Abstract
Complement protein C3 is crucial for immune responses in mucosal sites such as the lung, where it aids in microbe elimination and enhances inflammation. While trained immunity – enhanced secondary responses of innate immune cells after prior exposure – is well-studied, the role of the complement system in trained immune responses remains unclear. We investigated the role of C3 in trained immunity and found that in vivo, trained wild-type mice showed significantly elevated pro-inflammatory cytokines and increased C3a levels upon a second stimulus, whereas C3-deficient mice exhibited a blunted cytokine response and heightened evidence of lung injury. Ex vivo, C3-deficient alveolar macrophages (AMs) displayed reduced chemokine and cytokine output after training, which was restored by exogenous C3 but not by C3a. Inhibiting C3aR, both pharmacologically and with a genetic C3aR knockout, prevented this restoration, indicating the necessity of C3aR engagement. Mechanistically, trained WT AMs demonstrated enhanced glycolytic activity compared to C3-deficient AMs – a defect corrected by exogenous C3 in a C3aR-dependent manner. These findings reveal that C3 modulates trained immunity in AMs through C3aR signaling, affecting cytokine production and metabolic reprogramming, and highlight a novel role for C3 in trained immunity.
INTRODUCTION
The complement system is a crucial part of immunity, consisting of proteolytic enzymes that generate fragments which enhance antibody binding and help induce phagocytosis (Sahu et al., 2022). A central component of this system is the C3 protein, which is cleaved into its constituent components C3a and C3b upon activation (Kulkarni et al., 2018). C3b tags pathogens for phagocytosis and plays a vital role in the formation of the C3 convertase, which cleaves C5 and eventually forms the membrane attack complex that lyses targeted cells (Janssen et al., 2006). Meanwhile, C3a acts as an anaphylatoxin, binding to its cognate C3a receptor (C3aR) on and within cells, and modulates inflammatory responses (Kildsgaard et al., 2000). C3 activation has also been shown to favor an increase in glycolysis, suggesting a plausible mechanism for enhanced inflammatory activity (Friščić et al., 2021; Kang et al., 2024). Moreover, C3 is present at low levels in the bronchoalveolar lavage (BAL) fluid of uninjured mice as well as humans, indicative of localized production (Bolger et al., 2007). This local C3 production increases during injury, affecting mucosal responses to infection (Bolger et al., 2007). Additionally, the conversion of C3 to a conformationally altered C3(H2O) moiety increases during inflammation (Elvington et al., 2019). This C3(H2O) form is that which is internalized by various cell types, playing a key role in modulating survival and effector immune responses (Elvington et al., 2017; Kulkarni et al., 2019). Thus, local C3 activity plays an important role in modulating mucosal immune responses (Kulkarni et al., 2024).
A new concept of immune “memory” has recently emerged, whereby innate immune cells, particularly monocytes and macrophages, exhibit robust, enhanced inflammatory responses upon secondary stimulation after a prior insult (Quintin et al., 2012; Saeed et al., 2014). This form of “memory” is termed trained immunity and considered broadly antigen nonspecific, yet lasts several months after the initial stimulus (Netea et al., 2020). Underlying the induction of trained immunity are epigenetic and metabolic reconfigurations occurring after an initial stimulus, which prime transcriptional machinery for rapid activation after engaging secondary insults (Bekkering et al., 2014; Fanucchi et al., 2021). For instance, the bacterial and fungal cell wall component 1,3-D-β-glucan, binds the dectin-1 pattern recognition receptor, which induces metabolic changes favoring persistent glycolytic activity (Cheng et al., 2014; Earhart et al., 2023). This, in turn, promotes shifts in epigenetic modifications such as histone acetylation and methylation favoring strong pro-inflammatory gene expression upon restimulation, and can be long-lasting (Fok et al., 2019; Tercan et al., 2021). While knowledge of trained immunity is primarily systemic, there is growing evidence for site-specific effects such as in alveolar macrophages (AMs) (Chakraborty et al., 2023; Zahalka et al., 2022). Little is currently known about how the complement system affects trained immunity. Here, we sought to investigate whether the C3 protein – a key component of immune responses at mucosal sites such as the respiratory system – affects trained immunity in AMs and determine whether any effect is mediated by C3aR activity.
RESULTS AND DISCUSSION
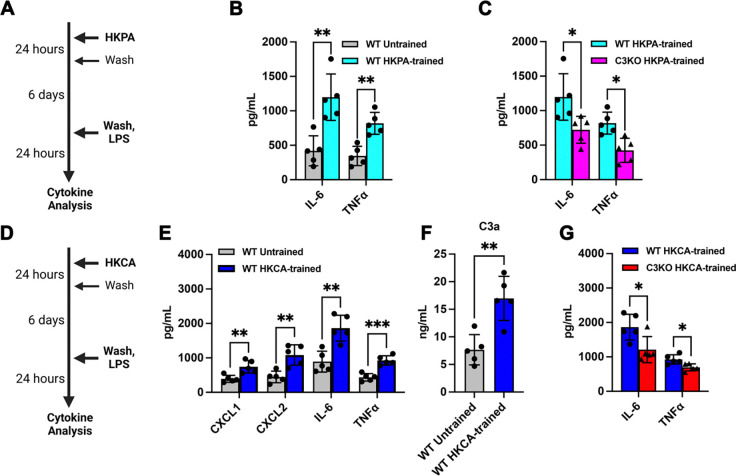
To investigate the role of C3 in pulmonary immune cell-trained immunity, we first inoculated C57BL/6J wild-type (WT) and B6.129S4-C3tm1Crr/J C3 knockout (C3KO) mice intranasally with heat-killed Pseudomonas aeruginosa (HKPA) for training or vehicle control (PBS, untrained). After 14 days, lipopolysaccharide (LPS) from Escherichia coli was also administered intranasally to both mouse strains to induce secondary stimulation for 24 h, followed by euthanasia and bronchoalveolar lavage (BAL) as previously described (Fig. 1A) (Sahu et al., 2023). BAL proinflammatory chemokines and cytokines (CXCL1, CXCL2, IL-6, and TNFα), total protein content (a marker of alveolar-capillary barrier disruption) and RAGE (a marker of epithelial injury), were quantified using ELISAs. Additionally, C3a, which is generated when C3 is activated and cleaved, was also measured using an ELISA specific to its neoepitope. CXCL1, CXCL2, IL-6, and TNFα were all significantly elevated in trained versus untrained WT BAL (Fig. 1B). Levels of C3a were increased in trained versus untrained WT BAL, indicating enhanced C3 activation is part of the trained immune response (Fig. 1C). The enhancement in BAL IL-6 and TNFα with training was blunted in C3-deficient mice compared to WT mice (Fig. 1D). In contrast to the blunted training response, BAL total protein and RAGE were elevated in trained C3KO versus WT mice post-LPS challenge, and alveolar damage was worse on histopathology in C3-deficient compared to WT mice (Figs. 1E, F). These results suggest C3 is important for trained immunity in vivo.
Figure 1. C3 deficiency predisposes to impaired pulmonary trained immunity and diminished protection from lung injury.
(A) Schematic representing the training of mice via the intranasal route with heat-killed Pseudomonas aeruginosa (HKPA) and subsequent restimulation with lipopolysaccharide (LPS), followed by bronchoalveolar lavage (BAL) and cytokine analysis. Created with BioRender.
(B) WT untrained compared against WT-trained BAL levels of CXCL1, CXCL2, IL-6, and TNFα.
(C) Comparison of BAL C3a levels, similar to (B).
(D) WT-trained versus C3-deficient (C3KO)-trained BAL concentrations of IL-6 and TNFα. WT-trained levels derived from (B) for comparison with C3KO-trained mice.
(E) Concentrations of protein and RAGE from WT-trained versus C3KO-trained mice.
(F) Representative histopathological slides of lungs showing increased tissue damage in C3KO-trained versus WT-trained mice (N=5 in each group), high power view from selected area. Data were compared with two-sided unpaired t-tests with (B, D) or without (C, E) Holm-Šidák correction for multiple hypothesis testing. Each point represents a measurement from one mouse, n = 5–10 for each group with mean ± SD shown. *p < 0.05, **p < 0.01, ***p < 0.001.
To examine how C3 affects trained immunity specifically in tissue-resident phagocytes, we set up an ex vivo culture system using primary alveolar macrophages (AM) from C3-deficient and wildtype mice (Gorki et al., 2022). Like the in vivo experiments (Fig. 1), we used HKPA to induce trained immunity in cultured AMs, followed days later by LPS challenge and analysis of cytokine secretion in the presence or absence of C3 deficiency (Fig. 2A). Supporting our in vivo results, HKPA was sufficient to induce trained immune responses from primary alveolar macrophages (Fig. 2B). However, C3-deficient AMs had blunted trained immune responses as measured by IL-6 and TNFα secretion (Fig. 2C). Employing heat-killed Candida albicans (HKCA) in place of HKPA produced similar results (Figs. 2D–G). These data suggest a specific role for C3 in AM immune training, consistent with our in vivo observations.
Figure 2: C3 deficiency results in impaired trained immune responses in ex vivo alveolar macrophages (AMs).
(A) Schematic representing in vitro training of AMs with HKPA, with later stimulation by LPS and subsequent cytokine analysis of the supernatants.
(B-C) Effects of HKPA-induced training in vitro on IL-6 and TNFα in supernatant from (B) WT AM, and (C) their comparison with C3KO-trained AMs.
(D) Schematic representing in vitro training of AMs with HKCA, with subsequent restimulation by LPS and cytokine analysis of the supernatants.
(E) Effects of heat-killed Candida albicans (HKCA)-induced training in vitro on CXCL1, CXCL2, IL-6 and TNFα in supernatant from WT AM.
(F) Comparison of C3a levels post-HKCA training, similar to (B).
(G) Comparison of IL-6 and TNFα post-HKCA training in WT versus C3KO AMs. WT-trained levels derived from (D) for comparison with C3KO-trained AMs.
Data were compared with two-sided unpaired t-tests with (B,C,E,G) or without (F) Holm-Šidák correction for multiple hypothesis testing. Each point is a technical replicate made by pooling AMs from at least n=4 mice in each group, with mean ± SD shown, and each experiment was repeated twice. *p < 0.05, **p < 0.01, ***p < 0.001.
To mechanistically validate the role of C3 or its products in trained immunity, we cultured C3-deficient AMs with exogenous C3 at a dose that leads to the cellular uptake of C3(H2O) (15 μg/mL, Fig 2A) (Elvington et al., 2017; Kulkarni et al., 2019). As a control, we incubated cells with C3a, which is not internalized (Fig. 3A) (Mogilenko et al., 2022). As measured by IL-6 and TNFα, exogenous C3 protein restored AM trained immunity to WT levels (Fig. 3B). In contrast, trained immunity was not restored by exogenous C3a, which stays outside the cell (Fig. 3C).
Figure 3. C3 uptake enhances trained immune responses in ex vivo alveolar macrophages via the C3a receptor (C3aR).
(A) Schematic representing in vitro training of AMs with HKCA, with pre-treatment of C3 or C3a prior to induction of training, and later stimulation by LPS and subsequent cytokine analysis of the supernatants.
(B) Effects of adding C3 prior to training on IL-6 and TNFα levels from C3KO AMs and their comparison with WT-trained AMs.
(C) Effects of adding C3a prior to training, similar to (B).
(D) Schematic representing addition of the C3aR antagonist prior to C3 treatment and in vitro training of AMs with HKCA, with later stimulation by LPS and subsequent cytokine analysis of the supernatants.
(E) Effects of C3aR antagonism on IL-6 and TNFα levels from trained WT and C3KO AMs treated with exogenous C3.
(F) Comparison of IL-6 levels post-HKCA-training in C3aR-deficient (C3aRKO), C3KO and WT AMs treated with exogenous C3.
Data were compared using one way ANOVA with Dunnett’s post hoc tests (B,C,F) or two-sided unpaired t-testing with Holm-Šidák correction for multiple testing (D). Each point is a technical replicate made by pooling AMs from at least n=4 mice in each group, with mean ± SD shown, and each experiment was repeated twice. *p < 0.05, **p < 0.01.
Upon internalization, C3 is cleaved to C3a (Elvington et al., 2017). Intracellular C3a binds to C3aR in lysosomes, and affects cytokine production in CD4+ T cells (Liszewski et al., 2013). To investigate whether a similar pathway could be important to trained immunity, we pre-treated AMs with a cell-permeable C3aR antagonist (SB290157) before immune training (Fig. 3D). C3aR antagonism blunted AM trained immunity in both WT and C3KO AMs that had previously been rescued with exogenous C3 (Fig. 3E). Importantly, genetic C3aR deficiency phenocopied the blunting of AM trained immune responses seen in C3-deficient cells (Fig. 3F). Altogether, these findings support a causal role for C3 in AM trained immunity via its intracellular binding of C3aR.
To explore transcriptomic changes relevant to how C3 influences trained immune responses, we performed bulk RNASeq comparing trained WT to trained C3KO AMs. C3-deficiency led to differential expression of not only innate immune genes but also several genes involved in metabolism, including those relevant to glycolysis such as gnpda1 and aldoc (Fig. 4A, Table S1). These metabolism-linked genes are significant because prior studies show that enhanced glycolytic metabolism is critical for trained immunity (Cheng et al., 2014). Therefore, we investigated whether the absence of C3 affects glycolytic flux in AMs (Fig. 4B). As measured by extracellular acidification rate, untrained C3-deficient AMs had comparable basal and maximum glycolytic flux compared to WT but failed to augment glycolysis upon training with HKCA (Fig. 4C). We next tested the ability of exogenous C3 to rescue the induction of training-associated glycolysis in C3-deficient AMs (Fig. 4D). Exogenous C3 returned glycolysis induction to the level of trained WT cells, suggesting intracellular processing of C3 is important for this effect (Fig. 4E). Like cytokines (Fig. 3), inhibiting C3aR prevented exogenous C3 from rescuing glycolysis induction during immune training (Fig. 4E).
Figure 4. C3-C3aR axis is required for glycolysis as a part of trained immune responses in alveolar macrophages.
(A) Principal component analysis (PCA, left) and EnrichR analysis of 391 genes (right, Table S1) downregulated in HKCA-trained C3KO vs WT AM by filtering genes (FDR step up ≤0.05). Arrow shows metabolism gene set in EnrichR; bars ranked by p-value.
(B) Schematic representing in vitro training of AMs with HKCA.
(C) Extracellular acidification rate (ECAR) from Seahorse analysis representing full glycolytic activity, and basal and maximum glycolysis in untrained and HKCA-trained WT and C3KO AMs.
(D) Schematic representing addition of the C3aR antagonist (SB290157) prior to C3 treatment and in vitro training of AMs with HKCA.
(E) Seahorse analysis in the presence and absence of exogenous C3 supplementation and C3aR antagonism. Each point is a technical replicate of pooled AMs from at least n=4 mice in each group, with mean ± SD shown. *p < 0.05, **p < 0.01 using an unpaired t-test.
Collectively, these data indicate that C3 makes a mechanistic contribution to trained immunity in AMs and implicate engagement of the C3aR as part of the pathway. Strengths of our study include in vitro and in vivo approaches to inducing trained immunity, orthogonal endpoints to quantify immune training (cytokine and metabolic), and mechanistic gain- and loss-of-function experimental approaches. Future work involves identifying the downstream effectors of C3 and C3aR in the AM training process and their connection to immunometabolism. The results may have clinical implications since trained immunity is linked to vaccine effectiveness (for example, with respect to the Bacillus Calmette-Guérin vaccine) and overall immune resilience (Arts et al., 2018; Kleinnijenhuis et al., 2015). Moreover, humans with C3 deficiency demonstrate impaired responses to vaccination (Kim et al., 2018; Pekkarinen et al., 2015). It is tempting to speculate that local augmentation of C3 and/or C3aR activity – could enhance vaccine effectiveness for hard-to-vaccinate pathogens like Mycobacterium tuberculosis.
MATERIALS AND METHODS
Details including mouse strains, design, models of trained immunity, readouts, and statistical analysis are described as per the ARRIVE guidelines and included in Supplementary Material.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. John Atkinson for his careful review of the manuscript and feedback, and the Genome Technology Access Center at the McDonnell Genome Institute for help with genomic analysis. The Center is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center. This publication is solely the responsibility of the authors.
Sources of support:
A.P.E. is supported by the NIH (T32HL125241); H.S.K. is supported by the NIH (R01HL169860) and the Longer Life Foundation.
DATA AVAILABILITY
Sequencing data have been deposited in GEO under the accession code GSE281001 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE281001.
REFERENCES
- Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang S-Y, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken CBEM, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, Van Crevel R, Netea MG. 2018. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host & Microbe 23:89–100.e5. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Bekkering S, Quintin J, Joosten LAB, Van Der Meer JWM, Netea MG, Riksen NP. 2014. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. ATVB 34:1731–1738. doi: 10.1161/ATVBAHA.114.303887 [DOI] [PubMed] [Google Scholar]
- Bolger MS, Ross DS, Jiang H, Frank MM, Ghio AJ, Schwartz DA, Wright JR. 2007. Complement levels and activity in the normal and LPS-injured lung. Am J Physiol Lung Cell Mol Physiol 292:L748–759. doi: 10.1152/ajplung.00127.2006 [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Singh A, Wang L, Wang X, Sanborn MA, Ye Z, Maienschein-Cline M, Mukhopadhyay A, Ganesh BB, Malik AB, Rehman J. 2023. Trained immunity of alveolar macrophages enhances injury resolution via KLF4-MERTK-mediated efferocytosis. Journal of Experimental Medicine 220:e20221388. doi: 10.1084/jem.20221388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. 2013. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14:128. doi: 10.1186/1471-2105-14-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, Van Der Veer BMJW, Deen PMT, Logie C, O’Neill LA, Willems P, Van De Veerdonk FL, Van Der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. 2014. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. doi: 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart AP, Karasseva NG, Storey KM, Olthoff B, Sarker MB, Laffey KG, Lange MJ, Rector RS, Schulz LC, Gil D, Neuhauser CM, Schrum AG. 2023. Lower female survival from an opportunistic infection reveals progesterone-driven sex bias in trained immunity. Cell Reports 42:113007. doi: 10.1016/j.celrep.2023.113007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JP. 2017. A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest 127:970–981. doi: 10.1172/JCI89412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington M, Liszewski MK, Liszewski AR, Kulkarni HS, Hachem RR, Mohanakumar T, Kim AHJ, Atkinson JP. 2019. Development and Optimization of an ELISA to Quantitate C3(H2O) as a Marker of Human Disease. Front Immunol 10:703. doi: 10.3389/fimmu.2019.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi S, Domínguez-Andrés J, Joosten LAB, Netea MG, Mhlanga MM. 2021. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 54:32–43. doi: 10.1016/j.immuni.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Fok ET, Davignon L, Fanucchi S, Mhlanga MM. 2019. The lncRNA Connection Between Cellular Metabolism and Epigenetics in Trained Immunity. Front Immunol 9:3184. doi: 10.3389/fimmu.2018.03184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friščić J, Böttcher M, Reinwald C, Bruns H, Wirth B, Popp S-J, Walker KI, Ackermann JA, Chen X, Turner J, Zhu H, Seyler L, Euler M, Kirchner P, Krüger R, Ekici AB, Major T, Aust O, Weidner D, Fischer A, Andes FT, Stanojevic Z, Trajkovic V, Herrmann M, Korb-Pap A, Wank I, Hess A, Winter J, Wixler V, Distler J, Steiner G, Kiener HP, Frey B, Kling L, Raza K, Frey S, Kleyer A, Bäuerle T, Hughes TR, Grüneboom A, Steffen U, Krönke G, Croft AP, Filer A, Köhl J, Klein K, Buckley CD, Schett G, Mougiakakos D, Hoffmann MH. 2021. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54:1002–1021.e10. doi: 10.1016/j.immuni.2021.03.003 [DOI] [PubMed] [Google Scholar]
- Gorki A-D, Symmank D, Zahalka S, Lakovits K, Hladik A, Langer B, Maurer B, Sexl V, Kain R, Knapp S. 2022. Murine Ex Vivo Cultured Alveolar Macrophages Provide a Novel Tool to Study Tissue-Resident Macrophage Behavior and Function. Am J Respir Cell Mol Biol 66:64–75. doi: 10.1165/rcmb.2021-0190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJC, Christodoulidou A, McCarthy A, Lambris JD, Gros P. 2006. Structure of C3b reveals conformational changes that underlie complement activity. Nature 444:213–216. doi: 10.1038/nature05172 [DOI] [PubMed] [Google Scholar]
- Kang S, Ko EY, Andrews AE, Shin JE, Nance KJ, Barman PK, Heeger PS, Freeman WM, Benayoun BA, Goodridge HS. 2024. Microglia undergo sex-dimorphic transcriptional and metabolic rewiring during aging. J Neuroinflammation 21:150. doi: 10.1186/s12974-024-03130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. 2000. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol 165:5406–5409. doi: 10.4049/jimmunol.165.10.5406 [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Kim K-H, Ko E-J, Kim M-C, Lee Y-N, Jung Y-J, Lee Y-T, Kwon Y-M, Song J-M, Kang S-M. 2018. Complement C3 Plays a Key Role in Inducing Humoral and Cellular Immune Responses to Influenza Virus Strain-Specific Hemagglutinin-Based or Cross-Protective M2 Extracellular Domain-Based Vaccination. J Virol 92:e00969–18. doi: 10.1128/JVI.00969-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Van Crevel R, Netea MG. 2015. Trained immunity: consequences for the heterologous effects of BCG vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene 109:29–35. doi: 10.1093/trstmh/tru168 [DOI] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90–W97. doi: 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni DH, Starick M, Aponte Alburquerque R, Kulkarni HS. 2024. Local complement activation and modulation in mucosal immunity. Mucosal Immunol 17:739–751. doi: 10.1016/j.mucimm.2024.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni HS, Elvington ML, Perng Y-C, Liszewski MK, Byers DE, Farkouh C, Yusen RD, Lenschow DJ, Brody SL, Atkinson JP. 2019. Intracellular C3 Protects Human Airway Epithelial Cells from Stress-associated Cell Death. Am J Respir Cell Mol Biol 60:144–157. doi: 10.1165/rcmb.2017-0405OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni HS, Liszewski MK, Brody SL, Atkinson JP. 2018. The complement system in the airway epithelium: An overlooked host defense mechanism and therapeutic target? J Allergy Clin Immunol 141:1582–1586.e1. doi: 10.1016/j.jaci.2017.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. 2008. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Storey JD. 2007. Capturing Heterogeneity in Gene Expression Studies by Surrogate Variable Analysis. PLoS Genet 3:e161. doi: 10.1371/journal.pgen.0030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, Arstila TP, Pekkarinen PT, Ma M, Cope A, Reinheckel T, Rodriguez de Cordoba S, Afzali B, Atkinson JP, Kemper C. 2013. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39:1143–1157. doi: 10.1016/j.immuni.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Holik AZ, Su S, Jansz N, Chen K, Leong HS, Blewitt ME, Asselin-Labat M-L, Smyth GK, Ritchie ME. 2015. Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res 43:e97–e97. doi: 10.1093/nar/gkv412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Brouwer C. 2013. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29:1830–1831. doi: 10.1093/bioinformatics/btt285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. 2009. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10:161. doi: 10.1186/1471-2105-10-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko DA, Danko K, Larionova EE, Shavva VS, Kudriavtsev IV, Nekrasova EV, Burnusuz AV, Gorbunov NP, Trofimov AV, Zhakhov AV, Ivanov IA, Orlov SV. 2022. Differentiation of human macrophages with anaphylatoxin C3a impairs alternative M2 polarization and decreases lipopolysaccharide-induced cytokine secretion. Immunol Cell Biol 100:186–204. doi: 10.1111/imcb.12534 [DOI] [PubMed] [Google Scholar]
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Stabell Benn C, Sun JC, Xavier RJ, Latz E. 2020. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20:375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano MB, Zhou H, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CTN. 2009. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation 119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14:417–419. doi: 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkarinen PT, Heikkilä N, Kisand K, Peterson P, Botto M, Daha MR, Drouet C, Isaac L, Helminen M, Haahtela T, Meri S, Jarva H, Arstila TP. 2015. Dysregulation of adaptive immune responses in complement C3-deficient patients. Eur J Immunol 45:915–921. doi: 10.1002/eji.201444948 [DOI] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg B-J, Wijmenga C, Joosten LAB, Xavier RJ, van der Meer JWM, Stunnenberg HG, Netea MG. 2012. Candida albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host & Microbe 12:223–232. doi: 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research 43:e47–e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR : a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JWM, Joosten LAB, Wijmenga C, Martens JHA, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086. doi: 10.1126/science.1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu SK, Kulkarni DH, Ozanturk AN, Ma L, Kulkarni HS. 2022. Emerging roles of the complement system in host-pathogen interactions. Trends Microbiol 30:390–402. doi: 10.1016/j.tim.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu SK, Ozantürk AN, Kulkarni DH, Ma L, Barve RA, Dannull L, Lu A, Starick M, McPhatter J, Garnica L, Sanfillipo-Burchman M, Kunen J, Wu X, Gelman AE, Brody SL, Atkinson JP, Kulkarni HS. 2023. Lung epithelial cell-derived C3 protects against pneumonia-induced lung injury. Sci Immunol 8:eabp9547. doi: 10.1126/sciimmunol.abp9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercan H, Riksen NP, Joosten LAB, Netea MG, Bekkering S. 2021. Trained Immunity: Long-Term Adaptation in Innate Immune Responses. Arterioscler Thromb Vasc Biol 41:55–61. doi: 10.1161/ATVBAHA.120.314212 [DOI] [PubMed] [Google Scholar]
- Wang L, Wang S, Li W. 2012. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28:2184–2185. doi: 10.1093/bioinformatics/bts356 [DOI] [PubMed] [Google Scholar]
- Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM, Jeon M, Ma’ayan A. 2021. Gene Set Knowledge Discovery with Enrichr. Current Protocols 1:e90. doi: 10.1002/cpz1.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y. 2012. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A Journal of Integrative Biology 16:284–287. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka S, Starkl P, Watzenboeck ML, Farhat A, Radhouani M, Deckert F, Hladik A, Lakovits K, Oberndorfer F, Lassnig C, Strobl B, Klavins K, Matsushita M, Sanin DE, Grzes KM, Pearce EJ, Gorki A-D, Knapp S. 2022. Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunology 15:896–907. doi: 10.1038/s41385-022-00528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Guo Y, Sheng Q, Shyr Y. 2014. Advanced Heat Map and Clustering Analysis Using Heatmap3. BioMed Research International 2014:1–6. doi: 10.1155/2014/986048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data have been deposited in GEO under the accession code GSE281001 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE281001.