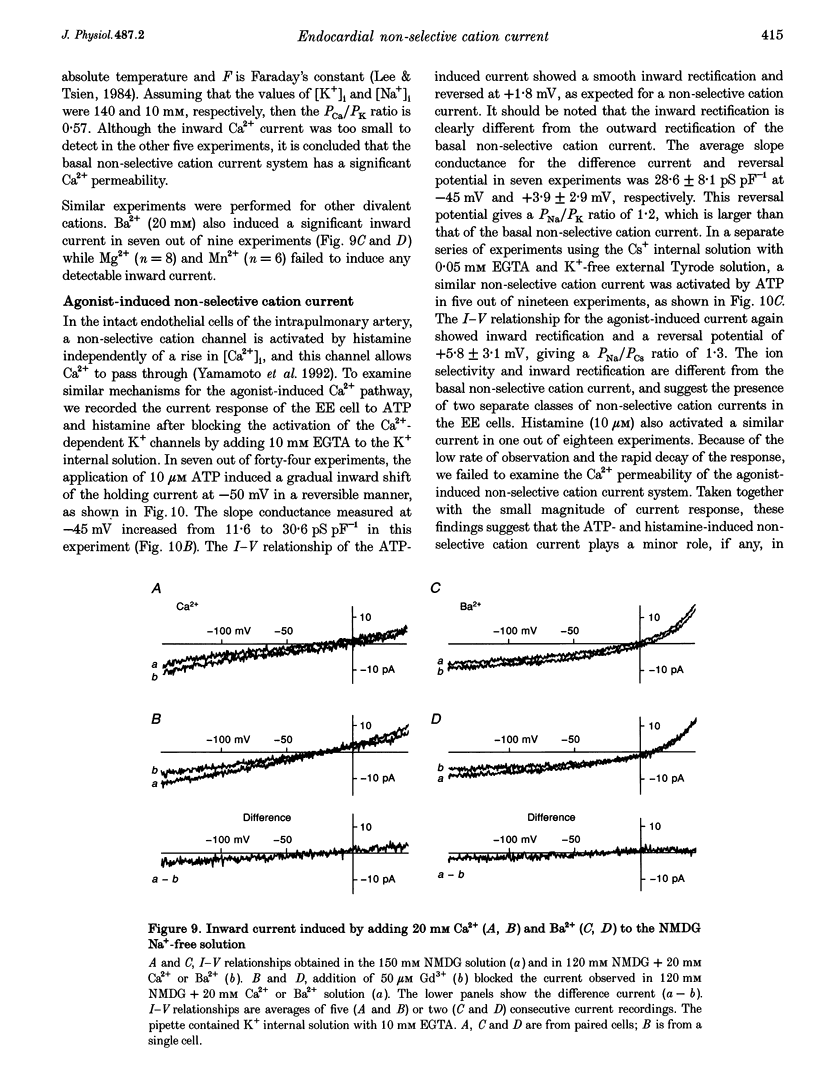
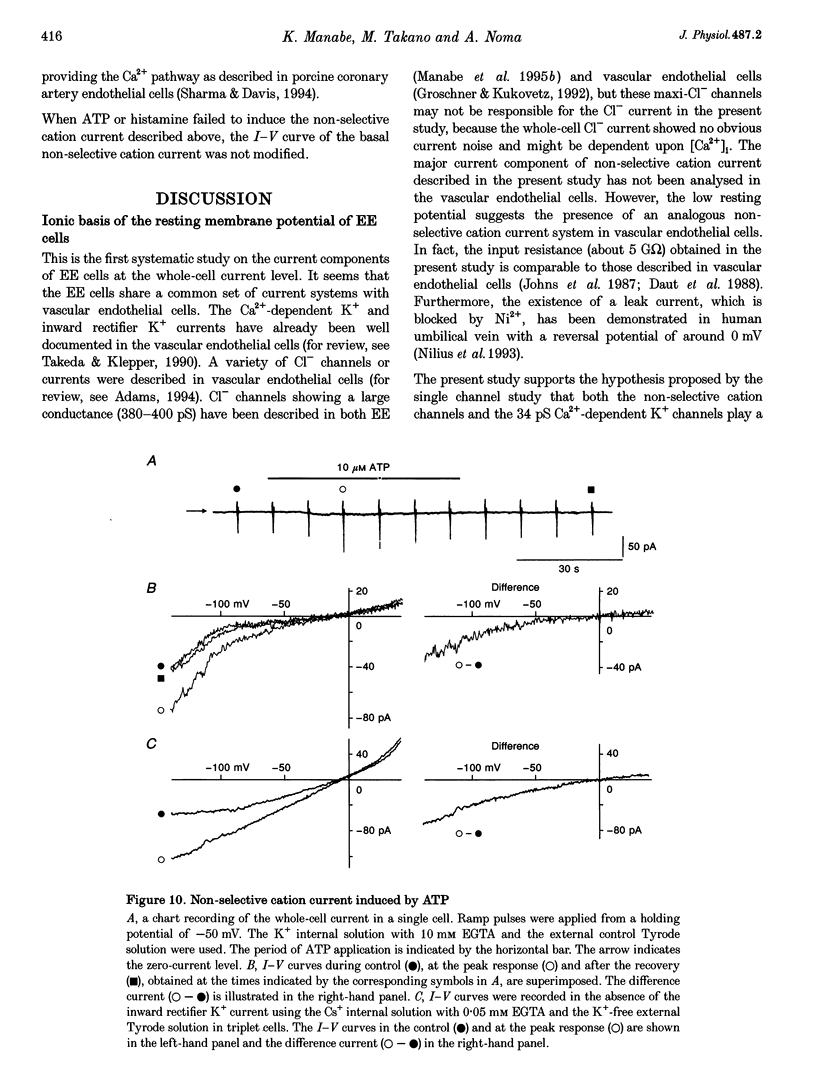
1. Endocardial endothelial (EE) cells, isolated by the enzymatic treatment of guinea-pig heart, were used for whole-cell voltage clamp experiments. 2. The inward rectifier K+ current was observed in about half of the experiments. The contribution of Ca(2+)-dependent K+ current to the resting membrane conductance was also suggested. 3. After the K+ conductances were suppressed, removal of external Na+ revealed an inward cation current (1.2 pA pF-1, at -45 mV), whose slope conductance was a saturable function of external Na+ concentration. When Na+ was totally replaced by various monovalent cations, the order of the membrane conductances was K+ > Rb+ > Cs+ > Na+ > Li+. 4. This basal non-selective cation current was blocked by either Gd3+ or La3+, and showed slight outward rectification. 5. Addition of 20 mM Ca2+ or Ba2+, but not Mg2+ or Mn2+, to the Na(+)-free solution, induced an inward current, indicating that this current possesses a significant Ca2+ permeability. 6. In approximately 15% of the experiments, ATP and histamine induced another type of non-selective cation current, which showed different ion selectivity (Na+ > K+, Cs+) and rectification (inward). 7. The basal non-selective cation current is responsible for both the low resting potential and the leak Ca2+ influx of EE cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Alloatti G., Serazzi L., Levi R. C. Prostaglandin I2 (PGI2) enhances calcium current in guinea-pig ventricular heart cells. J Mol Cell Cardiol. 1991 Jul;23(7):851–860. doi: 10.1016/0022-2828(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Bregestovski P., Bakhramov A., Danilov S., Moldobaeva A., Takeda K. Histamine-induced inward currents in cultured endothelial cells from human umbilical vein. Br J Pharmacol. 1988 Oct;95(2):429–436. doi: 10.1111/j.1476-5381.1988.tb11663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Andries L. J. The endocardial endothelium. Am J Physiol. 1992 Oct;263(4 Pt 2):H985–1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L. The endocardium. Annu Rev Physiol. 1989;51:263–273. doi: 10.1146/annurev.ph.51.030189.001403. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschner K., Kukovetz W. R. Voltage-sensitive chloride channels of large conductance in the membrane of pig aortic endothelial cells. Pflugers Arch. 1992 Jun;421(2-3):209–217. doi: 10.1007/BF00374829. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kasanuki H., Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992 Mar;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- James A. F., Xie L. H., Fujitani Y., Hayashi S., Horie M. Inhibition of the cardiac protein kinase A-dependent chloride conductance by endothelin-1. Nature. 1994 Jul 28;370(6487):297–300. doi: 10.1038/370297a0. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., van Breemen C. Cytosolic [Ca2+] measurements in endothelium of rabbit cardiac valves using imaging fluorescence microscopy. Am J Physiol. 1994 May;266(5 Pt 2):H2130–H2135. doi: 10.1152/ajpheart.1994.266.5.H2130. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B. N., O'Neill W. C. Ca(2+)-dependent and Ca(2+)-permeable ion channels in aortic endothelial cells. Am J Physiol. 1992 Dec;263(6 Pt 2):H1827–H1838. doi: 10.1152/ajpheart.1992.263.6.H1827. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Manabe K., Ito H., Matsuda H., Noma A. Hyperpolarization induced by vasoactive substances in intact guinea-pig endocardial endothelial cells. J Physiol. 1995 Apr 1;484(Pt 1):25–40. doi: 10.1113/jphysiol.1995.sp020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe K., Ito H., Matsuda H., Noma A., Shibata Y. Classification of ion channels in the luminal and abluminal membranes of guinea-pig endocardial endothelial cells. J Physiol. 1995 Apr 1;484(Pt 1):41–52. doi: 10.1113/jphysiol.1995.sp020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebazaa A., Martin L. D., Robotham J. L., Maeda K., Gabrielson E. W., Wetzel R. C. Right and left ventricular cultured endocardial endothelium produces prostacyclin and PGE2. J Mol Cell Cardiol. 1993 Mar;25(3):245–248. doi: 10.1006/jmcc.1993.1031. [DOI] [PubMed] [Google Scholar]
- Mebazaa A., Mayoux E., Maeda K., Martin L. D., Lakatta E. G., Robotham J. L., Shah A. M. Paracrine effects of endocardial endothelial cells on myocyte contraction mediated via endothelin. Am J Physiol. 1993 Nov;265(5 Pt 2):H1841–H1846. doi: 10.1152/ajpheart.1993.265.5.H1841. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D., Bacal K., Kunze D. L. Bradykinin-activated calcium influx pathway in bovine aortic endothelial cells. Am J Physiol. 1992 Apr;262(4 Pt 2):H942–H948. doi: 10.1152/ajpheart.1992.262.4.H942. [DOI] [PubMed] [Google Scholar]
- Nilius B., Droogmans G., Gericke M., Schwarz G. Nonselective ion pathways in human endothelial cells. EXS. 1993;66:269–280. doi: 10.1007/978-3-0348-7327-7_21. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N. R., Davis M. J. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am J Physiol. 1994 Jan;266(1 Pt 2):H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Lewis M. J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991 Aug;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Klepper M. Voltage-dependent and agonist-activated ionic currents in vascular endothelial cells: a review. Blood Vessels. 1990;27(2-5):169–183. doi: 10.1159/000158808. [DOI] [PubMed] [Google Scholar]
- Vaca L., Schilling W. P., Kunze D. L. G-protein-mediated regulation of a Ca(2+)-dependent K+ channel in cultured vascular endothelial cells. Pflugers Arch. 1992 Oct;422(1):66–74. doi: 10.1007/BF00381515. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Chen G., Miwa K., Suzuki H. Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. J Physiol. 1992 May;450:395–408. doi: 10.1113/jphysiol.1992.sp019133. [DOI] [PMC free article] [PubMed] [Google Scholar]



