Abstract
Hutchinson-Gilford progeria syndrome (HGPS) is a progeroid disorder characterized by multiple aging-like phenotypes, including disease in large arteries. HGPS is caused by an internally truncated prelamin A (progerin) that cannot undergo the ZMPSTE24-mediated processing step that converts farnesyl-prelamin A to mature lamin A; consequently, progerin retains a carboxyl-terminal farnesyl lipid anchor. In cultured cells, progerin and full-length farnesyl-prelamin A (produced in Zmpste24−/− cells) form an abnormal nuclear lamin meshwork accompanied by nuclear membrane ruptures and cell death; however, these proteins differ in their capacity to cause arterial disease. In a mouse model of HGPS (LmnaG609G), progerin causes loss of aortic smooth muscle cells (SMCs) by ~12 weeks of age. In contrast, farnesyl-prelamin A in Zmpste24−/− mice does not cause SMC loss—even at 21 weeks of age. In young mice, aortic levels of farnesyl-prelamin A in Zmpste24−/− mice and aortic levels of progerin in LmnaG609G/+ mice are the same. However, the levels of progerin and other A-type lamins increase with age in LmnaG609G/+ mice, whereas farnesyl-prelamin A and lamin C levels in Zmpste24−/− mice remain stable. Lmna transcript levels are similar, implying that progerin influences nuclear lamin turnover. We identified a likely mechanism. In cultured SMCs, the phosphorylation of Ser-404 by AKT (which triggers prelamin A degradation) is reduced in progerin. In mice, AKT activity is significantly lower in LmnaG609G/+ aortas than in wild-type or Zmpste24−/− aortas. Our studies identify that the accumulation of progerin in LmnaG609G aortas underlies the hallmark arterial pathology in HGPS.
One Sentence Summary:
The age-related accumulation of progerin in smooth muscle cells (SMCs) explains the loss of arterial SMCs in Hutchinson-Gilford progeria syndrome.
Introduction
Hutchinson-Gilford progeria syndrome (HGPS) is a pediatric progeroid disorder caused by point mutations that optimize a cryptic splice-donor site in exon 11 of LMNA (the gene for prelamin A and lamin C), resulting in aberrant mRNA splicing and the production of a prelamin A transcript lacking the last 150 nucleotides of exon 11 (1, 2). That transcript results in the production of a mutant prelamin A, commonly called progerin, containing an internal deletion of 50 amino acids. Children with HGPS can appear normal at birth, but they soon develop disease phenotypes that resemble, at least superficially, the phenotypes occurring with physiologic aging (3–5). Children with HGPS typically die during their teenage years from heart attacks or strokes (due to occlusive lesions in the cerebral and coronary arteries).
The products of the LMNA gene, prelamin A (the precursor to mature lamin A) and lamin C, are produced by alternative splicing (6). The lamin C transcript contains exon 1–10 sequences; prelamin A contains exon 1–12 sequences. Lamin C is identical to prelamin A through the first 566 amino acids but contains six unique residues at its C terminus. Prelamin A contains 98 unique C-terminal amino acids and terminates with a CaaX motif, which triggers protein farnesylation, followed by three additional processing steps [endoproteolytic release of the last three residues of the CaaX motif by RCE1 (“aaXing”), methylation of the newly exposed farnesylcysteine by ICMT, and endoproteolytic release of the last 15 amino acids (including the C-terminal farnesylcysteine methyl ester) by ZMPSTE24 (7)]. The ZMPSTE24-mediated processing step results in the production of mature lamin A.
The internal 50-amino acid deletion in progerin leaves its CaaX motif intact (and therefore does not affect protein farnesylation, aaXing, or methylation) but it eliminates the sequences required for ZMPSTE24 processing (8). Thus, progerin is farnesylated and methylated but cannot be processed to mature lamin A. Progerin’s C-terminal farnesylcysteine methyl ester enhances interactions with the inner nuclear membrane and limits the mobility of progerin. Fluorescence recovery studies following photobleaching revealed that the mobility of progerin is similar to that of lamin B1 (which is farnesylated and methylated) but slower than mature lamin A (which lacks the farnesyl lipid anchor) (9).
Progerin is the culprit molecule in disease pathogenesis. When progerin is expressed in cultured cells, it triggers misshapen nuclei, DNA damage, and cell senescence; these same abnormalities are observed in fibroblasts from patients with HGPS (1, 9–12). When progerin is expressed in mice, it triggers multiple disease phenotypes, including alopecia, bone fractures, and progressive weight loss (13–15). In addition, progerin causes the loss of smooth muscle cells (SMCs) in large arteries (16, 17). Loss of medial SMCs has also been documented in autopsy studies of aortic tissue from patients with HGPS (18, 19). Studies in mouse models of HGPS have provided valuable insights into the mechanisms of the vascular disease (15, 20–25). Of note, the high progerin–low lamin B1 nuclear lamin profile in aortic SMCs (which weakens the functional integrity of the nuclear envelope) combined with the rhythmic stretching/relaxation occurring in the wall of the aorta, play important roles in the tissue-specific loss of cells in the aorta.
Progerin’s ability to elicit disease phenotypes is often attributed to the fact that progerin, unlike mature lamin A or lamin C, contains a C-terminal farnesyl lipid. In support of that view, a protein farnesyltransferase inhibitor (FTI) improves nuclear shape in progerin-expressing cells and ameliorates disease in knock-in and transgenic models of HGPS (11–13, 16, 26–28). Also, disease phenotypes are absent in knock-in mouse models expressing a nonfarnesylated version of progerin (due to a single amino acid deletion in progerin’s CaaX motif) (29). Consistent with those observations, progerin in cultured cells results in the formation of a morphologically abnormal nuclear lamin meshwork (30, 31), whereas the meshwork formed by nonfarnesylated progerin is morphologically normal (31). The contribution of the internal deletion to the biological properties of progerin (other than eliminating ZMPSTE24-dependent processing) is less clear.
Studies of Zmpste24−/− mice have provided further insights into the pathogenesis of progeria (32). ZMPSTE24 deficiency abolishes the conversion of farnesyl-prelamin A to mature lamin A; consequently, tissues of Zmpste24−/− mice contain lamin C and farnesyl-prelamin A but lack mature lamin A. In humans, ZMPSTE24 deficiency causes restrictive dermopathy, a neonatal-lethal progeroid syndrome characterized by markedly impaired growth, rigid skin, joint contractures, and bone fractures (33). In mice, ZMPSTE24 deficiency causes many of the same phenotypes observed in gene-targeted models of HGPS (e.g., failure-to-thrive, bone fractures, loss of adipose tissue); those phenotypes are severe and invariably result in premature death (34, 35). However, in contrast to the gene-targeted HGPS mice, Zmpste24−/− mice or LmnaL648R/L648R knock-in mice (which produce full-length farnesyl-prelamin A due to the blockade of ZMPSTE24 processing) do not exhibit loss of medial SMCs in the aorta (15, 36).
Why the HGPS mouse models, but not Zmpste24−/− mice, exhibit vascular pathology is unknown, but this difference suggests that progerin and full-length farnesyl-prelamin A—despite both having identical C-terminal modifications—could have very distinct effects in the aorta. In the current study, we investigated that possibility. We discovered that progerin, but not farnesyl-prelamin A, accumulates progressively in SMCs in the mouse aorta, and that difference underlies their distinct capacities to cause arterial disease.
Results
Loss of vascular SMCs in HGPS knock-in mice but not Zmpste24−/− mice.
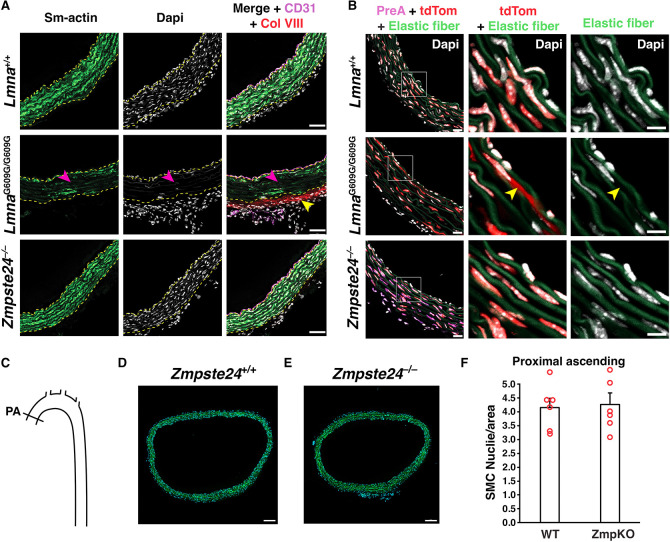
We examined aortas from wild-type mice (Lmna+/+), Zmpste24−/− mice, and a gene-targeted knock-in mouse model of HGPS (LmnaG609G/G609G). Cross sections of the proximal ascending thoracic aorta were stained with antibodies against α-smooth muscle actin [a marker of SMCs], CD31 (an endothelial cell marker), and collagen type VIII [a marker of HGPS-associated adventitial fibrous; (15)]. Confocal microscopy images of the inner curvature of the ascending aorta in LmnaG6096G/G609G mice revealed reduced α-smooth muscle actin staining, reduced numbers of SMCs, and increased collagen type VIII staining of the adventitia (Fig. 1a and Supplementary Fig. 1a). In contrast, aortas of Zmpste24−/− mice were normal, indistinguishable from those in Lmna+/+ mice. Consistent with those findings, nuclear membrane ruptures (identified by escape of a nuclear-targeted tdTomato into the cytoplasm) were frequent in the medial Ss of LmnaG6096G/G609G aortas but absent in Zmpste24−/− aortas (Fig. 1b and Supplementary Fig. 1b). As expected, farnesyl-prelamin A was detected in aortas of Zmpste24−/− mice but not in Lmna+/+ or LmnaG6096G/G609G mice (Fig. 1b and Supplementary Fig. 1b). Numbers of vascular SMCs in the medial layer of the aorta were the same in Zmpste24−/− mice and in wild-type mice (Figs. 1c–d and Supplementary Fig. 2).
Fig. 1. Loss of aortic smooth muscle cells (SMCs) occurs in HGPS knock-in mice but not Zmpste24−/− mice.
A. Confocal fluorescence microscopy images of the proximal ascending aorta from 16-week-old Lmna+/+, LmnaG609G/G609G, and Zmpste24−/− mice stained with antibodies against smooth muscle actin (Sm-actin, green), collagen type VIII (Col VIII, red), and CD31 (magenta). Nuclei were stained with Dapi (white). Scale bar, 50 μm. Yellow dotted lines mark the borders of the medial layer. Red arrowheads point to areas with reduced Sm-actin staining and reduced numbers of SMC nuclei. The yellow arrowhead points to collagen type VIII staining in the adventitia. Images of the entire tissue sections are shown in Supplementary Figure 1A. B. Confocal fluorescence microscopy images of the proximal ascending aorta from 13-week-old Lmna+/+ and LmnaG609G/G609G mice and a 21-week-old Zmpste24−/− mouse, all expressing a nuclear-targeted tdTomato transgene, stained with an antibody against farnesyl-prelamin A. The boxed regions are shown at higher magnification in the middle and far-right columns. The images show Dapi (white), elastic fibers (green), tdTomato (tdTom; red), and farnesyl-prelamin A (PreA; magenta). The yellow arrowhead (middle column) points to tdTomato outside of an SMC nucleus. Scale bar, 10 μm. Images of the entire tissue sections are shown in Supplementary Figure 1B. C. Diagram showing the location where SMC nuclei in the thoracic aorta were quantified (Proximal ascending, PA). D–E. Representative microscopy images of sections from the ascending thoracic aorta [stained with Dapi (blue)] from a 21-week-old Zmpste24+/+ mouse (D) and a Zmpste24−/− mouse (E). Scale bar, 100 μm. F. Bar graph showing the numbers of nuclei (relative to area) in the proximal ascending thoracic aorta in 21-week-old Zmpste24+/+ (WT) and Zmpste24−/− (ZmpKO) mice. Mean ± SEM (n = 5 mice/group). Student’s t test. P = 0.84. Images of the entire tissue sections used for quantification are shown in Supplementary Figure 2.
Progerin and farnesyl-prelamin A have similar effects in cultured SMCs.
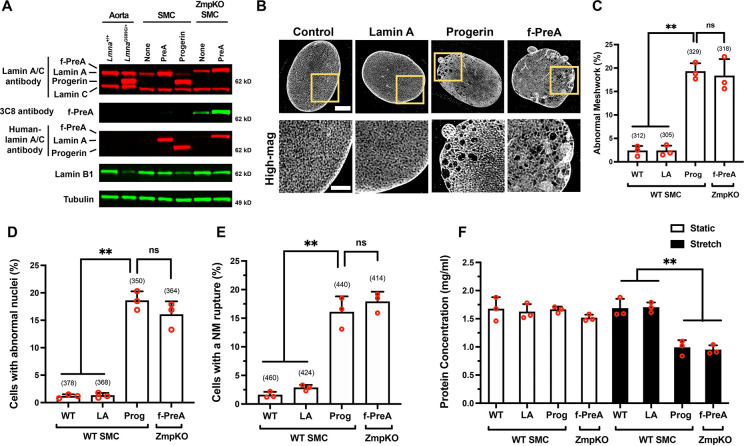
We used doxycycline (Dox)-inducible constructs to create mouse SMC clones that expressed human versions of progerin (Prog-SMC), mature lamin A (PreA-SMC), and farnesyl-prelamin A (PreA-ZMPKO-SMCs). The levels of nuclear lamin expression in the SMC clones were adjusted to match the amounts of progerin in aortas of LmnaG609G/+ mice (Fig. 2a). The expression of farnesyl-prelamin A in PreA-ZMPKO-SMCs was confirmed by western blotting with monoclonal antibody (mAb) 3C8, which binds preferentially to the farnesylated form of prelamin A (37) (Fig. 2a).
Fig. 2. Progerin and farnesyl-prelamin A have similar effects in cultured SMCs.
A. Western blot comparing the expression of nuclear lamins in the mouse aorta and in mouse SMCs expressing human lamin A, human progerin, and human farnesyl-prelamin A. Nuclear lamins were detected with antibodies that bind to mouse and human lamin A/C, farnesyl-prelamin A (clone 3C8), human lamin A/C, and lamin B1. Tubulin was measured as a loading control. B. Representative high-resolution confocal microscopy images showing the protein meshworks formed by human versions of lamin A, progerin, and farnesyl-prelamin A (f-PreA). Scale bar, 5 μm. The boxed regions are shown at higher magnification below. Scale bar, 2 μm. C. Nuclei with an abnormal meshwork in SMCs expressing human lamin A (LA), progerin (Prog), and farnesyl-prelamin A (f-PreA). Mean ± SEM (n = 3 experiments). ANOVA. **, P < 0.01. ns, not significant. D. Abnormal nuclear shape in SMCs expressing human versions of lamin A, progerin, and farnesyl-prelamin A. Mean ± SEM (n = 3 experiments). ANOVA. **, P < 0.01. ns, not significant. E. Nuclear membrane (NM) ruptures in SMCs expressing human versions of lamin A, progerin, and farnesyl-prelamin A. Mean ± SEM (n = 3 experiments). ANOVA. **, P < 0.01. ns, not significant. F. Cell death in SMCs expressing human versions of lamin A, progerin, or farnesyl-prelamin A. SMCs were cultured on PDMS membranes and exposed to static (open bars) or cyclical stretching conditions (closed bars) for 24 h. The fraction of cells remaining on the membranes were quantified by measuring protein concentration. Mean ± SEM (n = 3 experiments). ANOVA. **, P < 0.01. For the data reported in C–E, the number of nuclei or cells examined are shown in parentheses.
The nuclear lamin meshworks formed by progerin, farnesyl-prelamin A, and mature lamin A (in Prog-SMCs, PreA-ZMPKO-SMCs, and PreA-SMCs, respectively) were examined by high-resolution confocal fluorescence microscopy after staining with a human lamin A/C–specific antibody (31). The mature lamin A in PreA-SMCs formed a meshwork with small, uniform gaps and was morphologically indistinguishable from the mouse lamin A/C meshwork in wild-type SMCs (Fig. 2b). In contrast, progerin and farnesyl-prelamin A both formed an abnormal meshwork (with large and irregular-sized gaps) in SMCs (~17% for both) (Figs. 2b–c). Aside from triggering similar morphological abnormalities in the nuclear lamin meshwork, progerin and farnesyl-prelamin A triggered very similar frequencies of misshapen nuclei and nuclear membrane (NM) ruptures (Fig. 2d). Misshapen nuclei were present in 18% of Prog-SMCs and in 16% of PreA-ZMPKO-SMCs but were present in less than 1% of PreA-SMCs (≥ 350 cells/group). Similarly, NM ruptures [detected by live-cell imaging (21)] were present in 16% of Prog-SMCs and 18% in PreA-ZMPKO-SMCs, but were present in less than 3% of PreA-SMCs (≥ 414 cells/group) (Fig. 2e). In cells subjected to cyclical stretching (15), progerin and farnesyl-prelamin A increased the frequency of cell death to a similar degree (Fig. 2f). Cell death was observed in 40% of Prog-SMCs, 38% of PreA-ZMPKO-SMCs, but in less than 1% of PreA-SMCs.
Farnesyl-prelamin A does not accumulate with age in the aorta of Zmpste24−/− mice.
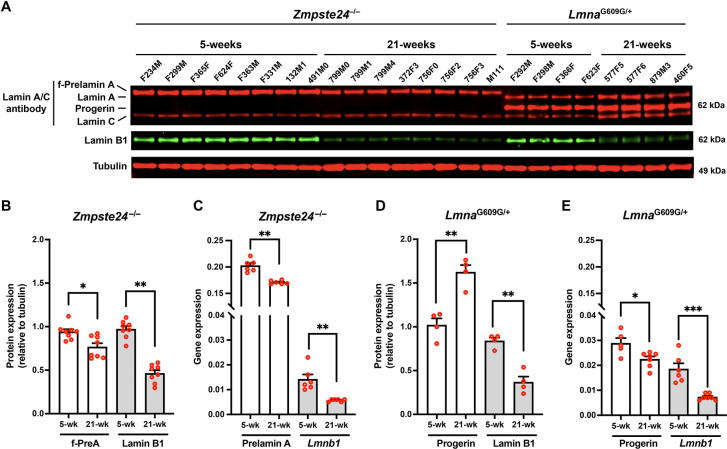
We hypothesized that the striking differences in aortic pathology in LmnaG609G/G609G and Zmpste24−/− mice (despite very similar toxicities of progerin and farnesyl-prelamin A in cultured SMCs) could be due to different amounts of progerin and farnesyl-prelamin A in aortas. To explore that possibility, we examined aortas of 5- and 21-week-old Zmpste24−/− and LmnaG609G/+ mice (Fig. 3a). Consistent with earlier findings (21), the levels of progerin in the aortas of LmnaG609G/+ mice increased with age, whereas the levels of lamin B1 fell. Levels of progerin increased by 62% between 5 and 21 weeks of age, while lamin B1 levels fell by 57% (n = 4 mice/group) (Fig. 3b). The age-related increase in progerin could not be explained by higher transcript levels; progerin transcript levels in the aorta fell by 22% with age (Fig. 3c). Lmnb1 transcripts in LmnaG609G/+ mice declined by 61% (Fig. 3e). In parallel, we examined aortas in age-matched Zmpste24−/− mice (n = 8 mice/group). In young animals, levels of farnesyl-prelamin A in Zmpste24−/− mice were equivalent to the progerin levels in LmnaG609G/+ mice. In contrast to the age-related increase in progerin levels in LmnaG609G/+ aortas, the levels of farnesyl-prelamin A in Zmpste24−/− aortas decreased by 19% (Fig. 3d). Lamin B1 protein levels in Zmpste24−/− aortas decreased by 53% (Fig. 3d). Prelamin A transcript levels in Zmpste24−/− aortas fell by 15% at 21 weeks; Lmnb1 transcript levels were reduced by 61% (Fig. 3e).
Fig. 3. Farnesyl-prelamin A does not accumulate with age in the aorta of Zmpste24−/− mice.
A. Western blot comparing the expression of farnesyl-prelamin A, progerin, and lamin B1 in aortas from young and old Zmpste24−/− and LmnaG609G/+ mice. Tubulin was measured as a loading control. The ages and mouse IDs are shown above each sample. B. Bar graph showing farnesyl-prelamin A (f-PreA) and lamin B1 expression (relative to tubulin) in aortas from young and old Zmpste24−/− mice. Mean ± SEM (n = 8 mice/group). Student’s t test. *, P < 0.05. **, P < 0.01. C. Quantitative RT-PCR studies showing prelamin A and Lmnb1 transcript levels in aortas from young and old Zmpste24−/− mice. Mean ± SEM (n = 6 mice). Student’s t test, **, P < 0.01. D. Bar graph showing progerin and lamin B1 protein expression (relative to tubulin) in aortas from young and old LmnaG609G/+ mice. Mean ± SEM (n = 4 mice/group). Student’s t test. **, P < 0.01. E. Quantitative RT-PCR studies showing progerin and Lmnb1 transcript levels in aortas from young and old LmnaG609G/+ mice. Mean ± SEM (n = 5–7 mice/group). Student’s t test. *, P < 0.05. ***, P < 0.001.
Progerin causes accumulation of A-type nuclear lamins in the aorta and heart.
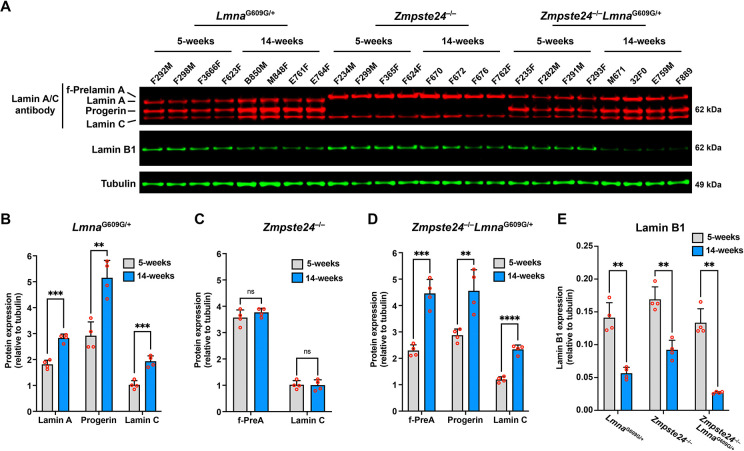
The fact that progerin levels increased with age in LmnaG609G/+ aortas without a corresponding increase in progerin transcripts [(21) and Figs. 3b–c] raised the possibility that progerin itself alters the turnover of nuclear lamins. To explore that possibility, we isolated aortas from young (5 week) and old (14 week) LmnaG609G/+ mice and quantified aortic levels of lamin A, lamin C, and progerin (i.e., all of the A-type nuclear lamins in LmnaG609G/+ mice). The levels of all three A-type nuclear lamins increased with age (Figs. 4a–b). We also examined aortas in age-matched Zmpste24−/− mice. The A-type nuclear lamins in Zmpste24−/− mice (farnesyl-prelamin A and lamin C) did not increase with age (Figs. 4a, 4c). To test whether the expression of progerin was responsible for the age-related increase in A-type nuclear lamin levels in the aorta, we bred Zmpste24-deficient mice that also express progerin (Zmpste24−/−LmnaG609G/+). In contrast to the observations in Zmpste24−/− mice, the levels of farnesyl-prelamin A and lamin C in Zmpste24−/−LmnaG609G/+ aortas increased with age (by 1.9- and 2-fold, respectively) (Figs. 4a, 4d). In these studies, aortas were examined at 14 weeks rather than 21 weeks of age because the disease phenotypes occurred earlier in Zmpste24−/−LmnaG609G/+ mice than in Zmpste24−/− mice (Supplementary Fig. 3a). Quantitative PCR studies showed that prelamin A, progerin, and lamin C transcript levels in the aorta did not increase with age (Supplementary Figs. 3b–d). Thus, the age-related increase in A-type nuclear lamins in both LmnaG609G/+ and Zmpste24−/−LmnaG609G/+ aortas could not be explained by increased Lmna transcripts. Lamin B1 protein levels in aortas of LmnaG609G/+, Zmpste24−/−, and Zmpste24−/− LmnaG609G/+ mice decreased with age (by 60%, 46%, and 79%, respectively; P < 0.01) (Figs. 4e).
Fig. 4. Progerin causes the accumulation of the A-type nuclear lamins in the aorta.
A. Western blot comparing the expression of lamin A, lamin C, farnesyl-prelamin A, progerin, and lamin B1 in aortas from 5- and 14-week-old LmnaG609G/+, Zmpste24−/−, and Zmpste24−/−LmnaG609G/+ mice. Tubulin was measured as a loading control. The ages and mouse IDs are shown above each sample. B. Bar graph showing lamin A, progerin, and lamin C expression (relative to tubulin) in aortas from young and old LmnaG609G/+ mice. Mean ± SEM (n = 4 mice/group). Student’s t test. **, P < 0.01. ***, P < 0.001. C. Bar graph showing farnesyl-prelamin A (f-PreA) and lamin C expression (relative to tubulin) in aortas from young and old Zmpste24−/− mice. Mean ± SEM (n = 4 mice/group). Student’s t test. ns, not significant. D. Bar graph showing f-PreA, progerin, and lamin C expression (relative to tubulin) in aortas from young and old Zmpste24−/−LmnaG609G/+ mice. Mean ± SEM (n = 4 mice/group). Student’s t test. **, P < 0.01. ***, P < 0.001. ****, P < 0.0001. E. Bar graph showing lamin B1 expression (relative to tubulin) in aortas from young and old LmnaG609G/+, Zmpste24−/−, and Zmpste24−/−LmnaG609G/+ mice. Mean ± SEM (n = 4 mice/group). Student’s t test. **, P < 0.01.
We also examined the impact of progerin on A-type nuclear lamins in the heart. Similar to the observations in aorta, amounts of A-type nuclear lamins in hearts of LmnaG609G/+ and Zmpste24−/− LmnaG609G/+ mice increased with age, whereas the amounts remained stable in Zmpste24−/− mice (Supplementary Figs. 4a–d). Lamin B1 levels in hearts of LmnaG609G/+ and Zmpste24−/− LmnaG609G/+ mice decreased with age or remained the same (Supplementary Fig. 4e). Transcript levels for the A-type nuclear lamins in hearts of LmnaG609G/+ and Zmpste24−/−LmnaG609G/+ mice did not increase with age (Supplementary Figs. 3e–g).
The loss of SMCs in aortas of Zmpste24-deficient mice can be induced by supraphysiologic levels of prelamin A production.
We suspected that Zmpste24−/− mice were protected from aortic SMC loss—despite the toxicity of farnesyl-prelamin A in cultured SMCs—was related to the absence of farnesyl-prelamin A accumulation with age. We reasoned that it might be possible to induce SMC loss in aortas of Zmpste24−/− mice by increasing prelamin A production. To test this possibility, we bred Zmpste24−/− mice harboring a prelamin A–only allele (LmnaPLAO), which channels all of the output from Lmna into prelamin A rather than into both lamin C and prelamin A (38). The levels of mature lamin A in the aorta of LmnaPLAO/+ mice (prelamin A in wild-type mice is processed to mature lamin A) were ~20% higher than in wild-type mice; the levels in LmnaPLAO/PLAO mice were increased by ~40% (Supplementary Figs. 5a–b). To our surprise, the levels of farnesyl-prelamin A in aortas of Zmpste24−/−LmnaPLAO/+ mice did not change (Supplementary Fig. 5c). Further increasing prelamin A production by breeding Zmpste24−/− LmnaPLAO/PLAO mice was not a useful strategy because those mice die from progressive multisystem disease by ~6–7 weeks of age (38).
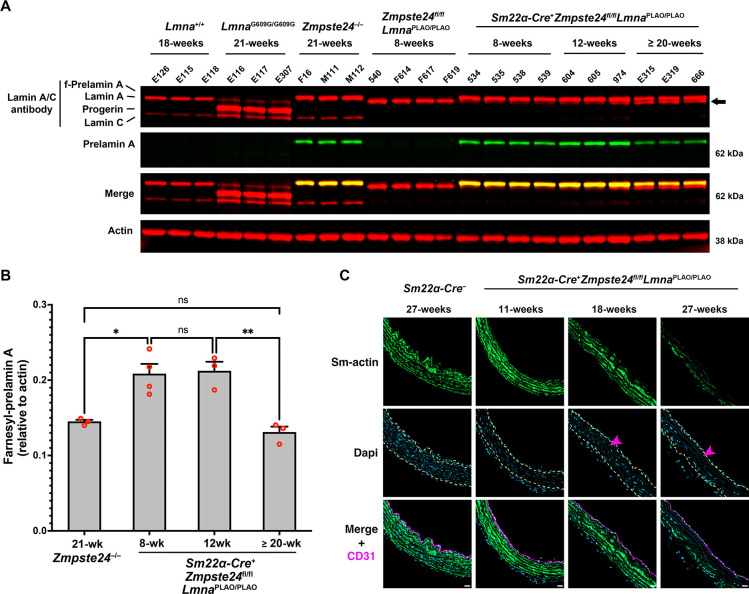
To circumvent this roadblock, we bred Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice to increase the levels of farnesyl-prelamin A selectively in SMCs. The expression of Sm22α-Cre in aortic SMCs was confirmed with a dual-fluorescence RosanT-nG reporter allele where tdTomato is expressed in the nucleus of cells but switches to EGFP after Cre-recombination (39). That approach makes it possible to visualize both Cre-positive and Cre-negative cells in the same sample. As expected, the two-color reporter showed Cre expression in aortic SMCs of Sm22α-Cre-positive mice (Supplementary Fig. 6a). Consistent with that finding, farnesyl-prelamin A synthesis was detected in aortic SMCs by immunohistochemistry (Supplementary Fig. 6b). However, the two-color reporter also revealed that many SMCs in Sm22α-Cre-positive mice do not express Cre (i.e., tdTomato-positive SMCs were still detected in the medial layer of the aorta). Thus, the expression of the Sm22α-Cre transgene in SMCs is variegated. Nonetheless, farnesyl-prelamin A levels were increased in Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice. At 8 and 12 weeks of age, the levels of farnesyl-prelamin A in the aorta were ~45% higher in Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice than in Zmpste24−/− mice. At 18 and 27 weeks of age, histopathology studies of Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice revealed loss of aortic SMCs along with reduced smooth muscle actin staining (Fig. 5c and Supplementary Figs. 7–8). Thus, supraphysiologic levels of farnesyl-prelamin A in SMCs had triggered aortic pathology. Interestingly, the aortic pathology developed even though the levels of farnesyl-prelamin A in 20-week-old Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice were lower than the levels at 12 weeks of age (Figs. 5a–b). The decreased farnesyl-prelamin A levels were accompanied by increased amounts of mature lamin A. The latter findings are almost certainly due to variegation in the expression of the Sm22α-Cre transgene in SMCs and a substantial competitive advantage of the subset of aortic SMCs that retained Zmpste24 expression (see Discussion).
Fig. 5. The loss of SMCs in aortas of Zmpste24-deficient mice can be induced by increasing prelamin A production.
A. Western blot comparing the expression of lamin A, lamin C, farnesyl-prelamin A, progerin, and lamin B1 in aortas from wild-type, LmnaG609G/G609G, Zmpste24−/−, Zmpste24fl/flLmnaPLAO/PLAO, and Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice. Farnesyl-prelamin A was detected with monoclonal antibody 3C8. Actin was measured as a loading control. The ages and mouse IDs are shown above each sample. The arrow points to mature lamin A in older Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice (detected with an anti-lamin A/C antibody). B. Bar graph comparing the expression of farnesyl-prelamin A (detected with antibody 3C8) relative to actin in 21-week-old Zmpste24−/− mice and Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice at 8, 12, and ≥ 20 weeks of age. ANOVA. *P < 0.05. **P < 0.01. ns, not significant. C. Confocal fluorescence microscopy images of the proximal ascending aorta from 11-, 18-, and 27-week-old Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice stained with antibodies against smooth muscle actin (Sm-actin, green) and CD31 (magenta). As a control, images from a 27-week-old Zmpste24fl/flLmnaPLAO/PLAO mouse are shown. Nuclei were stained with Dapi (blue). Scale bar, 20 μm. Yellow dotted lines mark the borders of the medial layer. Red arrowhead points to an area with reduced Sm-actin staining and reduced numbers of SMC nuclei. Images of the entire sections are shown in Supplementary Figure 7. Images from additional 27-week-old Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice are shown in Supplementary Figure 8.
Reduced phosphorylation of serine-404 in progerin.
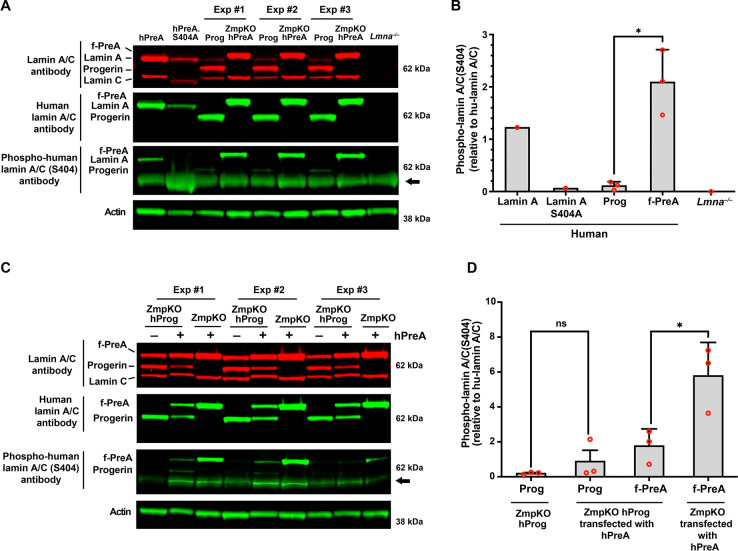
Earlier studies established that phosphorylation of prelamin A at serine-404 by AKT triggers prelamin A degradation by lysosomal enzymes (40). Considering the differences in levels of progerin and farnesyl-prelamin A in older mice, we hypothesized that the age-related accumulation of progerin in LmnaG609G/+ mice might result from lower levels of serine-404 phosphorylation. To test this possibility, we compared the phosphorylation of serine-404 in human progerin and human farnesyl-prelamin A expressed in SMCs by western blotting (with an antibody against human lamin A/C phosphoserine-404). The specificity of the antibody was confirmed in cells expressing a S404A-human lamin A mutant and in Lmna−/− SMCs (Fig. 6a–b). The phosphoserine lamin A antibody bound avidly to farnesyl-prelamin A in PreA-ZMPKO-SMCs, whereas the binding of the antibody to progerin in Prog-SMCs was very low (~5% of the binding to farnesyl-prelamin A) (Fig. 6a–b). To determine if the reduced phosphorylation was due to the impact of progerin expression, we transiently expressed human prelamin A in ZMPKO-SMCs or in ZMPKO-SMCs that expressed progerin, and then examined the phosphorylation of farnesyl-prelamin A at serine-404. In the ZMPKO-SMCs expressing progerin, the phosphorylation of farnesyl-prelamin A was significantly reduced (Figs. 6c–d).
Fig. 6. Reduced phosphorylation of serine-404 in progerin.
A. Western blot comparing the levels of serine-404 phosphorylation in human versions of lamin A, farnesyl-prelamin A (f-PreA), and progerin (Prog) expressed in mouse SMCs. Serine-404 phosphorylation was detected with an antibody against human lamin A/C phosphoserine-404. The specificity of the antibody was evaluated using extracts from SMCs expressing an S404A human lamin A mutant and Lmna−/− SMCs. The levels of expression of the human nuclear lamin proteins were measured with an anti-human lamin A/C antibody. Actin was measured as a loading control. The arrow points to a nonspecific band produced with the human lamin A/C phosphoserine-404 antibody. B. The bar graph compares the levels of serine-404 phosphorylation in the human versions of lamin A, lamin A-S404A, Prog, and f-PreA. Serine-404 phosphorylation was normalized to the level of human lamin protein expression. Mean ± SEM (n = 3). Student’s t test. *, P < 0.05. C. Western blot comparing the effects of progerin expression on the phosphorylation of serine-404 in human farnesyl-prelamin A. Human farnesyl-prelamin A synthesis was induced by transient transfection of human-prelamin A (hPreA) in Zmpste24−/− SMCs or Zmpste24−/− SMCs expressing progerin (ZmpKO hProg). Serine-404 phosphorylation in human farnesyl-prelamin A and progerin was detected as described in panel A. The arrow points to a nonspecific band produced with the human lamin A/C phosphoserine-404 antibody. D. The bar graph compares the effects of progerin expression on the levels of serine-404 phosphorylation in farnesyl-prelamin A. The levels of serine-404 phosphorylation were measured as described in panel B. Mean ± SEM (n = 3). ANOVA. *, P < 0.05. ns, not significant.
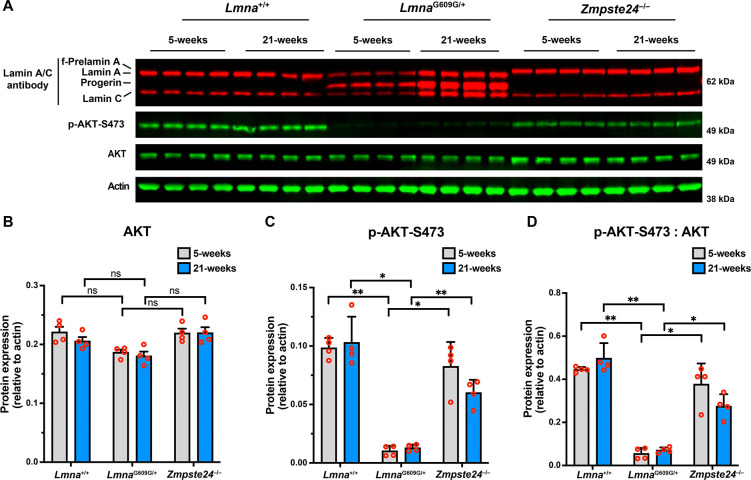
To determine if AKT activity is reduced in the aorta of LmnaG609G/+ mice, we compared the phosphorylation of AKT at serine-473 (41) in young and old wild-type (Lmna+/+), LmnaG609G/+, and Zmpste24−/− mice. Relative to total amounts of AKT, AKT phosphorylation at serine-473 was significantly lower in LmnaG609G/+ mice than in age-matched Lmna+/+ and Zmpste24−/− mice (Fig. 7). Consistent with reduced AKT activity in cells expressing progerin, AKT phosphorylation was also lower in 14-week-old Zmpste24−/−LmnaG609G/+ mice than in age-matched Zmpste24−/− mice (reduced by > 90%; ANOVA. P < 0.01).
Fig. 7. AKT activity is reduced in the aorta of LmnaG609G/+ mice.
A. Western blot comparing the levels of phosphorylated AKT at serine-473 (p-AKT-S473) in aortas from young and old Lmna+/+, LmnaG609G/+, and Zmpste24−/− mice. Actin was measured as a loading control. The ages and mouse IDs are shown above each sample. B. Bar graph shows the levels of total AKT (relative to actin) in young and old Lmna+/+, LmnaG609G/+, and Zmpste24−/− mice. Mean ± SEM (n = 4 mice/group). ANOVA. ns, not significant. C. Bar graph shows the levels of p-AKT-S473 (relative to actin) in young and old Lmna+/+, LmnaG609G/+, and Zmpste24−/− mice. Mean ± SEM (n = 4 mice/group). ANOVA. *, P < 0.05. **, P < 0.01. D. Bar graph shows the levels of p-AKT-S473 (relative to total AKT) in young and old Lmna+/+, LmnaG609G/+, and Zmpste24−/− mice. Mean ± SEM (n = 4 mice/group). ANOVA. *, P < 0.05. **, P < 0.01.
Discussion
The fact that progerin, an internally truncated and farnesylated prelamin A, triggers SMC loss in large arteries has been well documented (14, 16, 17). The causal relationship between progerin expression and SMC loss is substantiated by the observation that the extent of SMC loss is correlated with the amount of progerin expression (15, 25) and by the observation that progerin-triggered SMC loss in mice can be reversed by extinguishing progerin synthesis (42). Despite this progress, the reason that progerin triggers SMC loss in arteries but spares cells in other tissues has remained unclear. Also unclear is why SMC loss occurs in mouse models that express progerin but has not been observed in Zmpste24-deficient mice or LmnaL648R/L648R mice (15, 36), which express a mutant full-length farnesyl-prelamin A that is resistant to ZMPSTE24 cleavage. To gain insights into that difference, we compared the effects of progerin and full-length farnesyl-prelamin A in both cultured cells and genetically modified mice. We found that progerin and farnesyl-prelamin A, when expressed in identical amounts in mouse SMCs, induce virtually identical levels of toxicity (as judged by the morphology of nuclear lamin meshworks and by the frequencies of misshapen cell nuclei, nuclear membrane ruptures, and cell death during mechanical stretching). In contrast to the cell culture findings, the impacts of progerin and farnesyl-prelamin A expression in genetically modified mice are distinct. In young mice, the levels of progerin in aortas of “HGPS mice” (LmnaG609G) and the levels of farnesyl-prelamin A in Zmpste24−/− mice are similar. In older mice, however, progerin expression in LmnaG609G mice resulted in an age-related accumulation of progerin (along with lamin A and lamin C), whereas in Zmpste24−/− mice, the levels of farnesyl-prelamin A (and lamin C) did not accumulate with age. The accumulation of progerin could not be ascribed to changes in transcript levels, which either remained stable or declined with age, implying that progerin retards the turnover of A-type nuclear lamins. Consistent with that idea, AKT activity, which phosphorylates A-type nuclear lamins at serine-404 (43) and targets prelamin A for degradation (40), is reduced in the aortas of LmnaG609G/+ mice. These studies identify a distinct property of progerin that underlies the high levels of progerin in the aorta and the progerin-induced SMC loss in HGPS.
Our earlier studies identified an age-related accumulation of progerin in aortas of LmnaG609G/+ mice and raised the possibility of reduced progerin turnover (21). Building on that observation, we have, in the current study, shown that progerin levels in the aorta (and heart) not only accumulated with age but that this accumulation was accompanied by progerin-induced accumulation of the other A-type nuclear lamins. In contrast, full-length farnesyl-prelamin A in Zmpste24−/− mice did not affect the levels of A-type nuclear lamins. In a recent report (44), Hasper and colleagues examined “lifetimes” of nuclear lamins in a knock-in mouse model of HGPS. They found that the turnover of progerin in the aorta was reduced in the HGPS mice, consistent with our results. However, they did not detect an accumulation of progerin. In those studies, they measured the abundance of all A-type nuclear lamins (lamin A, lamin C, progerin) as a group in both young (n = 3) and old (n = 2) HGPS mice. While aortic levels of the A-type lamins tended to be higher in HGPS mice than in wild-type mice, this difference was not judged to be significant “due to variability across samples.” Higher levels of the A-type nuclear lamins were observed in the heart of HGPS mice, but since the A-type nuclear lamins were measured as a group, they could not determine whether the higher levels were due to an accumulation of progerin alone, or to lamin A and lamin C, or to all three nuclear lamins.
We suspected that the absence of vascular pathology in Zmpste24−/− mice was due to the fact that the levels of farnesyl-prelamin A in Zmpste24−/− aortas were lower than the levels of progerin in LmnaG609G/G609G aortas. To test whether increasing the levels of farnesyl-prelamin A expression were capable of eliciting aortic pathology, we bred Zmpste24−/−LmnaPLAO/+ mice. Because those mice die earlier than Zmpste24−/− mice (38), we suspected that they might have significantly higher levels of farnesyl-prelamin A in the aorta, but this was not the case. The levels of farnesyl-prelamin A did not change. This was surprising given that the LmnaPLAO allele increased the levels of lamin A in the aortas of wild-type mice (i.e., 20% with one LmnaPLAO allele and 40% with two LmnaPLAO alleles). Breeding Zmpste24−/− mice with two LmnaPLAO alleles was not an option because those mice die by ~6-weeks of age (38). We therefore produced SMC-specific Zmpste24 knockout mice harboring two LmnaPLAO alleles (Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO). At 8 and 12 weeks of age, the aortic levels of farnesyl-prelamin A in those mice were ~45% higher than in Zmpste24−/− mice. At 18 weeks of age, we observed substantial loss of aortic SMCs in Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice, demonstrating that supraphysiologic levels of farnesyl-prelamin A are toxic to aortic SMCs in vivo. Unexpectedly, the levels of farnesyl-prelamin A did not remain stable. Instead, there was a decrease in farnesyl-prelamin A levels, accompanied by increased amounts of mature lamin A, in older Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice. We suspect that those findings were secondary to the variegated expression of the Sm22α-Cre transgene. Because of the incomplete expression of the transgene in aortic SMCs, the aortas of Sm22α-CreZmpste24fl/flLmnaPLAO/PLAO mice contain a mixture of wild-type and Zmpste24-deficient SMCs. We believe that the supraphysiologic levels of farnesyl-prelamin A in the Zmpste24-deficient SMCs led to the death of those cells, resulting in increased numbers of SMCs expressing mature lamin A. Consistent with this interpretation, western blot studies revealed an age-related fall in farnesyl-prelamin A levels accompanied by increased levels of mature lamin A.
Initially, we suspected that the differences in vascular pathology in LmnaG609G and Zmpste24−/− mice could be related to different amounts of lamin B1 in SMCs. In earlier studies (15, 21), we observed that lamin B1 levels in the mouse aorta are very low. In wild-type mice, the low levels of lamin B1 remained stable with advancing age, while in LmnaG609G mice, the lamin B1 levels fell with increasing age. In cultured cells, we observed that increased expression of lamin B1 reduces some of the toxic effects of progerin (e.g., abnormal meshwork, nuclear membrane ruptures, stress-induced cell death), whereas reducing lamin B1 expression has the opposite effect (15, 21, 31). Given these observations, we suspected that we might find significantly higher aortic levels of lamin B1 in Zmpste24−/− mice than in LmnaG609G mice, but this was not the case. The aortic levels of lamin B1 were low in Zmpste24−/− mice, and the levels in Zmpste24−/−and LmnaG609G/+ mice declined with age to a similar degree. Instead, we found that the greater susceptibility of LmnaG609G mice to vascular pathology was due to a greater age-related accumulation of progerin. Although the levels of the other A type nuclear lamins also increased with age in LmnaG609G mice (which might be expected to mitigate disease in the aorta), it is likely that the dominant-negative effects of progerin limit any beneficial effects provided by the increased levels of lamin A and lamin C. Progerin levels also increased in the heart with age, but in contrast to the aorta, we could not detect an extensive loss of cardiomyocytes in the heart. The key difference between the heart and the aorta is that the levels of Lmna expression are far lower in the heart. Thus, even though progerin accumulates with age in the heart of LmnaG609G/G609G mice, the levels of progerin in the heart never approach the extremely high levels observed in the aorta. Quantitative western blots have demonstrated that the progerin-to-lamin B1 ratio is ~10-fold higher in the aorta than in the heart (15).
The phosphorylation/dephosphorylation of nuclear lamins (45) has long been recognized to be crucial for the disassembly and assembly of the nuclear lamina during mitosis [reviewed in (46)], but nuclear lamin phosphorylation also affects the interaction of nuclear lamins with other proteins, the subcellular localization of nuclear lamins, and nuclear lamin levels (47, 48). The phosphorylation of serine-404 in prelamin A by AKT affects the localization of prelamin A and triggers its degradation in lysosomes (40, 43). Progerin is also phosphorylated at serine-404 (48), but it has not been clear whether that phosphorylation regulates progerin levels. Given that AKT activity is perturbed in both cell culture and mouse models of HGPS (41, 49), we hypothesized that the age-related accumulation of progerin in the aorta could be due to reduced phosphorylation by AKT. In cultured cells, serine-404 was strongly phosphorylated in lamin A and farnesyl-prelamin A, but weakly phosphorylated in progerin. The reduced phosphorylation could theoretically be due to differences in protein structure (as a consequence of the missing 50-amino acid segment) (50), thereby reducing AKT-dependent phosphorylation, but the fact that progerin expression also reduced the phosphorylation of farnesyl-prelamin A suggested that progerin might affect AKT activity. We were encouraged by these findings and hoped to investigate the phosphorylation of progerin in the aorta of mice, but antibodies against mouse lamin A/C phosphoserine-404 were not available. We did, however, examine AKT activity as judged by AKT phosphorylation at serine-473 (41, 49). AKT activity was lower in aortas of LmnaG609G/+ mice than in aortas of wild-type or Zmpste24−/− mice. Consistent with the ability of progerin to reduce AKT activity, AKT phosphorylation in aortas of Zmpste24−/−LmnaG609G/+ mice was lower than in aortas of Zmpste24−/− mice.
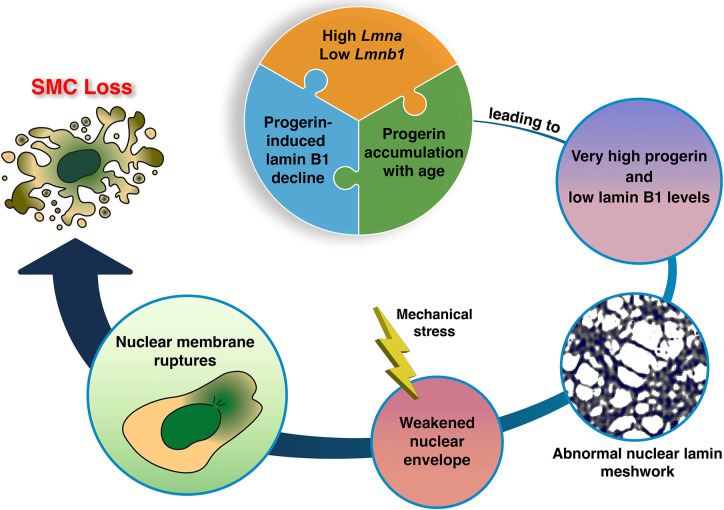
Our studies suggest a model for the loss of aortic SMCs in HGPS (Fig. 8). In SMCs of the mouse aorta, Lmna expression is high and Lmnb1 expression is low. In mouse models of HGPS, this expression pattern translates into high levels of progerin and low levels of lamin B1 in aortic extracts (15). This high progerin:lamin B1 ratio results in gaps and irregularities in the nuclear lamina meshwork, reduced structural integrity of the nuclear lamina, and increased susceptibility to mechanical stress (15, 21, 31). As the mice age, the progerin:lamin B1 ratio in the aorta is even higher (21), a consequence of both progerin accumulation and declining levels of lamin B1. That combination leads to frequent nuclear membrane ruptures, DNA damage, and ultimately to the loss of aortic SMCs.
Fig. 8. SMC loss in a mouse model of HGPS.
A high Lmna and low Lmnb1 expression profile in the mouse aorta results in a high lamin A:lamin B1 protein profile—ranking at the very top compared to 10 other mouse tissues (e.g., ~10-fold higher than in the kidney) (15). In the setting of HGPS, the high progerin:lamin B1 ratio increases with age and reaches extremely high levels (e.g., ~30-fold higher than in the kidney) (15). This increase is due to two factors. First, progerin, the toxic molecule in HGPS, increases the turnover of lamin B1, thereby reducing lamin B1 levels in the aorta (21). Lamin B1 acts in vitro to reduce the toxicity of progerin (21, 31). Second, progerin causes the accumulation of progerin itself as well as the other A-type nuclear lamins (shown in the current studies). We propose that the very high progerin:lamin B1 profile in aortic SMCs underlies the morphologically abnormal nuclear lamin meshwork and the reduced integrity of the nuclear envelope (31). In the setting of HGPS and mechanical stress (e.g., pulsatile flow in the aorta), the structural abnormalities in the nuclear lamin meshwork lead to nuclear membrane ruptures, DNA damage, and ultimately to loss of SMCs in the large arteries (15, 21).
Methods
Mice.
Zmpste24−/− (51), LmnaPLAO (38), LmnaG609G (17), and Zmpste24fl/fl (37) mouse strains have been described previously. The Sm22α-Cre (stock no. 017491) and ROSAnT-nG (stock no. 023035) mouse strains were purchased from The Jackson Laboratory (Bar Harbor, ME). Zmpste24−/− LmnaG609G/+ (and littermate Zmpste24−/− mice) were produced by intercrossing Zmpste24+/− LmnaG609G/+ mice. All mice were housed in a specific pathogen–free barrier facility with a 12-h light/dark cycle. The mice were provided pelleted mouse chow (NIH31) and water ad libitum. Both male and female mice were used for experimental studies.
Immunohistochemical analysis of aortic tissue.
Mice were perfused in situ with PBS followed by fixative solution (3% paraformaldehyde in PBS). The thoracic aorta was dissected free and incubated in fixative solution at 4°C for 1–2 h. Aortic rings (~2-mm) from the proximal ascending aorta, proximal descending, and mid-descending aorta were embedded in OCT, and frozen sections (4–6-μm) collected onto glass slides. Tissue sections were incubated as described previously (15, 21) with antibodies and concentrations listed in Supplementary Table 1. Nuclei were stained with Dapi. The stained sections were coded, and images were captured on a Zeiss LSM800 confocal microscope with a Plan-Apochromat 20×/0.8 NA objective. The coded images (tiff format) were imported into ImageJ, and the numbers of nuclei in the media were counted by trained observers and expressed relative to media area.
Zmpste24-deficient SMCs.
Guide RNAs targeting exon 6 of Zmpste24 (5′-GGTAAGGCTACCTGGAGGTG-3′) and (5′-ACAACATACACCTTAGTCAA-3′) were designed with Synthego’s gRNA design tool. Double-stranded gRNAs were subcloned into pX458-GFP CRISPR/Cas9 vector linearized with BbsI. A Nucleofector II apparatus (Lonza) and the Cell line T Nucleofector kit (Lonza) were used to electroporate 2 μg of pX458 vectors containing the gRNAs into 2 × 106 SMCs. After 48 h, transfected SMCs were cell sorted for the top 10% of GFP intensity by flow cytometry. Individual clones (20–30) were isolated by limiting dilution. Genomic DNA was extracted from SMC clones with the DNeasy kit (Qiagen) and subjected to PCR analysis. PCR primers outflanking the gRNA cut sites (5′-ATTGCCTGTGTCTGCCCTTCTGCT-3′ and 5′-GAACACTGGTTTTGTTTTGCAGCC-3′) were used to amplify the gene fragment from the genomic DNA. Sequencing primer (5′-TCCACCAGCATGAACAAGGGTGTGT-3′) was used to verify the sequence deletion between the two guide RNA cut sites. Clonal cell lines were further tested by qPCR and western blotting to confirm the absence of ZMPSTE24 activity.
Lmna-deficient SMC.
Lmna-deficient SMCs were generated in a similar fashion as Zmpste24-deficient SMCs. Guide RNAs targeting the 5′ and 3′ UTRs of Lmna (5′-GGATTGGCCGCTTCTGTGCG-3′) and (5′-CCAATCGCCGCACCTCTAGA-3′) were designed. Individual clones were isolated, and genomic DNA was extracted for sequencing. Deletion of the entire Lmna gene was confirmed by sequencing, qPCR, immunofluorescence, and western blotting.
Measurement of nuclear membrane (NM) ruptures in live SMCs.
SMCs stably expressing nls-GFP were seeded into 2-well chamber slides with #1.5 glass coverslip bottom (ThermoFisher Scientific) and cultured in complete media. To induce cell-cycle arrest, mitomycin C (1 μg/ml) was added to the media. The inhibition of mitosis greatly facilitated quantification of NM ruptures. Doxycycline was added to induce nuclear lamin expression for 24 h before examining the cells by confocal microscopy. The chamber slides were placed on a Zeiss LSM800 confocal laser-scanning microscope with a CO₂ and temperature-controlled stage, operated with Zen Blue 2.3 software (Zeiss). Cells were imaged for 48 h at 37°C with 5% CO₂ using a Plan-Apochromat 20×/0.8 NA objective. Images from randomly selected fields were captured every 1–2 min. The ratio of cells with a NM rupture, identified by the presence of GFP in the cytoplasm, was calculated by dividing the number of cells with a rupture by the total number of cells in the field.
High-resolution confocal fluorescence microscopy.
A total of 50,000 cells were seeded in a chambered slide with a #1.5H (170 μm ± 5 μm) glass bottom (ibidi USA). After 48 h, the cells, with or without doxycycline treatment, were fixed using 4% paraformaldehyde in PBS for 10 min at room temperature, followed by permeabilization with 0.3% Triton X-100 in PBS for 10 min. Immunofluorescence microscopy was performed as previously described (31). The antibodies and their concentrations are detailed in Supplementary Table 1. Airyscan images were captured using a Zeiss LSM980 equipped with Airyscan2 in super-resolution (SR) imaging mode, with a scan speed of 5 and with a Plan-Apochromat 63×/1.4 NA oil-immersion objective. The excitation wavelengths and filter settings for each dye were chosen based on the integrated dye presets in ZEN Blue 2.3 software, and consistent settings were maintained throughout the imaging session. Z-stacks were acquired at an optimal section thickness of 0.14 μm, starting from near the glass bottom to the top of the nucleus. Images collected in SR mode were further enhanced with Airyscan Joint Deconvolution (Zeiss) with the following parameters: sample structure set to standard, maximum iterations at 10, and a quality threshold of 0.00. SR confocal fluorescence images were processed with ZEN Blue 2.3 software to create maximum intensity projection images from the equatorial plane to the top of the nucleus (furthest from the glass bottom).
Quantification of lamina meshwork gap.
Z-axis images from the equator to the top of a nucleus were compiled and converted to 8-bit grayscale in ImageJ (NIH). Minor adjustments to brightness and contrast were made to optimize visualization of the nuclear lamina meshwork. The meshwork was outlined with the “Overlay” tool with a paintbrush set to 1-pixel width. A threshold was applied to define the meshwork, and the “Analyze Particles” function was used to quantify the areas of the gaps within the meshwork. A global scale was set using the original image’s scale bar. Meshwork gaps adjacent to the image borders were excluded from the analysis. The distribution of lamina meshwork gap areas was plotted for each nucleus. Nuclei exhibiting gap sizes greater than five times the average gap area were classified as abnormal.
Measurement of cell death in stretched SMCs.
Wild-type SMCs (1 × 105) expressing human prelamin A, SMCs expressing human progerin, or ZMPSTE24-deficient SMCs expressing human prelamin A were seeded onto polydimethylsiloxane (PDMS) membranes and cultured for 48 h. The membranes were clamped in a custom-built biaxial cell stretching device (15) and stretched 3-mm at 0.5 Hz for 24 h. To measure protein concentration of surviving cells, membranes were rinsed with PBS and digested with 0.1 N NaOH. Protein content was measured with the DC protein assay kit (Bio-Rad).
Statistical analysis.
Statistical analyses were performed with Microsoft Excel for Mac 2021 and GraphPad Prism software. Experimental groups were analyzed by unpaired 2-tailed Student’s t test, or one-way and two-way ANOVA with Tukey’s multiple comparisons test. Statistical differences were considered significant when the P value was < 0.05. Red circles in bar graphs show the average values of independent experiments or values for individual animals.
Supplementary Material
Acknowledgments
We thank Dino Di Carlo (UCLA) for the use of the plasma cleaner. This work was supported by the National Institutes of Health grants HL171737 and HL139725.
Footnotes
The authors have declared that no conflict of interest exists.
Study approval. All animal studies were approved by UCLA’s Animal Research Committee.
Data availability.
All data are available in the main text or the Supplementary Materials.
References
- 1.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin A truncation in Hutchinson–Gilford progeria. Science. 2003;300:2055. [DOI] [PubMed] [Google Scholar]
- 3.Debusk FL. The Hutchinson-Gilford progeria syndrome. J Pediatr. 1972;80:697–724. [DOI] [PubMed] [Google Scholar]
- 4.Gordon LB, McCarten KM, Giobbie-Hurder A, Machan JT, Campbell SE, Berns SD, et al. Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics. 2007;120(4):824–33. [DOI] [PubMed] [Google Scholar]
- 5.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358(6):592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin F, and Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268(22):16321–6. [PubMed] [Google Scholar]
- 7.Young SG, Fong LG, and Michaelis S. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria—New evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res. 2005;46:2531–58. [DOI] [PubMed] [Google Scholar]
- 8.Wood KM, Spear ED, Mossberg OW, Odinammadu KO, Xu W, and Michaelis S. Defining substrate requirements for cleavage of farnesylated prelamin A by the integral membrane zinc metalloprotease ZMPSTE24. PLoS One. 2020;15(12):e0239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, and Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2006;103(27):10271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101(24):8963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glynn MW, and Glover TW. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet. 2005;14:2959–69. [DOI] [PubMed] [Google Scholar]
- 12.Mallampalli MP, Huyer G, Bendale P, Gelb MH, and Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2005;102(40):14416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest. 2006;116(8):2115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osorio FG, Navarro CL, Cadinanos J, Lopez-Mejia IC, Quiros PM, Bartoli C, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3(106):106ra7. [DOI] [PubMed] [Google Scholar]
- 15.Kim PH, Luu J, Heizer P, Tu Y, Weston TA, Chen N, et al. Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci Transl Med. 2018;10(460)(460):eaat7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103(9):3250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Nobumori C, Tu Y, Choi C, Yang SH, Jung HJ, et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest. 2016;126(4):1592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehbens W, Wakdfield S, Gilbert-Barness E, Olson R, and Ackerman J. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol. 1999;8:29–39. [DOI] [PubMed] [Google Scholar]
- 19.Stehbens W, Delahunt B, Shozawa T, and Gilbert-Barness E. Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc Pathol. 2001;10:133–6. [DOI] [PubMed] [Google Scholar]
- 20.Hamczyk MR, Villa-Bellosta R, Quesada V, Gonzalo P, Vidak S, Nevado RM, et al. Progerin accelerates atherosclerosis by inducing endoplasmic reticulum stress in vascular smooth muscle cells. EMBO Mol Med. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim PH, Chen NY, Heizer PJ, Tu Y, Weston TA, Fong JL, et al. Nuclear membrane ruptures underlie the vascular pathology in a mouse model of Hutchinson-Gilford progeria syndrome. JCI Insight. 2021;6(16)(16):e151515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coll-Bonfill N, Mahajan U, Shashkova EV, Lin CJ, Mecham RP, and Gonzalo S. Progerin induces a phenotypic switch in vascular smooth muscle cells and triggers replication stress and an aging-associated secretory signature. Geroscience. 2023;45(2):965–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitrez PR, Estronca L, Monteiro LM, Colell G, Vazão H, Santinha D, et al. Vulnerability of progeroid smooth muscle cells to biomechanical forces is mediated by MMP13. Nat Commun. 2020;11(1):4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squarzoni S, Schena E, Sabatelli P, Mattioli E, Capanni C, Cenni V, et al. Interleukin-6 neutralization ameliorates symptoms in prematurely aged mice. Aging Cell. 2021;20(1):e13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabral WA, Tavarez UL, Beeram I, Yeritsyan D, Boku YD, Eckhaus MA, et al. Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome. Aging Cell. 2021;20(9):e13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci USA. 2005;102(36):12873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci USA. 2005;102(29):10291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capell BC, Erdos MR, Madigan JP, Fiordalisi JJ, Varga R, Conneely KN, et al. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2005;102(36):12879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang SH, Chang SY, Ren S, Wang Y, Andres DA, Spielmann HP, et al. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum Mol Genet. 2011;20(3):436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxboim A, Kronenberg-Tenga R, Salajkova S, Avidan N, Shahak H, Thurston A, et al. Scaffold, mechanics and functions of nuclear lamins. FEBS Lett. 2023;597(22):2791–805. [DOI] [PubMed] [Google Scholar]
- 31.Kim PH, Kim JR, Tu Y, Jung H, Jeong JYB, Tran AP, et al. Progerin forms an abnormal meshwork and has a dominant-negative effect on the nuclear lamina. Proc Natl Acad Sci U S A. 2024;121(27):e2406946121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worman HJ, and Michaelis S. Prelamin A and ZMPSTE24 in premature and physiological aging. Nucleus. 2023;14(1):2270345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, et al. Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy. J Invest Dermatol. 2005;125(5):913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA. 2002;99:13049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pendás AM, Zhou Z, Cadiñanos J, Freije JMP, Wang J, Hultenby K, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase–deficient mice. Nat Genet. 2002;31:94–9. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Shilagardi K, Hsu T, Odinammadu KO, Maruyama T, Wu W, et al. Abolishing the prelamin A ZMPSTE24 cleavage site leads to progeroid phenotypes with near-normal longevity in mice. Proc Natl Acad Sci U S A. 2022;119(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heizer PJ, Yang Y, Tu Y, Kim PH, Chen NY, Hu Y, et al. Deficiency in ZMPSTE24 and resulting farnesyl-prelamin A accumulation only modestly affect mouse adipose tissue stores. J Lipid Res. 2020;61(3):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies BS, Barnes RH 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, et al. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum Mol Genet. 2010;19(13):2682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prigge JR, Wiley JA, Talago EA, Young EM, Johns LL, Kundert JA, et al. Nuclear double-fluorescent reporter for in vivo and ex vivo analyses of biological transitions in mouse nuclei. Mamm Genome. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertacchini J, Beretti F, Cenni V, Guida M, Gibellini F, Mediani L, et al. The protein kinase Akt/PKB regulates both prelamin A degradation and Lmna gene expression. Faseb j. 2013;27(6):2145–55. [DOI] [PubMed] [Google Scholar]
- 41.Jiang B, Wu X, Meng F, Si L, Cao S, Dong Y, et al. Progerin modulates the IGF-1R/Akt signaling involved in aging. Sci Adv. 2022;8(27):eabo0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sánchez-López A, Espinós-Estévez C, González-Gómez C, Gonzalo P, Andrés-Manzano MJ, Fanjul V, et al. Cardiovascular Progerin Suppression and Lamin A Restoration Rescue Hutchinson-Gilford Progeria Syndrome. Circulation. 2021;144(22):1777–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cenni V, Bertacchini J, Beretti F, Lattanzi G, Bavelloni A, Riccio M, et al. Lamin A Ser404 is a nuclear target of Akt phosphorylation in C2C12 cells. J Proteome Res. 2008;7(11):4727–35. [DOI] [PubMed] [Google Scholar]
- 44.Hasper J, Welle K, Swovick K, Hryhorenko J, Ghaemmaghami S, and Buchwalter A. Long lifetime and tissue-specific accumulation of lamin A/C in Hutchinson-Gilford progeria syndrome. J Cell Biol. 2024;223(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerace L, and Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19(1):277–87. [DOI] [PubMed] [Google Scholar]
- 46.Liu SY, and Ikegami K. Nuclear lamin phosphorylation: an emerging role in gene regulation and pathogenesis of laminopathies. Nucleus. 2020;11(1):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack CG, et al. Interphase phosphorylation of lamin A. J Cell Sci. 2014;127(Pt 12):2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho S, Abbas A, Irianto J, Ivanovska IL, Xia Y, Tewari M, et al. Progerin phosphorylation in interphase is lower and less mechanosensitive than lamin-A,C in iPS-derived mesenchymal stem cells. Nucleus. 2018;9(1):230–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim MX, Sayin VI, Akula MK, Liu M, Fong LG, Young SG, et al. Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science. 2013;340(6138):1330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin EW, Brady GF, Kwan R, Nesvizhskii AI, and Omary MB. Genotype-phenotype analysis of LMNA-related diseases predicts phenotype-selective alterations in lamin phosphorylation. Faseb j. 2020;34(7):9051–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung GK, Schmidt WK, Bergo MO, Gavino B, Wong DH, Tam A, et al. Biochemical studies of Zmpste24-deficient mice. J Biol Chem. 2001;276:29051–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the Supplementary Materials.