Abstract
CRISPR-Cas genome editing tools enable precise, RNA-guided modification of genomes within living cells. The most clinically advanced genome editors are Cas9 nucleases, but many nuclease technologies provide only limited control over genome editing outcomes. Adenine base editors (ABEs) and cytosine base editors (CBEs) enable precise and efficient nucleotide conversions of A:T-to-G:C and C:G-to-T:A base pairs, respectively. Therapeutic use of base editors (BEs) provides an avenue to correct approximately 30% of human pathogenic variants. Nonetheless, factors such as protospacer adjacent motif (PAM) availability, accuracy, product purity, and delivery limit the full therapeutic potential of BEs. We previously developed Nme2Cas9 and its BE derivatives, including ABEs compatible with single adeno-associated virus (AAV) vector delivery, in part to enable editing near N4CC PAMs. Further engineering yielded domain-inlaid BEs with enhanced activity, as well as Nme2Cas9/SmuCas9 chimeras that target single-cytidine (N4C) PAMs. Here we further enhance Nme2Cas9 and Nme2SmuCas9 editing effectors for improved efficiency and vector compatibility through site-directed mutagenesis and deaminase linker optimization. Finally, we define the editing and specificity profiles of the resulting variants by using paired guide-target libraries.
Introduction
By enabling the alteration and correction of genes that contribute to disease, CRISPR-Cas genome editing tools are quickly advancing in the field of genetic medicine 1,2. Emerging tools have provided unparalleled control over the types of genetic manipulations that are possible 3. Base editors (BEs), composed of a nickase nCas9 fused to a nucleotide deaminase, are precision genome editors that enable single-nucleotide conversions 4–6. The most commonly used classes of BEs include adenine BEs (ABEs) and cytidine BEs (CBEs) that enable A:T-to-G:C or C:G-to-T:A base pair conversions, respectively 3. In principle, these two classes of BEs can together correct a large majority of disease-causing single nucleotide polymorphisms 3.
Despite the promise of genome editors to correct diseases at their root causes, in many cases in vivo delivery represents a major hurdle for the application of BEs and other editing systems 7,8,8. Adeno-associated virus (AAV) vectors are valuable delivery vehicles for genetic cargo to extra-hepatic tissues but are constrained by packaging capacity, among other limitations. Commonly used Cas9 variants such as SpyCas9 are large enough to greatly complicate AAV delivery, especially when used in combination with deaminases 4–6,8 or other fusion domains. The use of compact Cas9s such as Nme2Cas9 for nuclease 9 and BE 10,11 systems enhance the utility of gene editing tools from a variety of standpoints, including: (1) compact size, streamlining in vivo delivery through the use of single AAV vectors; (2) a cytidine dinucleotide PAM requirement, enabling access to pyrimidine-rich genome targets, and (3) editing specificity. Despite these benefits, initial Nme2Cas9-derived ABE systems exhibited modest editing efficiencies 10,11. Although the N4CC dinucleotide PAM provided additional flexibility when compared to other natural compact Cas9 variants 12–15, the PAM still restricts accessible BE editing windows.
Our initial efforts to improve the activity and targeting scope of Nme2Cas9 editing systems focused on BE applications 16. These efforts included internal rather than terminal deaminase domain insertion, which improved editing efficiencies while providing additional control over editing windows. We also developed Nme2SmuCas9 ABE systems in which the single-cytidine PAM-interacting domain (PID) of SmuCas9 17 was transplanted into Nme2Cas9 to enable targeting of N4C PAMs 16. Independently, phage evolution also yielded enhanced Nme2 variants (the eNme2-C ABE and the eNme2-C.NR nuclease) with N4C PAM compatibility 18. Despite these advances, when targeting sites with an N4CC PAM, Nme2Smu-ABEs suffered from diminished activity when compared to their WT PID counterparts. Other groups developing their own Nme2SmuCas9 systems for nuclease and BE applications have reported similar findings 19,20. Interestingly, this decrease in activity at target sites with canonical PAMs is a common trend observed among PAM-relaxed Cas9 variants 21–25. Although our use of the transplanted PID from SmuCas9 increased PAM targetability, for ABE applications it came with a modest increase in size, potentially affecting single-vector AAV delivery with our format. Here we have further optimized domain-inlaid Nme2SmuCas9 ABE systems to improve activity and minimize size for AAV deliverability. We also show that our editing activity improvements extend to Nme2SmuCas9 nuclease as well as Nme2Cas9 systems with native PAM specificities.
Results
Rational design of Nme2SmuCas9 nuclease and base editing mutants with increased activity
Nme2SmuCas9’s subpar activities at N4CC targets sites prompted us to improve its activities through rational engineering. First, to recapitulate these findings, we compared Nme2Cas9 and Nme2SmuCas9 editors in either their nuclease format or the domain-inlaid ABE8e -i1 architecture using a small subset of N4CC target sites (Supplementary Fig. 1a, b). Following plasmid transfection in HEK293T cells across three N4CC target sites, we observed a 2.2- and 2-fold decrease in editing efficiencies for the nuclease and ABE8e-i1 Nme2SmuCas9 editors vs. their WT PID counterparts, respectively.
The enhancement of non-specific interactions between Cas effectors and the target nucleic acid is a common strategy employed to improve the activities of various genome editing reagents 21,24,26,27. We hypothesized that introducing arginine residues in proximity to either the DNA heteroduplex [target strand (TS) or non-target strand (NTS)] or the sgRNA could improve Nme2SmuCas9 editing activity at N4CC and potentially N4CD (D = not C) target sites. To test this hypothesis, we selected candidate Nme2SmuCas9 residues within 5–10 angstroms of guides or substrates using our previously generated SWISS homology model (Supplementary Fig. 1c; Supplementary Table 1). In addition to the Arg mutants, we also included a T72Y substitution analogous to the L58Y substitution of enCjeCas9 and reported to enhance base stacking interactions with the sgRNA 27. T72Y is located in the bridge helix and situated within the core of the Nme2Cas9/sgRNA/DNA ternary structure 28, likely forming a base-stacking interaction with U64 of the sgRNA. We incorporated a total of 33 single mutations into plasmids encoding the domain-inlaid Nme2Smu-ABE8e-i1 effector, and transfected them along with sgRNA-expressing plasmids into an established HEK293T ABE mCherry reporter cell line 11,29.
Among the TS-DNA interacting mutants, the E520R and D873R mutations substantially outperformed Nme2Smu-ABE8e-i1 (WT), increasing activation of the fluorescent reporter (Supplementary Fig. 2a). In addition, the panel of sgRNA and NTS-DNA proximity mutants produced eight variants with higher editing activities (Supplementary Fig. 2a). Mutations of note include the E932R, D56R and D1048R substitutions. Following our initial experiment, we re-evaluated the top two performing mutants from the TS-DNA panel and top eight variants from the sgRNA and NTS-DNA panel at additional N4CD PAM target sites within the ABE mCherry reporter (Supplementary Fig. 2b). In agreement with our pilot experiments, all mutations outperformed wildtype Nme2Smu-ABE8e-i1 (WT).
Encouraged by these results, we tested whether these mutations would increase the nuclease activity of Nme2SmuCas9. We cloned the E520R and D873R substitutions, in addition to the entire panel of sgRNA and NTS-DNA mutants, into plasmids encoding Nme2SmuCas9 nuclease. Subsequently, we transfected the panel of mutants along with sgRNA-expressing plasmids targeting the integrated traffic light reporter TLR-MCV1 in HEK293T cells 30 to report on DSB activity. For this assay, we screened editing activities at four target sites, varying the nucleotide in the 6th PAM position to cover all N4CN PAMs targetable by the SmuCas9 PID. As an additional benchmark of activity, we included Nme2Cas9 nuclease with the WT PID. As expected, Nme2Cas9 outperformed Nme2SmuCas9 at the N4CC PAM target site (Supplementary Fig. 2c).
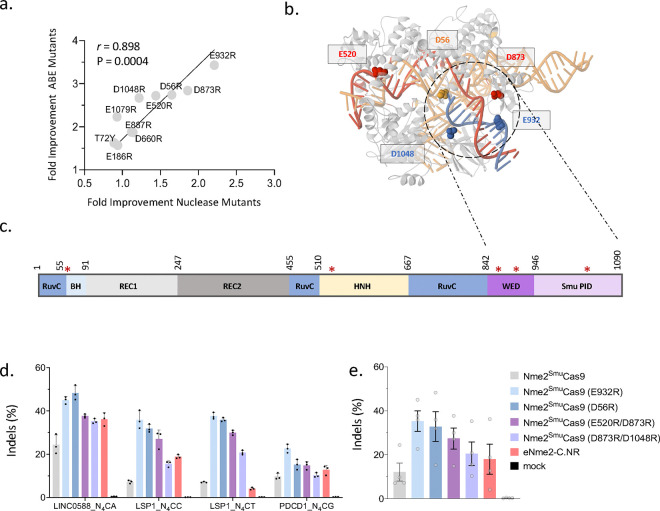
Encouragingly, the top-performing variants identified in our initial ABE8e screens also enhanced nuclease Nme2SmuCas9 performance across all tested target sites, displaying a positive correlation in activity increase compared to the WT effector (Pearson r = 0.898) (Fig. 1a). Subsequently, we combined the top five mutations identified from our preliminary screens: E932R, D873R, D56R, E520R and D1048R. Intriguingly, 4 out of the 5 mutants spatially clustered around the PAM DNA duplex, while E520R was located closer to the PAM-distal end of the TS-DNA (Fig. 1b, 1c). We then screened the Nme2SmuCas9 combinatorial variants in nuclease format. We anticipated that the enhancements that improved DSB activity would be most likely to translate to our domain-inlaid BE architectures. We repeated the TLR-MCV experiments for nuclease activity with 31 out of the 32 possible Nme2SmuCas9 permutations including WT (Supplementary Fig. 3). As additional activity benchmarks, we included Nme2Cas9 (WT) and the nuclease eNme2-C.NR. To enable controlled comparisons, eNme2-C.NR was either assessed with the originally described NLS-linker architecture (eNme2-C.NR vLiu)18 or with an NLS-linker architecture identical to that of our Nme2SmuCas9 plasmids (eNme2-C.NR vEJS). As previously demonstrated, Nme2Cas9 performed best at the N4CC target site, with background levels of activity at the N4CD target sites (Supplementary Fig. 3). In contrast, eNme2-C.NR and Nme2SmuCas9 derivatives performed well at all N4CN PAM target sites. Notably, at N4CD PAM target sites, we observed similar TLR-MCV activation when comparing Nme2SmuCas9 (WT) and eNme2-C.NR (Supplementary Fig. 3). Lastly, 23 out of the 30 Nme2SmuCas9 combination mutants performed comparably to or better than their WT counterpart. The top performers in the first quartile consisted of single and double mutants (Supplementary Fig. 3).
Figure 1. Arginine mutagenesis improves Nme2SmuCas9 nuclease and BE activity.
(a) Fold improvement of top 10 single-arginine mutants for Nme2SmuCas9 nuclease and Nme2Smu-ABE8e-i1 vs. WT variants. Data represent mean activities of four guide RNAs activating either the TLR-MCV reporter (nuclease activity) or mCherry ABE reporter (ABE activity) at N4CC, N4CT, N4CA or N4CG PAM targets. Editing efficiencies were measured by flow cytometry (n = 2–3 biological replicates per guide; compiled from Supplementary Figures 2a-2c). r = Pearson correlation. (b) Homology model of Nme2SmuCas9/sgRNA/DNA ternary structure and (c) linear domain organization (not drawn to scale) depicting the relative positions of the top 5 arginine mutants (red asterisks) within Nme2SmuCas9. (d) Nuclease editing by Nme2SmuCas9 (WT and E932R, D56R, E520R/D873R, and D873R/D1048R variants) and eNme2-C.NR at endogenous HEK293T genomic loci. The editing efficiency for each target was plotted. Editing efficiencies were measured by amplicon deep sequencing (n = 3 biological replicates; data represent mean ± SD). (b) Data from (a) were aggregated and replotted, with each data point representing the editing efficiency of an individual target site, as measured by amplicon deep sequencing (n = 3 biological replicates; data represent mean ± SEM).
We next selected four single or double Nme2SmuCas9 mutants exhibiting increased activity for additional characterization: E932R, E520R/D873R, D873R/D1048R, and D56R. As an initial test, we measured editing efficiencies across endogenous targets within HEK293T cells. Plasmids encoding the Nme2SmuCas9 variants and eNme2-C.NR were transfected along with sgRNA into HEK293T cells (Fig. 1d, 1e). In agreement with our screening assays, all Nme2SmuCas9 mutants were more active than the WT nuclease. Furthermore, the E932R and D56R mutants outperformed eNme2-C.NR across all target sites (Fig. 1d, 1e).
Activities of Nme2- and Nme2SmuCas9 and nuclease variants
To better elucidate the activities of the enhanced Nme2SmuCas9 mutants, we assessed editing at a battery of target sites in the context of a self-targeting library. We previously generated HEK293T cells with an integrated 200-member library expressing Nme2Cas9 sgRNAs targeted to adjacent N4CN PAM sites 16. In this experiment, we analyzed indel activities at 173 target sites, following plasmid transfection and antibiotic selection of cells expressing Nme2SmuCas9 (WT, E932R, D56R and D873R/D1048R) variants, Nme2Cas9 (WT) or eNme2-C.NR. Four days post-transfection, we collected cells and measured editing activities by amplicon sequencing. Across the entire panel of N4CN PAM targets, the activity ranks determined by mean indel production tracked consistently with our endogenous target site data: Nme2SmuCas9 (E932R), 12.58% > Nme2SmuCas9 (D56R), 12.48% > Nme2SmuCas9 (WT), 7.78% > Nme2SmuCas9 (D873R/D1048R), 6.76% > eNme2-C.NR, 5.55% > Nme2Cas9 (WT), 1.52% (Supplementary Fig. 4). Across the N4CD PAM target sites, the trends remained the same with Nme2SmuCas9 (E932R) and (D56R) variants respectively ranking first and second for activity. In contrast, across N4CC PAM target sites, Nme2Cas9 (WT) and eNme2-C.NR ranked 3rd and 4th for activity respectively, outperforming Nme2SmuCas9 (WT and D873R/D1048R) (Supplementary Fig. 4). Overall, the Nme2SmuCas9 (E932R) and (D56R) variants both demonstrated ~1.6-fold increase in activity compared to their otherwise WT counterpart.
We considered whether the increases in activity observed with the D56R and E932R mutations would carry over to Nme2Cas9 (WT) 9. To test this hypothesis, we measured efficiencies of indel formation using the HEK293T activity library across 191 N4CN PAM target sites for Nme2- and Nme2SmuCas9 variants (WT, E932R or D56R) in addition to eNme2-C.NR (Fig. 2a). As expected, eNme2-C and the Nme2Smu PID-swapped variants demonstrated the highest editing efficiencies at N4CD PAM target sites, while Nme2Cas9 variants exhibited minimal activities at these PAMs (Fig. 2a). Conversely, across target sites bearing canonical N4CC PAMs, Nme2Cas9 variants exhibited mean editing efficiencies of 3.47% (WT), 9.2% (E932R) and 7.05% (D56R), respectively, yielding a 2.6- and 2-fold increase in activity in comparison to the WT protein (Fig. 2a). When comparing Nme2Cas9 vs. Nme2SmuCas9 variants with the same background mutation, the WT PID effector still performed best at N4CC PAM target sites (Fig. 2a). These results indicate that the enhancing mutations improve the editing activity of Nme2SmuCas9 and extend to Nme2Cas9, enabling enhanced genome editing potential at a range of single- and double-cytidine PAM target sites.
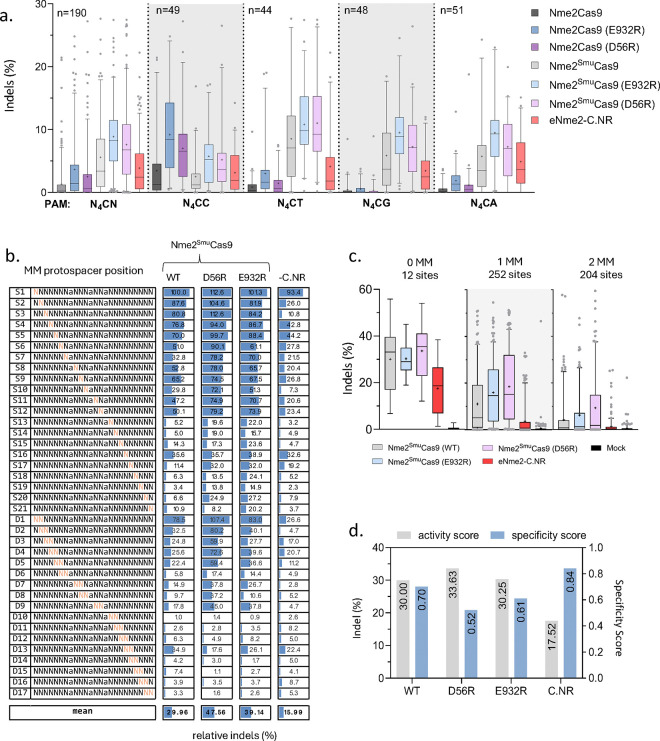
Figure 2. Activities and specificities of Nme2- and Nme2SmuCas9 nuclease variants.
(a) Nuclease-induced indels in the nuclease experimental panel 2 of the guide-target activity library following plasmid transfection of Nme2Cas9 (WT and E932R, D56R, E520R/D873R variants), Nme2SmuCas9 (WT and E932R, D56R, and E520R/D873R variants) or eNme2-C.NR into HEK293T cells with integrated guide-target sites harboring N4CN PAMs. The editing efficiencies for 190 target sites were plotted. (b) Average indel frequencies of Nme2SmuCas9 or eNme2-C.NR across single- (S) or di-nucleotide (D) mismatched target sites within the guide-target mismatch library. Activities for each mismatched target were normalized to the activity of their respective perfectly matched target site. Orange nucleotides represent the protospacer position(s) of the transversion mutation(s) present within the mismatched target site. (c) Bulk indel frequencies of the nuclease variants within the mismatch library for 12 perfectly matched target sites (0 MM), 252 single-mismatched target sites (1 MM), or 204 double-mismatched target sites (2 MM). (d) Indel vs. specificity scores for Nme2SmuCas9 variants and eNme2-C.NR across the mismatched guide-target library. Indel efficiency was compiled from data for the 12 perfectly matched target sites (0 MM) in (c). The specificity score was calculated as one minus the tiled mismatched editing mean in (b), normalized to a scale of one to 100. Data were measured by amplicon sequencing (n = 3 biological replicates; boxplots represent median and interquartile ranges; whiskers indicate 5th and 95th percentiles and the cross represents the mean).
Specificities of Nme2- and Nme2SmuCas9 and nuclease variants
Following the characterization of nuclease activity, we next examined the specificities of the enhanced Nme2Cas9 and Nme2SmuCas9 variants. For this experiment, we developed a library to assess the mismatch tolerance profile of Nme2Cas9 variants at varied positions across its protospacer (Fig. 2b). The library consisted of 12 guide RNAs, paired with either a perfectly matched protospacer target or mismatched targets. Each of the 12 guide RNAs were paired with a total of 40 target sites (two identical perfectly-matched targets, 21 single-nucleotide mismatches, and 17 dinucleotide mismatches), for a total of 480 library members (Fig. 2b). To mitigate potential PAM-specific effector preferences, three guides per possible N4CN PAM sequences were used. In anticipation of using the library for ABE specificity experiments, we incorporated adenines into protospacer positions A8, A12 and A15 and held them constant, as they fall within the editing windows of previously validated Nme2Cas9 derived base editors 16,18,20.
After Tol2 transposon-mediated integration of the mismatch library into HEK293T cells 16,31, we assessed the mismatch tolerance profiles of Nme2Cas9, Nme2SmuCas9, their derivatives, and the eNme2-C.NR nuclease. Following nuclease plasmid transfection and selection, we measured editing via deep sequencing at an average depth of ~2,300 reads per library member (Fig. 2b). At the 12 perfectly matched N4CN PAM target sites, the Nme2SmuCas9 variants performed comparably, with mean indel formation ~30%, while eNme2-C.NR was slightly less efficient, inducing ~17% indels on average (Fig. 2c). Similar trends were observed at the three N4CC target sites, with Nme2- and Nme2SmuCas9 arginine mutants (E932R and D56R) performing comparably to or better than their WT counterparts. eNme2-C.NR again had the lowest rates of indel formation for the perfectly matched N4CC PAM target sites. In line with previous reports for Nme2Cas9 and other Cas9 effectors, single and double mismatches within the protospacer decreased editing efficiencies 9,22, with double mismatches having a more pronounced effect than single mismatches (Fig. 2b, 2c, Supplementary Fig. 5a), as expected.
Next, we turned our attention to relative efficiencies of indel formation at mismatched targets (compared to their perfectly matched counterparts) across protospacer positions. In agreement with previous reports 9,22, all nucleases tested had general trends for increased tolerance to mismatches at the PAM-distal ends of the protospacer, and decreased mismatch tolerance within the PAM-proximal or seed regions of the protospacer (Fig. 2b, Supplementary Fig. 5a). Using the mismatch library data, we calculated nuclease specificity scores that enable comparisons between the activity and specificity of the respective editors (Fig. 2d, Supplementary Fig. 5b). As expected, we observed a negative correlation between indel efficiency and specificity. For example, eNme2-C.NR was the most sensitive to mismatches with the highest specificity score (~0.8) and the lowest indel efficiency (~18%), whereas Nme2SmuCas9 (D56R) had the lowest specificity score (0.52) and the highest mean indel efficiency (~34%) (Fig. 2d, Supplementary Fig. 5b).
Optimizing linker lengths for domain-inlaid Nme2Smu-ABEs
In our initial development of domain-inlaid Nme2Cas9 BEs 16, the larger SmuCas9 PID added 24 bp [8 amino acids (AA)] to the length of the ORF. Consequently, while WT PID Nme2-ABEs could be packaged within a single AAV vector, our design using the 251bp U1a promoter and Nme2SmuCas9-ABEs exceeds the ~5kb packaging limit of AAV (Fig. 3a). Although the use of alternative short promoters, such as EF-1α short (EFS, 212bp) 10 could enable packaging of these larger ABEs, we decided to augment this strategy with a more generalized approach to minimize the overall size of the editing reagent. In our initial design of the domain-inlaid Nme2-ABEs, we introduced 20AA linkers between the N- and C-termini of the inserted deaminase domain and the nickase Cas9 (Fig. 3b). Previous research has demonstrated that optimization of linker property and length is important for engineering Cas9 fusion proteins 4,5,32,33. We anticipated that truncation of the N- and C-terminal linkers flanking the inlaid deaminase domains would enable a reduction in overall protein size to facilitate single-vector AAV packaging (Fig. 3a, 3b).
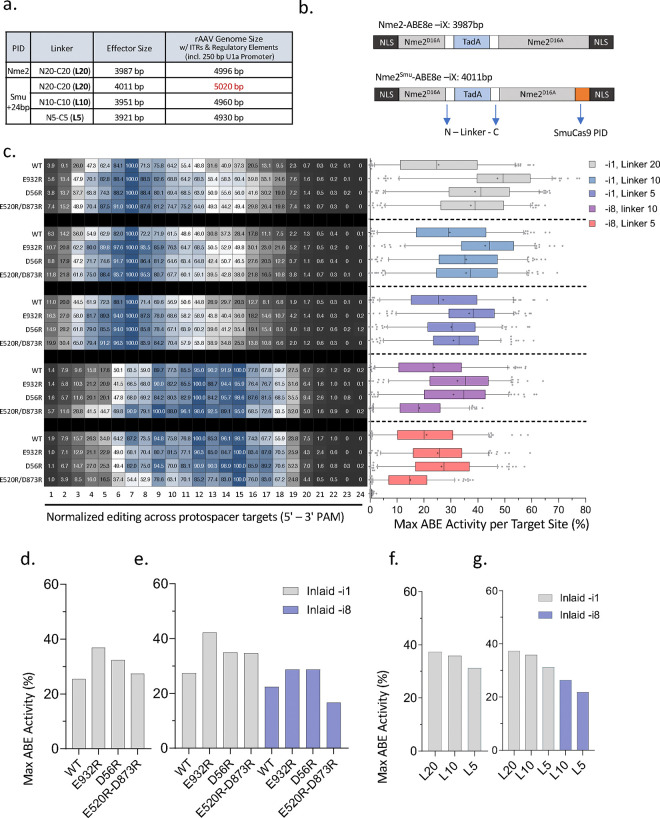
Figure 3. Editing windows and activities of domain-inlaid Nme2Smu-ABE8e variants.
(a) Table depicting rAAV genome size in bp for respective domain-inlaid editors with linker variants and associated regulatory elements (right). Regulatory and coding elements for a representative all-in-one AAV vector include ITRs, U1a promoter, ABE8e editor, U6 promoter, and sgRNA cassette. Cartoon schematic depicting open reading frame length (in bp) of domain-inlaid Nme2-ABE8e with Nme2Cas9 PID (left, top) or Nme2Smu-ABE8e with the SmuCas9 PID (left, bottom) with 20AA linkers flanking the N- and C-termini of Tad8e. (c) Assessment of editing windows and activities from ABE experimental panel 1 of the guide-target activity library (183 sites) for Nme2Smu-ABE8e –i1 or –i8, with or without arginine mutations (E932R, D56R, E520R/D873R), in combination with varied deaminase linker lengths (L20, L10, L5). Following plasmid transfection of the ABE variants into HEK293T cells with the integrated guide-target library, editing activities were measured by amplicon sequencing. Left: average editing windows across the target sites, normalized on a scale of 0 – 100 (%) normalized to the adenine position with the highest observed edited efficiencies within the window. Right: editing activities at the maximally edited adenine for each target were plotted (n = 3 biological replicates; boxplots represent median and interquartile ranges; whiskers indicate 5th and 95th percentiles and the cross represents the mean). (d-g) Summary data from self-targeting library maximal activity, aggregated from (a). (d) Nme2Smu-ABE8e and arginine mutant activity independent of domain insertion site and linker length, or (e) separated by the position of domain insertion. (f) Nme2Smu-ABE8e and linker variant activity independent of domain insertion site and arginine mutation, or (g) separated by the position of domain insertion.
To probe the effects of linker minimization, we designed and cloned a panel of domain-inlaid Nme2Smu-ABE8e (WT) plasmids in -i1 or -i8 formats with combinations of N- or C-terminal linker lengths (Nx - Cx) ranging from 20 to 5 AAs in 5AA steps (Supplementary Fig. 6). In addition to the 8 combinations of Nx – Cx linkers ranging from 20 to 5 AA, we included a N0 - C0 linker variant as an extra test subject and the N20-C20 variant as a benchmark. Following testing in HEK293T cells at four endogenous target sites, we observed that most of the linker variants performed comparably to the original N20-C20 linker for both -i1 and -i8 domain-inlaid ABE8e formats (Supplementary Fig. 6). With marginal impact on the editing activity at the tested targets, linker minimization of domain-inlaid Nme2Smu-ABEs will facilitate single-vector AAV packaging.
To more closely examine size-minimized Nme2Smu-ABE effectors, we combined the N10-C10 (L10) and N5-C5 (L5) linker variants with the top-performing enhancing mutations (E932R, D56R, E520R/D873R and D873R/D1048R). Using the same endogenous target sites from our nuclease test, we transfected plasmids encoding combinations of the Nme2Smu-ABE8e -i1 or -i8, the L5 or L10 linkers, and the Arg mutants for initial activity testing (Supplementary Fig. 7a, 7b). In addition to our domain-inlaid variants, eNme2-C with its original NLS and linker architecture was used for comparison 18. Across the four target sites, we observed that all combinations of Tad8e insertion sites, linkers, and mutations were functional with varied rates of activity depending on the combination (Supplementary Fig. 7a, 7b). When compared to eNme2-C, the domain-inlaid editors performed comparably across the four target sites on average; however, there were target site-specific differences in activity (Supplementary Fig. 7a, 7b). Overall, independent of the site of deaminase insertion or linker length, the E932R and D56R mutations ranked first and second with 1.7- and 1.4-fold activity increases, respectively, compared with their non-mutated counterparts (Supplementary Fig. 7c). When reassessing the data for each deaminase insertion site and arginine mutation, independent of linker length, we observed differences in the effect of different effector mutants. For example, in the domain-inlaid -i1 format, E932R and the E520R/D873R mutations ranked first and second for activity, respectively, while in the -i8 format the top two performers were the E932R and D56R mutations (Supplementary Fig. 7d).
Evaluating size-optimized and activity-enhanced domain-inlaid Nme2Smu-ABE8e effectors
We next used the guide-target library to investigate the impact of arginine mutation and shortened linkers on the editing windows and activities of domain-inlaid Nme2Smu-ABE8e variants. For this experiment we assessed the editing efficiencies for a range of Nme2Smu-ABE8e variants across 183 target sites within the library. The effector variants included in this panel consisted of a combination of the following domain-inlaid Nme2Smu-ABE8e formats: -i1 or -i8 inlaid architecture, WT or arginine mutants (E932R, D56R, or E520R/D873R), and deaminase linker lengths (L20, L10, L5). For WT Nme2Smu-ABE8e editors not bearing arginine mutations, linker variation had minimal impact on the editing window (positions exhibiting activity >50% of the maximum editing efficiency of any adenine within the window) (Fig. 3c). A small increase in the mean maximum observed activity were observed for these WT linker variants. For example, Nme2Smu-ABE8e-i1 (WT) with L20, L10 or L5 linkers had editing windows spanning protospacer nucleotide (nt) positions 4–11, +/−1 nt. Simultaneously, the mean maximum activity marginally increased with L10 performing the best (~29%) and L20 the worst (~25%) (Fig. 3c). In contrast, comparison of the truncated linkers with the same arginine mutant background slightly reduced activity. For instance, linker variants L20, L10 and L5 of Nme2Smu-ABE8e-i1 (E932R) had mean maximum activities of approximately 47%, 43% and 37% respectively (Fig. 3c). A similar trend was also observed with the -i8 architecture in the background of the E932R and D56R mutations (Fig. 3c). Despite this, arginine mutations generally had positive effects on domain-inlaid Nme2Smu-ABE8e’s for both the -i1 and -i8 architectures. The effects of the enhancing mutations followed a consistent trend for both the editing window and activity. For example, across the inlaid -i1 linker variants, the E932R mutation led to a widened window accompanied by a considerable increase in activity (+4nt window and 1.9-fold activity in the -i1, L20 format) (Fig. 3c). Installation of D56R and E520R/D873R also led to editing window and activity increases for Nme2Smu-ABE8e-i1 editors, with these changes being less pronounced (Fig. 3c). In the -i8 architecture, both the E932R and D56R improved activity ~1.4-fold compared to the WT effector. By contrast, the D520/E873R double mutant performed poorly in the -i8 architecture, exhibiting comparable or lower activity than the WT counterpart (Fig. 3c).
Overall, within the library editing experiments, the E932R and D56R mutations resulted in the highest increases in activities (~1.5- and 1.3-fold respectively) when assessing editing outcomes independent of the deaminase insertion site or linker length (Fig. 3d). Importantly, although the E520R/D873R double mutant in the -i1 architecture performed comparably to the D56R substitution, it performed poorly for the -i8 architecture independent of the linker used (Fig. 3e).
In summary, the installation of arginine mutants in the domain-inlaid Nme2Smu-ABE8e variants enhanced their activities at endogenous target sites and in the context of guide-target libraries. Additionally, when considering linker length for applications without cargo size limits, the L20 variants are likely the variant of choice (Fig. 3c, 3f, 3g) based upon their marginally increased activities. Nevertheless, when applying domain-inlaid Nme2Smu-ABEs in size-constrained applications, the minimized linkers provide additional flexibility, with minimal losses in overall activity (Fig. 3f, 3g).
Specificities of size-optimized and activity-enhanced domain-inlaid Nme2Smu-ABE8e effectors
We repeated the mismatch library experiments to determine the specificities of the size- and activity-optimized domain-inlaid Nme2-ABE8e variants. The effector variants included a combination of the following domain-inlaid formats: Nme2- or Nme2SmuCas9, -i1 or -i8 inlaid architecture (with L10 linkers), WT or arginine mutants (E932R and D56R), and eNme2-C as an additional benchmark.
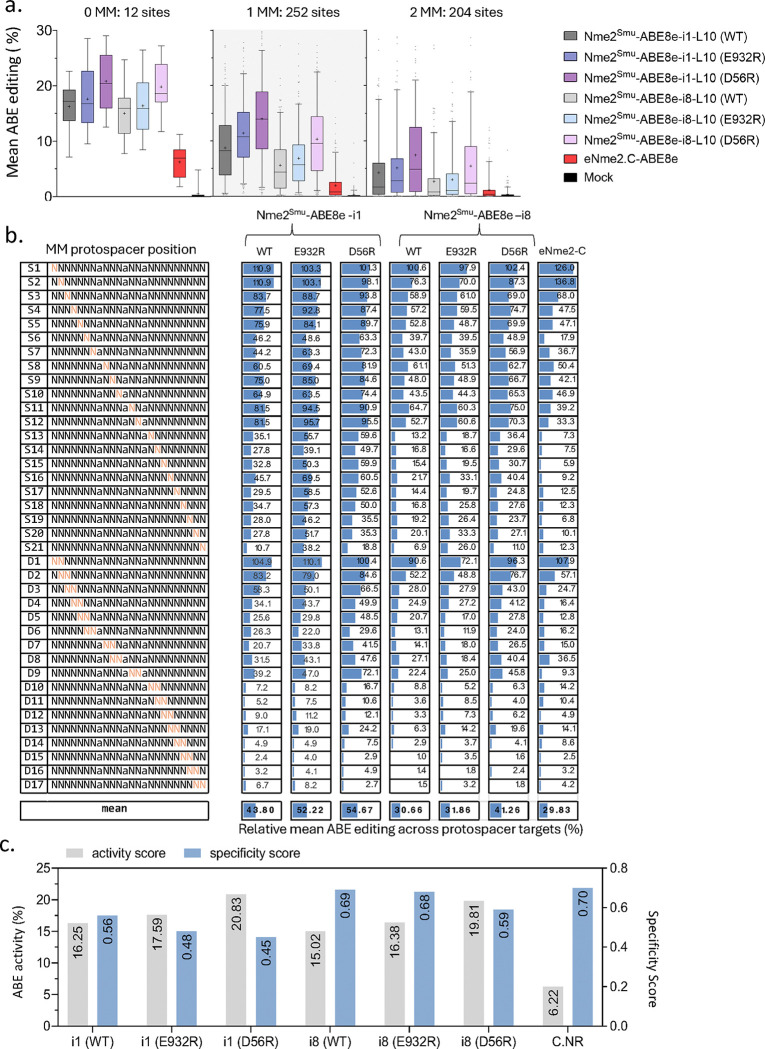
At the 12 perfectly matched N4CN target sites, base editors with arginine mutations outperformed their WT counterparts. Inlaid architectures with the -i1 format performed better than those in the -i8 format, and all domain-inlaid Nme2Smu-ABE8e variants induced higher editing efficiencies than eNme2-C (Fig. 4a). Similar to the nuclease panel, we observed an inverse correlation with editing efficiency when more mismatches are present, and a similar trend of decreased mismatch tolerance within the seed region (protospacer positions 15–24 counting from the 5’ end of the guide (Fig. 4b). Finally, the rankings of specificity scores were also similar to those of the nucleases; eNme2-C exhibited the best specificity and worst editing activity, whereas Nme2Smu-ABE8e (D56R) mutants in the -i1 and -i8 architectures had the best activities and worst specificity scores (Fig. 4b). Another trend we observed was the unusually high specificity of the -i8 inlaid variants in comparison to the -i1 inlaid variants (Fig. 4b). In some cases, -i8 editors exhibited some of the best activities across the 12 perfectly matched target sites with specificity scores (~0.7), approaching that of eNme2-C (which had the lowest overall ABE activity) (Fig. 4c). Assessment of Nme2- and Nme2Smu-ABE8e variants with only the N4CC targeting guides within the library revealed similar trends to the entire N4CN mismatch library (Supplementary Fig. 8a). However, Nme2Cas9 variants were generally more effective than Nme2Smu variants at the three N4CC perfectly matched targets, though with similar or better specificity scores (Supplementary Fig. 8b).
Figure 4. Specificities of domain-inlaid Nme2Smu-ABE8e variants.
(a) Mean A-to-G editing efficiency across the targets within the mismatch library for domain-inlaid Nme2Smu-ABE8e variants or eNme2-C. Data was separated by the number of mismatches between guide and target site: 12 perfectly matched sites (0 MM), 252 single-mismatched sites (1 MM), and 204 double-mismatched sites (2 MM). Each data point represents the average A-to-G editing observed across a protospacer of an individual library member. (b) Mean A-to-G editing frequencies of domain-inlaid Nme2Smu-ABE8e variants or eNme2-C across single- (S) or di-nucleotide (D) mismatched target sites within the guide-target mismatch library. Activities for each mismatched target were normalized to the mean efficiency of their respective perfectly matched target site. Orange nucleotides represent the protospacer position(s) of the transversion mutation(s) present within the mismatched target site. (d) ABE activity vs. specificity scores for base editing variants in (a-b) across the mismatched guide-target library. ABE activity was compiled from editing data for the perfectly matched target sites (0 MM) in (a). The specificity score was calculated as one minus the tiled mismatched editing mean in (b), normalized to a scale of one to 100. Data were measured by amplicon sequencing (n = 3 biological replicates; boxplots represent median and interquartile ranges; whiskers indicate 5th and 95th percentiles and the cross represents the mean).
Testing narrow-window deaminases with the domain-inlaid Nme2Smu-ABE architecture
Although we were able to improve the performance of domain-inlaid Nme2Smu-ABE8e, in cases where bystander editing is unavoidable, wide editing windows could become problematic. Engineered variants of TadA8e have been established that narrow the editing window by altering residues within or near the catalytic pocket of the deaminase 34,35. We tested both Tad9 34 and Tad9e 35 variants in combination with our compact Nme2Smu-ABE architectures using the self-targeting library. For this experimental panel, we assessed the editing windows and activities across 193 target sites (Supplementary Fig. 9). In our hands, the Tad9 34 variants failed to exhibit editing activity above background (data not shown). The other Nme2Smu-ABE variants tested included a combination of variants: Tad8e or Tad9e deaminase, -i1 or -i8 inlaid architecture, arginine mutants (E932R or D56R), and shortened deaminase linker lengths (L10, L5). Analysis of ABE editing outcomes for the Tad9e effectors confirmed that they have substantially smaller editing windows. This came at the cost of editing activity, a trend consistent with all formats tested (Supplementary Fig. 9). For example, the editing window and activity of Nme2Smu-ABE8e (L10, E932R), spanned nucleotides 3 – 15 with mean maximum activity of ~30% (Supplementary Fig. 9). In contrast, the Nme2Smu-ABE9e (L10, E932R), exhibited an editing window across positions 5 −10, with ~15% maximum activity (Supplementary Fig. 9). Likewise, Nme2Smu-ABE9e (-i8, L10, E932R) exhibited a narrower window (pos. 10 −18) than its ABE8e counterpart, though with a further shift to editing PAM-proximal nucleotides (Supplementary Fig. 9). These results demonstrate that Tad9e is compatible with domain-inlaid Nme2Smu-ABEs and can increase their precision. Although ABE9e effectors exhibited reduced activity in the domain-inlaid format, in cases where bystander editing must be avoided, they represent a potentially valuable option in base editing reagent.
Assessment of arginine mutations with WT PID domain-inlaid Nme2-ABEs
Next, we wanted to determine whether the arginine mutations also enhance Nme2Cas9-derived ABEs that natively target an N4CC PAM, and how they compare to Nme2Smu chimeric effectors. In addition to the arginine mutations, we also tested whether Nme2Cas9-ABEs (WT PID) were compatible with the minimal linker format (L10). We first constructed plasmids expressing domain-inlaid Nme2-ABE8e effectors in the following formats: inlaid architecture (-i1 and -i8), the L10 linker, and the E932R or D56R mutants. After cloning, we used the self-targeting library to compare domain-inlaid Nme2-ABEs, Nme2Smu-ABEs, and eNme2-C (used as a benchmark) across 192 N4CN targets (Supplementary Fig. 10a).
We first assessed the editing windows and activities of the Nme2-ABE8e derivatives across the 49 canonical N4CC PAM target sites within the self-targeting library (Supplementary Fig. 10a). While editing windows were somewhat more variable among this subset of target sites, the assay adequately captured the previously described window of eNme2-C 18. For editing window analysis, we noticed that in most cases, arginine mutants led to an increase in the editing windows for both Nme2- and Nme2Smu-ABEs, a trend consistent with the previous experiments (Fig. 3a, Supplementary Fig. 10a). For example, the observed editing window for Nme2-ABE-i1 (WT) and the E932R variant ranged from positions 3–13 and 3–16 respectively (Supplementary Fig. 10a). Although the windows for Nme2-ABE-i8 (WT and E932R) didn’t change dramatically in size (spanning positions 8–17 and 9–18 respectively), the arginine mutants increased editing near window boundaries (Supplementary Fig. 10a). Again, when installing the arginine mutants, we observed higher editing activities associated with an increased window size. For illustration, the mean maximum activities of Nme2Cas9-ABEs with the inlaid -i1 format were approximately 21% (WT), 31% (E932R), and 26% (D56R) (Supplementary Fig. 10a). Similarly, Nme2Cas9 ABEs with the inlaid -i8 format had mean maximum activities of approximately 15% (WT), 27% (E932R), and 20% (D56R) (Supplementary Fig. 10a). Altogether, at N4CC PAM targets, the E932R and D56R mutants increased editing of the domain-inlaid Nme2-ABE8es over their non-mutant counterparts by 1.6- and 1.2-fold. Furthermore, at all N4CC PAMs, the average activities of Nme2-ABE8e effectors were higher than the PID-chimeric Nme2Smu-ABEs and eNme2-C.
We also assessed the editing activities of the inlaid Nme2-ABE8e effectors at N4CD PAM target sites. Similar to our nuclease experiments, installation of the E932R and D56R mutants increased the activities of inlaid Nme2-ABEs at non-canonical (N4CD) PAM target sites (Supplementary Fig. 10b). Intriguingly, although inlaid Nme2Smu-ABE8e effectors outperformed the Nme2Cas9 (WT PID) counterparts at all N4CD PAM targets, the Nme2Cas9-ABE-i1 (E932R) variant approached similar activity levels across N4CT targets, albeit with less consistency than the corresponding PID-swapped variant [Nme2 PID, (~20% ± SD of 14%) vs. Smu PID (~24% ± SD of 10%)] (Supplementary Fig. 10b). These results demonstrate that the enhancing mutations are also applicable to domain-inlaid Nme2-ABE8e effectors and potentially relax their PAM requirements.
Activities and windows of engineered Nme2Cas9 variants in various ABE8e formats
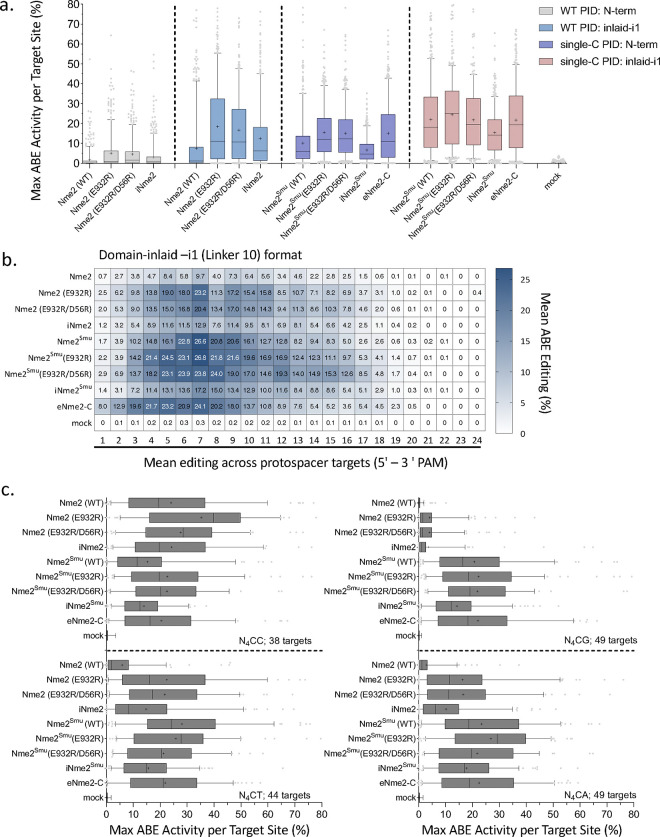
While this work was in progress, other groups reported on alternative engineering avenues to increase the efficiency and alter the PAM targeting scope of Nme2Cas9 editing systems towards N4CN PAM targets 18,36. We thus wanted to compare these PAM-relaxed nickase Cas9 domains for base editing applications at N4C PAM target sites. The Cas9 variants in this panel consisted of Nme2 (WT), Nme2Smu (WT), and their mutant derivatives (E932R, E932R/D56R), in addition to eNme2-C and a rationally engineered Nme2Cas9 variant with enhanced DNA unwinding capabilities iNme2 36. Since iNme2 had no mutations within the PID domain, we also cloned an iNme2Smu Cas9 derivative, to determine whether the alternate PID would further enhance efficiency at N4CD PAM target sites. In this experiment, we tested the engineered Cas9 base editing variants in either the N-terminal or domain-inlaid -i1 (linker L10) ABE8e architectures using the aforementioned activity library across 181 N4CN target sites (Fig. 5, Supplementary Figs. 11, 12).
Figure 5. Activities and editing windows of engineered Nme2Cas9 variants in various ABE8e formats.
Assessment of editing activities and windows from experimental panel 4 of the guide-target activity library (181 target sites) for Nme2-, Nme2Smu-, iNme2-, iNme2Smu-, and eNme2-C variants in either the N-terminally fused or inlaid-i1 (linker 10) format. (a) Efficiency at the maximally edited adenine for each target was plotted for all N4CN PAM target sites. ABEs with a WT Nme2Cas9 PID (WT PID) or N4CN targeting PID (single -C PID) are depicted by color. (b) Mean A-to-G editing activities and windows across protospacer positions in the activity guide-target library for engineered Nme2-ABE8e variants in the domain-inlaid -i1 (linker 10) format. (c) Data in (a) separated by target site PAM identity (N4CC, N4CT, N4CG, N4CA) for the engineered Nme2-ABE8e variants in the domain-inlaid -i1 (linker 10) format. The editing efficiency of the maximally edited adenine for each target was plotted. The number of target sites per PAM is indicated (n = 3 biological replicates; boxplots represent median and interquartile ranges; whiskers indicate 5th and 95th percentiles and the cross represents the mean).
In all cases, Nme2SmuCas9 PID variants performed better than their WT PID counterparts across the N4CN PAM targets. In a similar fashion, the domain-inlaid -i1 architecture also increased the editing efficiency of all Cas9 variants tested within the panel (Fig. 5a). We also observed that the -i1 inlaid architecture mediated shifts in editing windows towards PAM-distal adenines, an effect most prominent with the eNme2-C Cas9 domain (Fig. 5b, Supplementary Fig. 11). Since the ABE8e editors in the -i1 inlaid format had the highest activities, we focused our subsequent analyses on these variants. The top-ranking editor for mean maximum ABE activity across all N4CN target sites was Nme2Smu(E932R) (~24%), closely followed by Nme2Smu (WT, E932R/D56R) and eNme2-C, with approximate editing efficiencies ~22% (Fig. 5a). iNme2 had average ABE editing activity approximately 12%, while iNme2Smu performed marginally better (~15%) (Fig. 5a).
Next, we looked more closely at the average ABE editing activities of different Cas9 editors when varying the 6th nucleotide of the N4CN PAM. For the N4CC target sites, Cas9 mutants with the WT Nme2 PID performed best (Fig. 5c). For example, Nme2-ABE8e-i1 (E932R) induced mean maximum activity ~35%, whereas its PID-swapped counterpart had lower activity (~22%). For these target sites, the Nme2 (E932R/D56R) and iNme2 Cas9 editors ranked second (~28%) and third (~24%) respectively (Fig. 5c). Top-performing Cas9 variants for mean maximum ABE editing at N4CD target sites were Nme2Smu (E932R) > Nme2Smu (WT) > eNme2-C, with respective mean maximum editing rates of ~25%, ~24% and 22% (Fig. 5c). Across N4CT target sites, Nme2Smu (WT) ranked first, whereas Nme2Smu (E932R) performed the best at N4CG and N4CA target sites (Fig. 5c). In agreement with our previous results, Nme2 (E932R) had high activity across N4CT target sites (~22%), ranking third for activity against the other Cas9 editors (Fig. 5c). These results establish deaminase domain insertion as a viable approach to improve the general activities for a variety of engineered Nme2Cas9-derived base-editing systems.
Discussion
In an effort to relax the PAM preference of Nme2Cas9 editors from N4CC to N4CN, we 16 and others 19,20 engineered Nme2Cas9 nuclease and BE derivatives with a transplanted PAM interacting domain (PID) from SmuCas9 17. Although active at N4CN PAM target sites, the chimeric effectors are often outperformed by Nme2Cas9 at target sites with a canonical N4CC PAM. This trend is commonly observed with other PAM-relaxed Cas9 variants 21,24,23,22,25. Furthermore, when considering in vivo ABE applications, the SmuCas9 PID swap increases the sizes of our domain-inlaid architectures, which are already very close to the packaging capacities of AAV vector capsids.
We continued our advances to optimize Nme2Smu nuclease and ABE systems for activity and single-vector AAV deliverability. We first used arginine mutagenesis to enhance interactions with either sgRNA, TS DNA, or NTS DNA. Of note, the E932R and D56R mutations significantly improved the editing efficiencies of Nme2Smu nuclease systems on all N4CN PAM targets. After finding Nme2Smu mutants with improved activity, we transferred them into our domain-inlaid ABE architectures, optimizing for linker length in the process to facilitate single-vector AAV compatibility. Finally, we defined the editing activities, PAM compatibilities, editing windows, and specificities of the resulting editors using endogenous genomic sites as well as self-targeting guide-target libraries. In addition to the wide windows of ABE8e deaminases, we found that ABE9e variants are also compatible with our domain-inlaid ABE architectures, allowing for narrow-window applications (albeit at the cost of somewhat reduced editing efficiency).
As an additional benefit, the activity-enhancing properties of the arginine mutants also extended to WT Nme2Cas9 effectors, increasing their activities at N4CC PAM targets. In contrast to the self-targeting library nuclease results (Fig. 2a), we observed a particularly stark activity increase at N4CW (W = A or T) PAM target sites for domain-inlaid Nme2-ABE-i1 (Fig. 5c, Supplementary Fig. 10b). This suggests that the enhancing mutations may relax the PAM requirements of this ABE. Nonetheless, in terms of PAM selection, Nme2SmuCas9 effectors are the variants of choice for N4CD targets, while Nme2Cas9 effectors likely have the best activity at targets with N4CC PAMs. Finally, although inlaid Nme2-ABEs with a WT PID can already be packaged into single AAV vectors 10,11, the additional space saved with minimized linkers may further their utility when delivered via AAV vectors.
In terms of alternative engineering approaches for Nme2Cas9 systems, the Liu group used phage-assisted evolution to develop eNme2-C base editing systems 18, whereas the Doudna group employed a mixture of rational design and homology modelling focused on WED domain engineering to develop iNme2Cas9 36. Notably, our top-performing arginine mutant (E932R) overlaps with one of the identified amino acid positions observed to improve Nme2Cas9 activity during PACE evolution (E932K for eNme2-C) for nuclease and base editing (Huang et al., 2022); or during a bacterial evolution campaign combined with rational design (E932K) for iNme2Cas9 nuclease 36. Biochemical characterization in the iNme2Cas9 study 36 points to increased unwinding rates as a prospective mechanism for the enhanced editing activity seen with some nucleic acid interacting mutants (E932R, D873R, D1048R).
Enhanced R-loop formation also potentially explains the PAM relaxation effect observed with WT PID Nme2-ABE8e-i1. iNme2Cas9 was shown to have robust activity at the non-canonical PAMs N4CT and N4TC at a limited number of target sites, without mutation of residues directly interacting with the PAM 36. It is interesting to speculate whether activating mutations such as E932R or those found within the iNme2Cas9 editors could further relax the preferences of Nme2SmuCas9 effectors. Finally, although the activity-specificity balance of Nme2Cas9-derived editors leads to intrinsically reduced off-target editing than many other Cas9 orthologs, the broadening of PAM compatibility paired with improved activity are likely to be inversely correlated with specificity 37,38. Additional studies will be necessary to carefully define the off-target profiles of these enhanced Nme2Cas9 and Nme2SmuCas9 variants, especially in cases of therapeutic application.
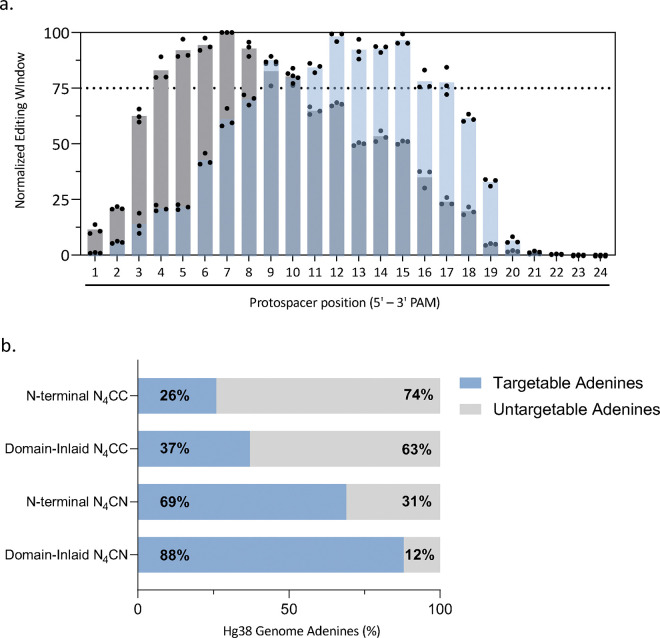
In summary, we have improved the editing potential of Nme2Cas9- and Nme2SmuCas9- derived editors for nuclease and ABE applications. Altogether, the domain-inlaid Nme2Smu-ABE8e’s (-i1 and -i8) with editing windows that enable targeting from positions 4 – 18 (counting from the 5’ end of the protospacer NTS, Fig. 6a), in combination with a single-cytidine PAM preference, enables targeting of 88% of adenines within the hg38 reference genome (Fig. 6b). The expanded windows of the domain-inlaid variants resulted in a 62% and 19% increase in targeting scope compared to N-terminally fused variants targeting dinucleotide N4CC PAMs (Nme2-ABE8e) 10,11 or single-cytidine N4CN PAMs (eNme2-C) 18 (Fig. 6b). Furthermore, we were able to port our domain-inlaid designs for use with eNme2-C and iNme2 Cas9 domains. The use of alternately engineered Nme2Cas9 ABE editing systems could prove beneficial when fine-tuning parameters such as editing window, activity, and off-target profile during therapeutic implementation. Overall, the minimal PAM requirements, tunable editing windows, and AAV compatibilities of these enhanced domain-inlaid ABE systems provide greater control over targetable genomic adenines with improved utility for in vivo applications.
Figure 6. Summary of editing windows and genomic targetable adenines by various Nme2Cas9-derived ABEs.
(a) Summary of editing windows of Nme2Smu-ABE8e-i1 or Nme2Smu-ABE8e-i8 with the L10 linker format and E932R mutation. The data represent the normalized editing rates across the window from three independent self-targeting library experimental panels, compiled from Fig. 3a and Supplementary Figures 9 and 10a. Each experimental panel consisted of 3 biological replicates. (b) Adenines targetable within the hg38 reference human genome by Nme2Cas9-derived ABE8e variants in various formats. Editing windows to calculate the targetable adenines within the reference genome consisted of the previously described window for N-terminally fused Nme2-ABE8e 10, or the editing windows observed here with the guide-target library assay for N-terminally fused eNme2-C or domain-inlaid –i1 or –i8 Nme2Smu-ABE8e editors from (a). Targetable adenine calculations were also made for whether the ABE uses dinucleotide (N4CC) or single nucleotide cytidine (N4CN) PAMs. Editing activity above 75% of the maximum position in the window was the cutoff criteria for window selection. Code used to generate this data was adapted from 10.
Methods
Molecular cloning.
Nucleotide sequences of key nucleases and base editors described in this manuscript are provided in the Supplementary Notes. Effectors used for endogenous target site editing were cloned into a CMV promoter plasmid backbone (Addgene #201510) for expression. Conversely, for the guide-target library experiments, nucleases and base editors were cloned into the p2T-CMV-ABEmax-BlastR backbone (Addgene, #152989) via Gibson assembly. Plasmids expressing nuclease and base editor arginine mutants were generated by introduction of point mutations by site-directed mutagenesis (SDM) oligos in the Supplementary Oligonucleotides file, using NEB’s KLD enzyme mix (NEB #M0554S) with the appropriate Nme2Cas9 effector plasmid used as a template. The truncated Nx-Cx linker variants were constructed via Gibson assembly with ssDNA bridge oligos containing the linker of interest, as described in the Supplementary Notes.
Transient transfection for fluorescent reporter and endogenous target site experiments.
HEK293T (ATCC #CRL-3216) cells and the fluorescent reporter derivatives described in this manuscript were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Genesee Scientific #25–500) with 10% fetal bovine serum (FBS; Gibco #26140079). For transient plasmid transfection, ~15,000 cells were seeded in 96-well plates and incubated overnight. The following day, approximately 0.75 μl Lipofectamine 2000 (ThermoFisher #11668019) was used to transfect cells with ~100 ng of the editor plasmid and ~100 ng of sgRNA according to the manufacturer’s recommended protocols. In all cases cells were incubated at 37°C with 5% CO2.
Flow cytometry.
For measurements of editing activities with fluorescent reporters, cells were trypsinized, collected, and washed with FACS buffer (chilled PBS and 3% fetal bovine serum) 72 hours post-transfection. After washing, cells were resuspended in 300 μl FACS buffer for flow cytometry analysis using the MACSQuant VYB system. A total of 10,000 cells were counted for analysis with Flowjo v10.
Amplicon sequencing and data analysis of endogenous target sites.
Amplicon sequencing, library preparation, and analysis were performed as previously described16. We used NEBNext Ultra II Q5® Master Mix (NEB #M0544) to amplify genomic DNA for library preparation, followed by pooling and two rounds of left-sided size selection with SPRIselect beads (Beckman Coulter #B23317) and subsequent agarose gel analysis. Pooled amplicons were sequenced on an Illumina MiniSeq system (300 cycles, Illumina sequencing kit #FC-420–1004) following the manufacturer’s protocol. Sequencing data was analyzed with CRISPResso248 (version 2.0.40) in nuclease or BE output batch mode with the following flags: Nuclease (-wc −3, -w 1, -q 30), BE (-w 12, -wc −12, -q 30).
Transient transfection for guide-target library experiments.
Construction and editing experiments for the activity guide-target library assay were as previously described 16. In brief, ~200,000 (activity library) or ~400,000 (mismatch library) cells were plated into 12- or 6-well plates, respectively, in non-selective medium and incubated overnight. The next day, cells were transfected with ~1.8 μg (activity library) or ~3.6 μg (mismatch library) of editing effector plasmid using Lipofectamine 2000 following the standard manufacturer-recommended protocol. One day post-transfection, culture media were supplemented with Blasticidin S [10 μg/ml]. After 3 days, genomic DNA was extracted from cells with QuickExtract (Lucigen #QE0905), column-purified (Zymo Research #C1102–50), and used for NGS library preparation.
Amplicon sequencing and data analysis of library target sites.
NGS library preparation was performed as described previously 16 and akin to the Amplicon sequencing and data analysis of endogenous target sites section listed above, with the following amendments. 1 μg (activity library) or 2 μg (mismatch library) of input DNA was used for library PCRs and sequenced on an Illumina NextSeq 2000 system (200 cycles, Illumina sequencing kit #20046812). Sequencing data for the activity library was demultiplexed using our previously published custom script 16 while sequencing data for the mismatch library was demultiplexed with a new custom script (see Supplemental Code). Demultiplexed files were analyzed with CRISPResso2 (version 2.0.40) in the standard batch output modes as described above. Library members with <40 reads were omitted from analysis in all samples. The following flags were used: Nuclease (-wc −3, -w 1, -q 10), BE (-w 12, -wc −12, -q 10).
Statistical analysis.
Data analysis and plotting was performed in GraphPad Prism 9.4.0.
Supplementary Material
Acknowledgments
We thank members of the Sontheimer, Watts, Wolfe, Xue, and Khvorova laboratories for their time, advice, and feedback during the preparation of this manuscript. Support for this work was provided by the National Institutes of Health (F31GM143879 and R25GM113686 to N.B. and R01GM150273 to E.J.S.), the Rett Syndrome Research Trust (E.J.S. and J.K.W.), and the Leducq Foundation (to E.J.S.).
Footnotes
Competing Interests
The authors declare competing financial interests. The authors have filed patent applications on technologies related to this work. E.J.S. is a co-founder and scientific advisor of Intellia Therapeutics and a member of the Scientific Advisory Board of Tessera Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare no competing non-financial interests.
Data Availability
Sequencing data that support the findings of this study will be made available in the NCBI SRA (bioproject #PRJNA1098767) upon publication. Source data for figures are provided within the Source Data and Supplementary Data files. Sequences of target sites and oligonucleotides (primers, guide-target library oligos) used in this study are provided in Supplementary Data 9, Oligonucleotides file. Key plasmids described in this paper are being made available via Addgene. All other data will be made available upon request.
References
- 1.Villiger L. et al. CRISPR technologies for genome, epigenome and transcriptome editing. Nat Rev Mol Cell Biol 25, 464–487 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Wang J. Y. & Doudna J. A. CRISPR technology: A decade of genome editing is only the beginning. Science 379, eadd8643 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Anzalone A. V., Koblan L. W. & Liu D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol (2020) doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 4.Gaudelli N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komor A. C., Kim Y. B., Packer M. S., Zuris J. A. & Liu D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Madigan V., Zhang F. & Dahlman J. E. Drug delivery systems for CRISPR-based genome editors. Nat Rev Drug Discov 22, 875–894 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Wang D., Zhang F. & Gao G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 181, 136–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edraki A. et al. A Compact, High-Accuracy Cas9 with a Dinucleotide PAM for In Vivo Genome Editing. Molecular Cell 73, 714–726.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis J. R. et al. Publisher Correction: Efficient in vivo base editing via single adenoassociated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng 6, 1317–1317 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H. et al. Adenine Base Editing In Vivo with a Single Adeno-Associated Virus Vector. GEN Biotechnology 1, 285–299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou Z. et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. U.S.A. 110, 15644–15649 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esvelt K. M. et al. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods 10, 1116–1121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ran F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E. et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 8, 14500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamidele N. et al. Domain-inlaid Nme2Cas9 adenine base editors with improved activity and targeting scope. Nat Commun 15, 1458 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J. et al. Potent Cas9 Inhibition in Bacterial and Human Cells by AcrIIC4 and AcrIIC5 Anti-CRISPR Proteins. mBio 9, e02321–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang T. P. et al. High-throughput continuous evolution of compact Cas9 variants targeting single-nucleotide-pyrimidine PAMs. Nat Biotechnol (2022) doi: 10.1038/s41587-022-01410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J. et al. Closely related type II-C Cas9 orthologs recognize diverse PAMs. eLife 11, e77825 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao D. et al. Engineered domain-inlaid Nme2Cas9 adenine base editors with increased on-target DNA editing and targeting scope. BMC Biology 21, 250 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimasu H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H. K. et al. High-throughput analysis of the activities of xCas9, SpCas9-NG and SpCas9 at matched and mismatched target sequences in human cells. Nat Biomed Eng 4, 111–124 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Kim N. et al. Prediction of the sequence-specific cleavage activity of Cas9 variants. Nat Biotechnol 38, 1328–1336 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Walton R. T., Christie K. A., Whittaker M. N. & Kleinstiver B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 368, 290–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H. et al. Rapid two-step target capture ensures efficient CRISPR-Cas9-guided genome editing. 2024.10.01.616117 Preprint at 10.1101/2024.10.01.616117 (2024). [DOI] [Google Scholar]
- 26.McGaw C. et al. Engineered Cas12i2 is a versatile high-efficiency platform for therapeutic genome editing. Nat Commun 13, 2833 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa R. et al. Engineered Campylobacter jejuni Cas9 variant with enhanced activity and broader targeting range. Commun Biol 5, 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W. et al. Structures of Neisseria meningitidis Cas9 Complexes in Catalytically Poised and Anti-CRISPR-Inhibited States. Molecular Cell S1097276519307300 (2019) doi: 10.1016/j.molcel.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P. et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat Commun 12, 2121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer S. et al. Efficient Homology-Directed Repair with Circular Single-Stranded DNA Donors. The CRISPR Journal 5, 685–701 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbab M. et al. Determinants of Base Editing Outcomes from Target Library Analysis and Machine Learning. Cell 182, 463–480.e30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen Tran M. T. et al. Engineering domain-inlaid SaCas9 adenine base editors with reduced RNA off-targets and increased on-target DNA editing. Nat Commun 11, 4871 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villiger L. et al. Replacing the SpCas9 HNH domain by deaminases generates compact base editors with an alternative targeting scope. Molecular Therapy - Nucleic Acids 26, 502–510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L. et al. Engineering a precise adenine base editor with minimal bystander editing. Nat Chem Biol 19, 101–110 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Tu T. et al. A precise and efficient adenine base editor. Molecular Therapy 30, 2933–2941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggers A. R. et al. Rapid DNA unwinding accelerates genome editing by engineered CRISPR-Cas9. Cell 187, 3249–3261.e14 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D., Luk K., Wolfe S. A. & Kim J.-S. Evaluating and Enhancing Target Specificity of Gene-Editing Nucleases and Deaminases. Annual Review of Biochemistry 88, 191–220 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Bisaria N., Jarmoskaite I. & Herschlag D. Lessons from Enzyme Kinetics Reveal Specificity Principles for RNA-guided nucleases in RNA Interference and CRISPR-based Genome Editing. Cell Syst 4, 21–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data that support the findings of this study will be made available in the NCBI SRA (bioproject #PRJNA1098767) upon publication. Source data for figures are provided within the Source Data and Supplementary Data files. Sequences of target sites and oligonucleotides (primers, guide-target library oligos) used in this study are provided in Supplementary Data 9, Oligonucleotides file. Key plasmids described in this paper are being made available via Addgene. All other data will be made available upon request.