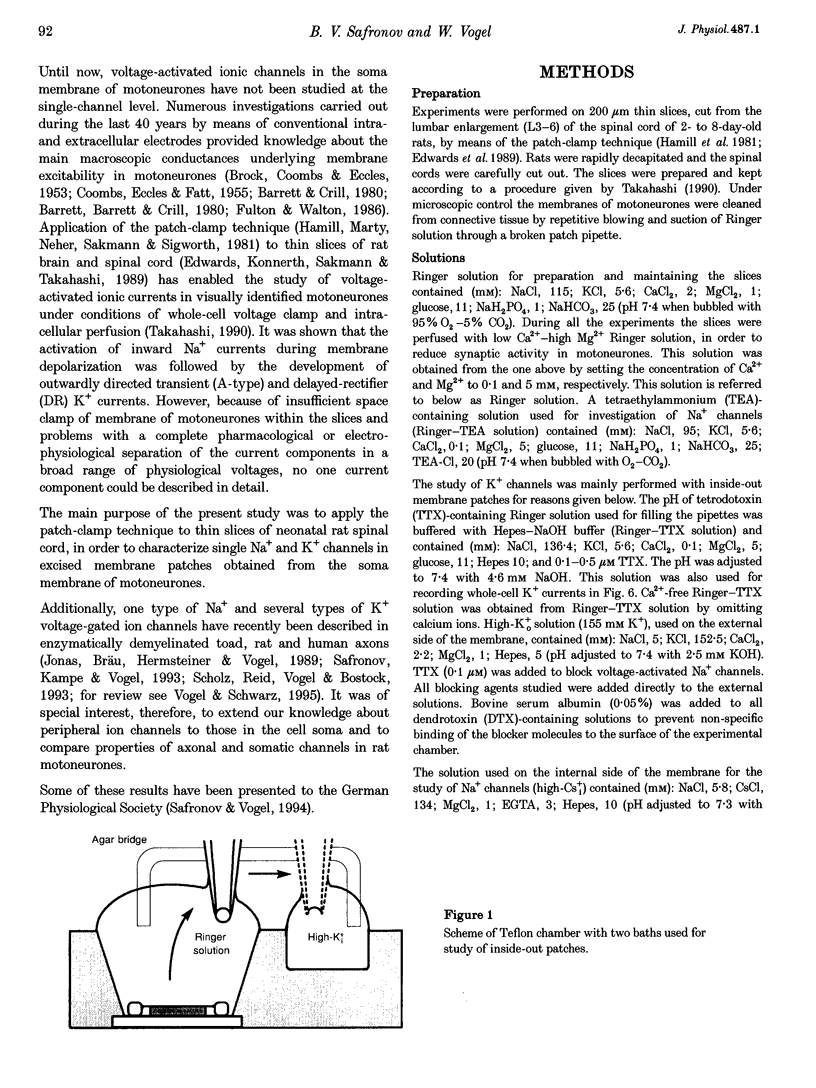
Abstract
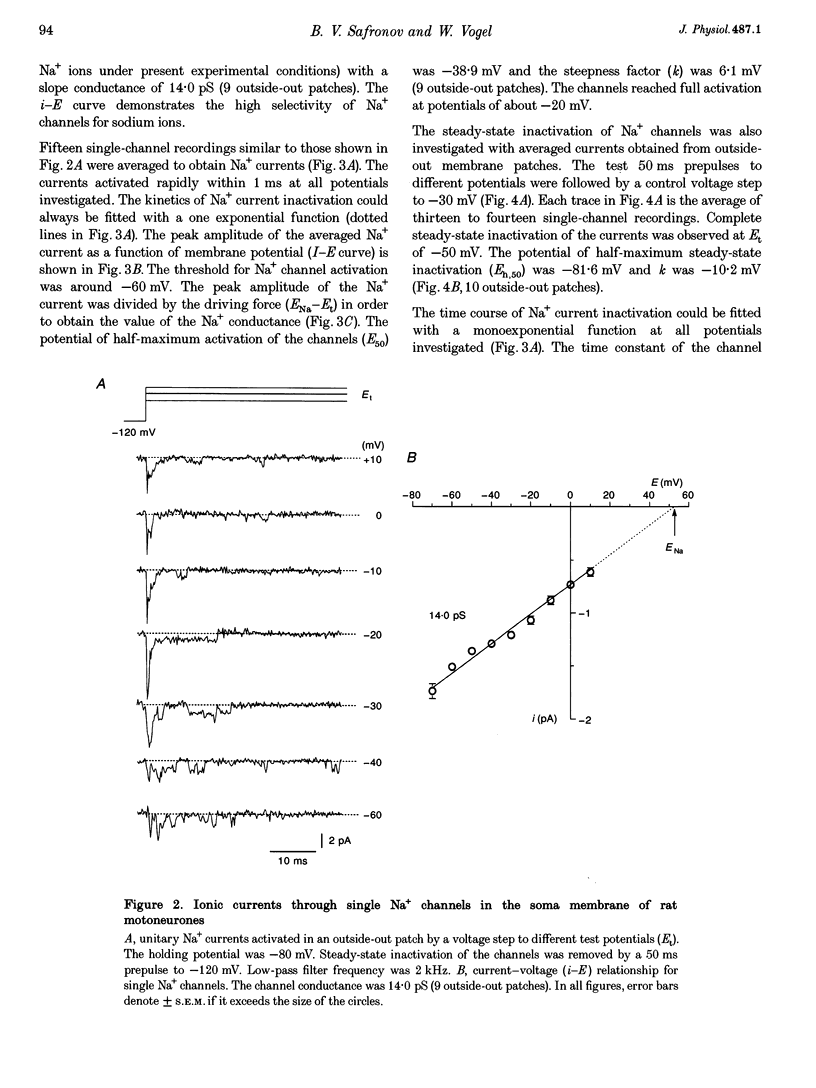
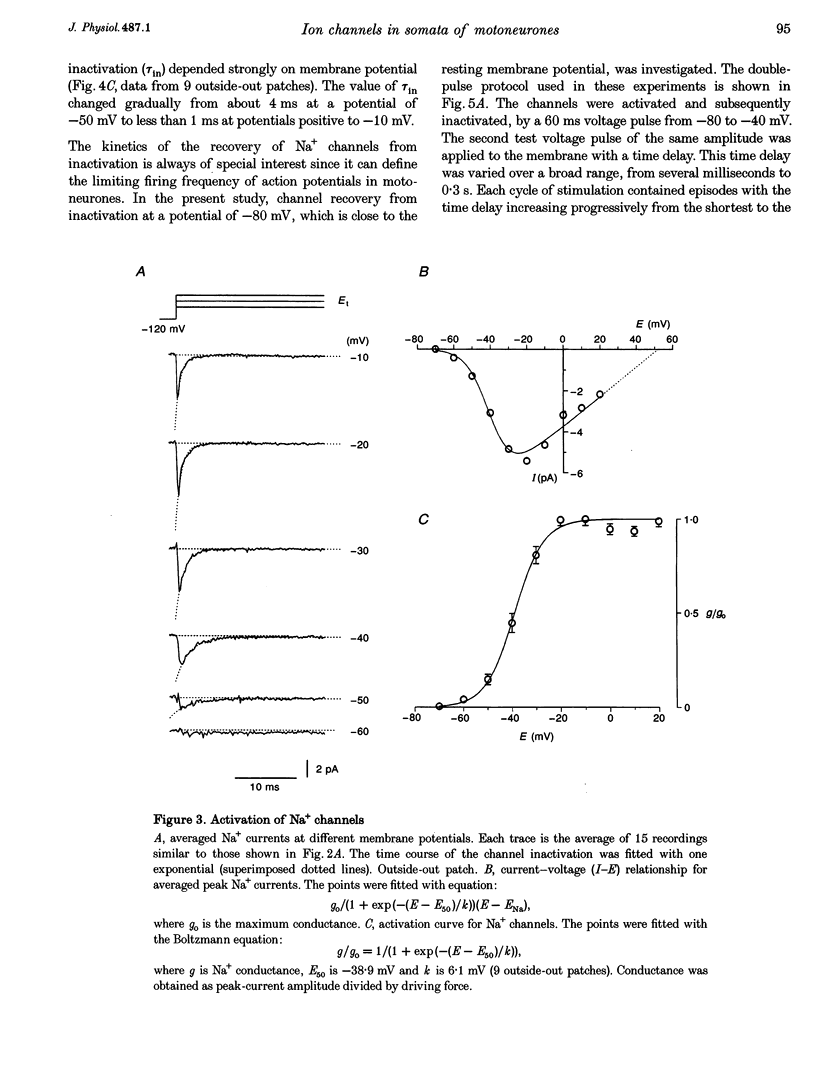
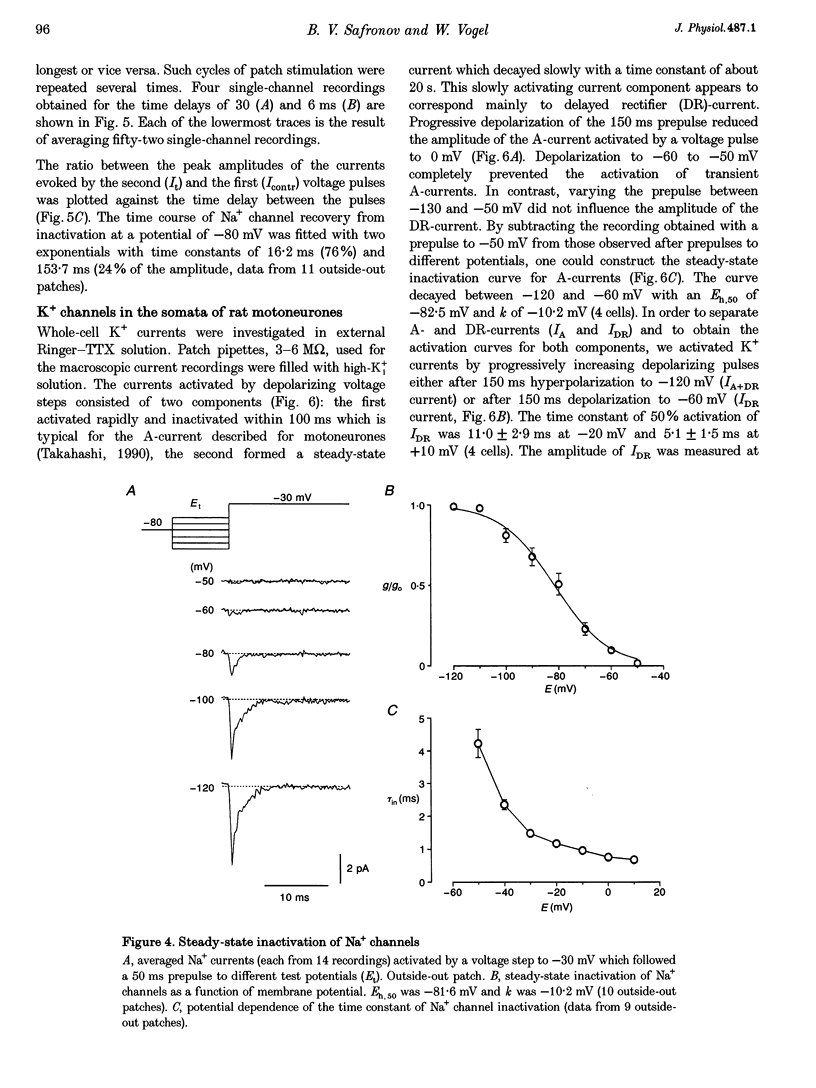
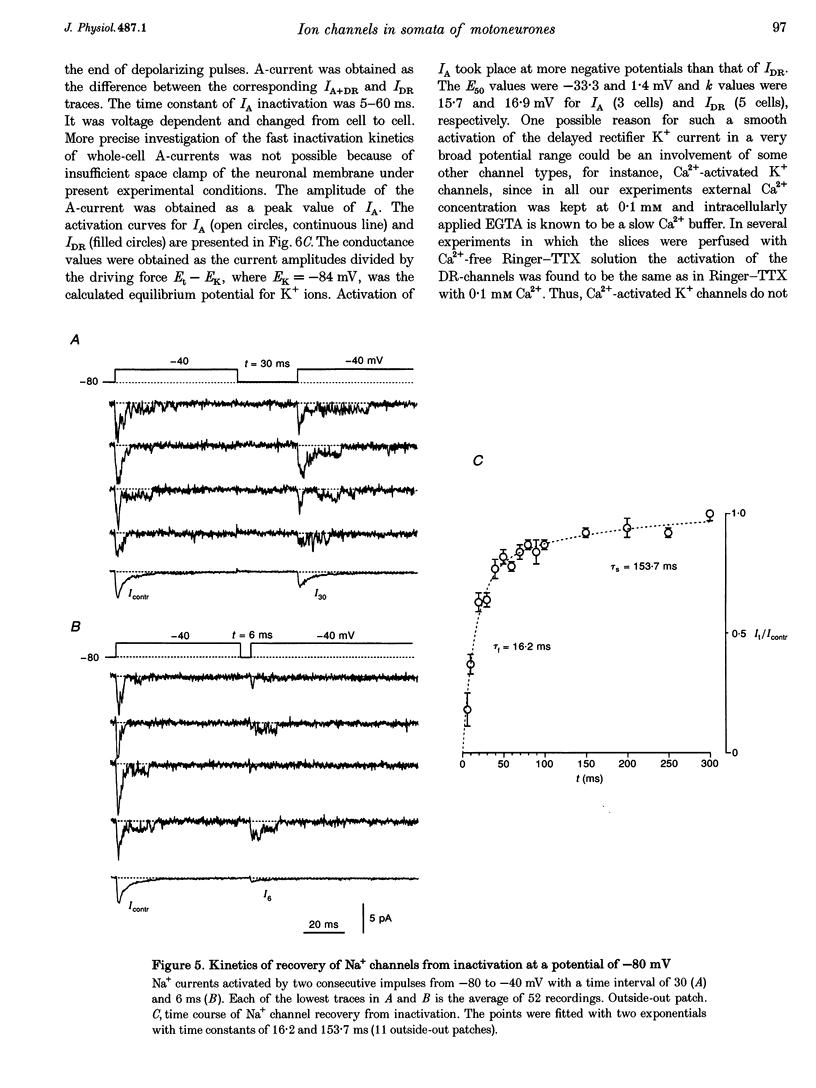
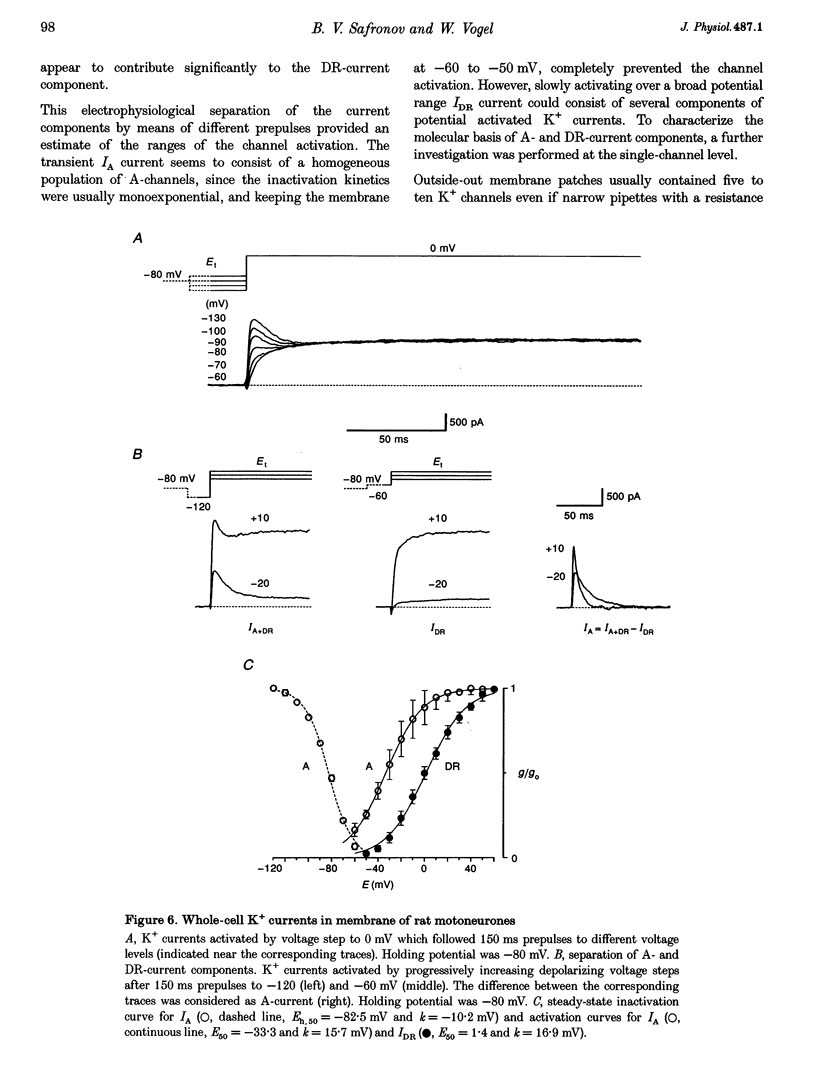
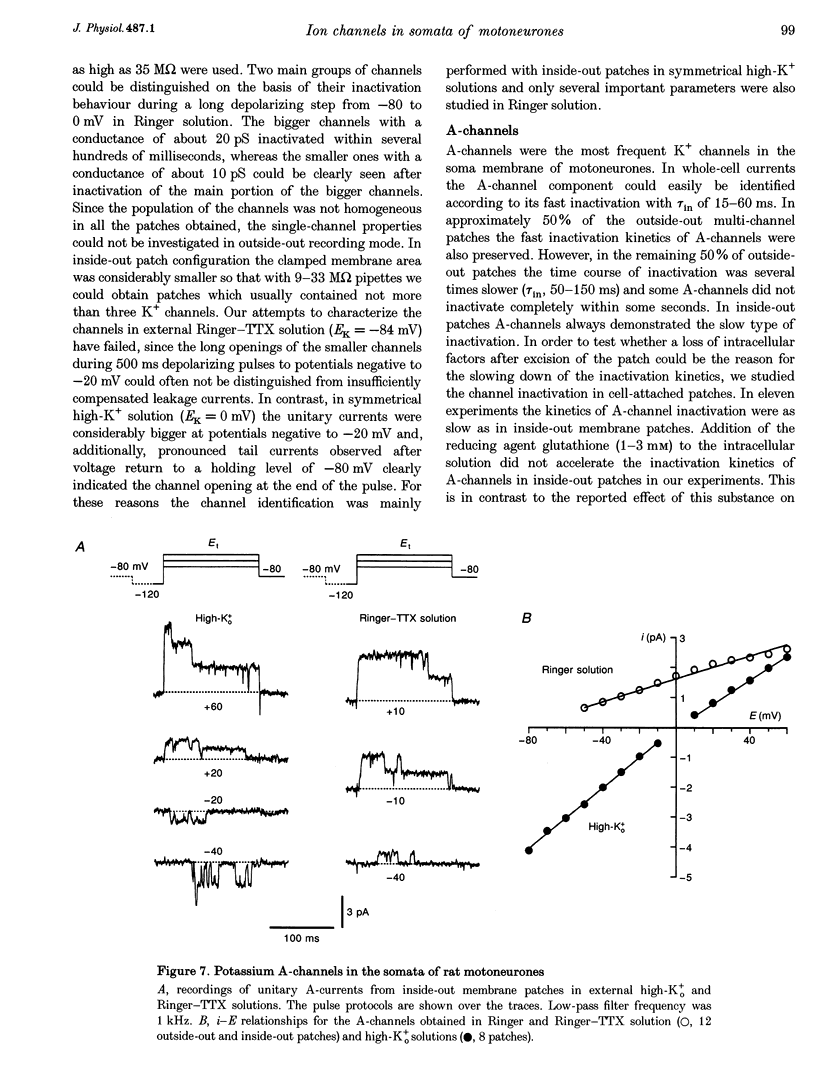
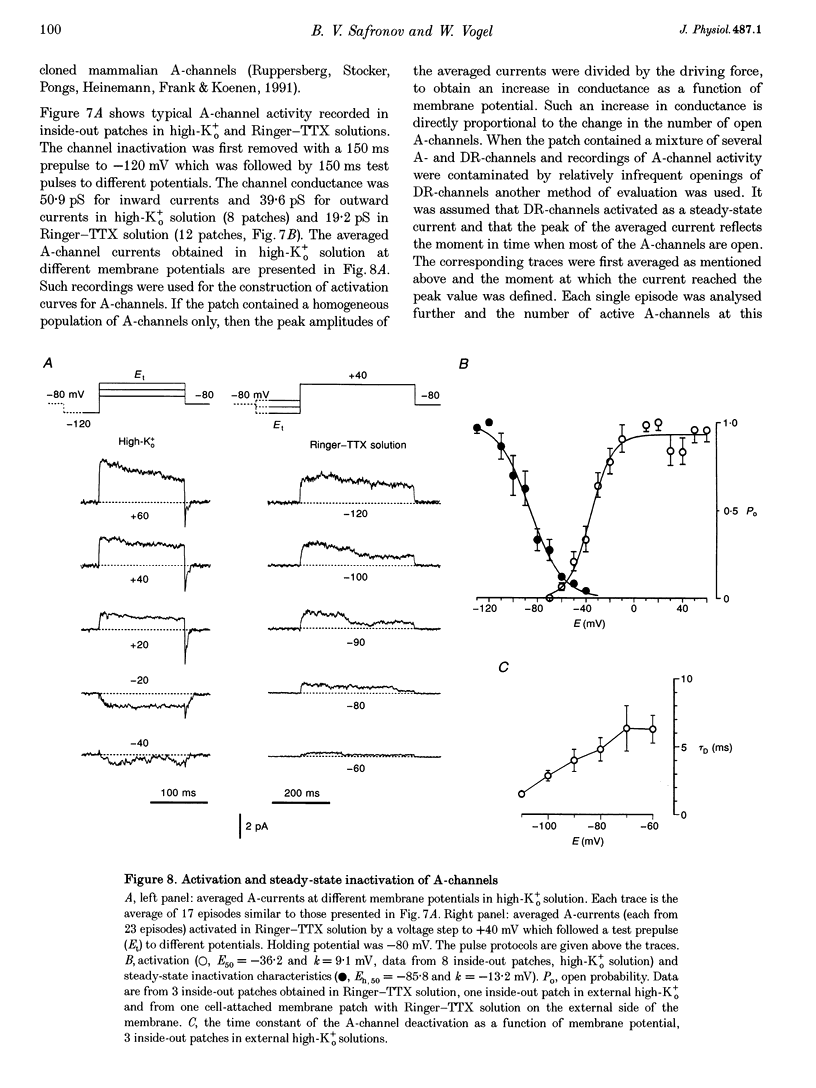
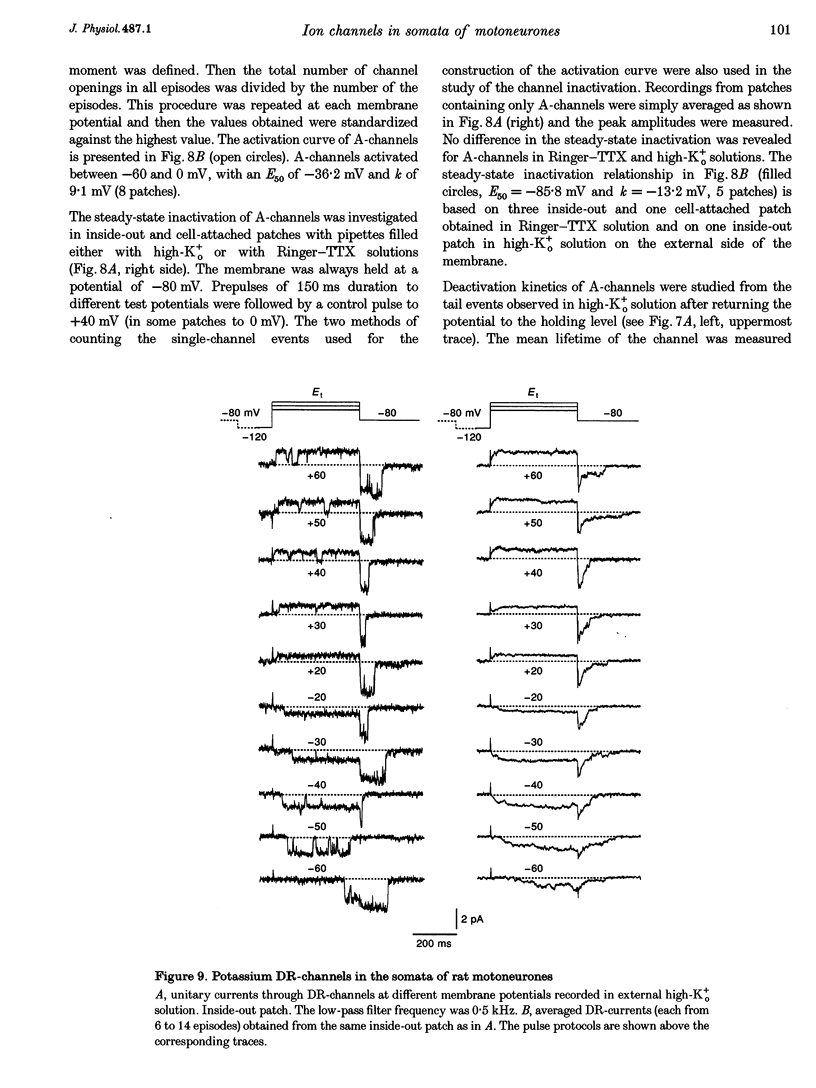
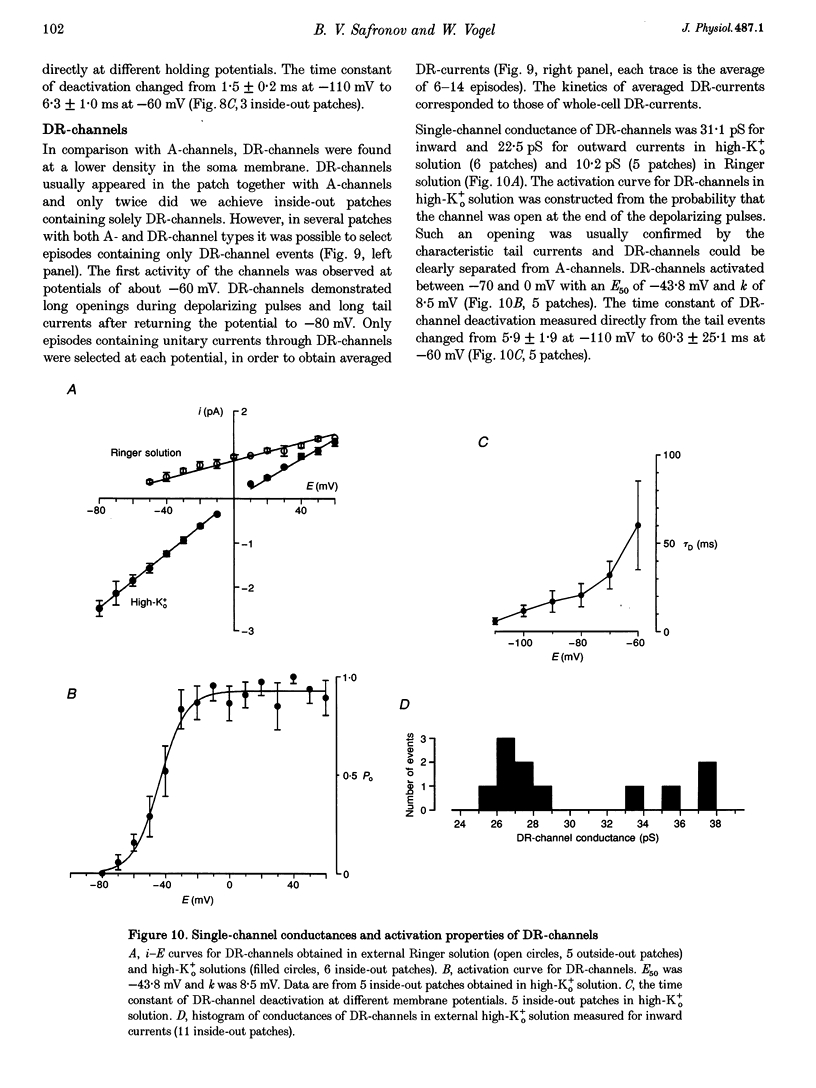
1. Voltage-activated Na+ and K+ channels were investigated in the soma membrane of motoneurones using the patch-clamp technique applied to thin slices of neonatal rat spinal cord. 2. One type of TTX-sensitive Na+ channel, with a conductance of 14.0 pS, was found to underlie the macroscopic Na+ conductance in the somata of motoneurones. These channels activated within a potential range between -60 and -20 mV with a potential of half-maximal activation (E50) of -38.9 mV and steepness factor (k) of 6.1 mV. 3. Kinetics of Na+ channel inactivation could be fitted with a single exponential function at all potentials investigated. The curve of the steady-state inactivation had the following parameters: a half-maximal potential (Eh,50) of -81.6 mV and k of -10.2 mV. 4. Kinetics of recovery of Na+ channels from inactivation at a potential of -80 mV were double exponential with fast and slow components of 16.2 (76%) and 153.7 ms (24%), respectively. It is suggested that the recovery of Na+ channels from inactivation plays a major role in defining the limiting firing frequency of action potentials in motoneurones. 5. Whole-cell K+ currents consisted of transient (A)- and delayed-rectifier (DR)-components. The A-component activated between -60 and +20 mV with an E50 of -33.3 mV and k of 15.7 mV. The curve of steady-state inactivation was best fitted with an Eh,50 of -82.5 mV and k of -10.2 mV. The DR-component of K+ current activated smoothly at more positive potentials. E50 and k for DR-currents were +1.4 and 16.9 mV, respectively. 6. The most frequent single K+ channel found in the somata of motoneurones was the fast inactivating A-channel with a conductance of 19.2 pS in external Ringer solution. In symmetrical high-K+ solutions the conductance was 50.9 and 39.6 pS for inward and outward currents, respectively. The channel activation took place between -60 and +20 mV. The curve of steady-state inactivation of single A-channels had an Eh,50 of -87.1 mV and k of -12.8 mV. In high-Ko+ solution A-channels demonstrated a rapid deactivation at potentials between -110 and -60 mV. The time constant of the channel deactivation depended on the membrane potential and changed from 1.5 ms at -110 mV to 6.3 ms at -60 mV. 7. Delayed-rectifier K+ channels were found in the soma membrane at a moderate density.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., Schwindt P. C., Crill W. E. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993 Feb;13(2):660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Voltage clamp of cat motoneurone somata: properties of the fast inward current. J Physiol. 1980 Jul;304:231–249. doi: 10.1113/jphysiol.1980.sp013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräu M. E., Dreyer F., Jonas P., Repp H., Vogel W. A K+ channel in Xenopus nerve fibres selectively blocked by bee and snake toxins: binding and voltage-clamp experiments. J Physiol. 1990 Jan;420:365–385. doi: 10.1113/jphysiol.1990.sp017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. Single-channel current/voltage relationships of two kinds of Na+ channel in vertebrate sensory neurons. Pflugers Arch. 1993 Jun;423(5-6):492–496. doi: 10.1007/BF00374946. [DOI] [PubMed] [Google Scholar]
- Corrette B. J., Repp H., Dreyer F., Schwarz J. R. Two types of fast K+ channels in rat myelinated nerve fibres and their sensitivity to dendrotoxin. Pflugers Arch. 1991 May;418(4):408–416. doi: 10.1007/BF00550879. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993 Apr;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MOORE L. E. THE EFFECT OF TEMPERATURE ON THE SODIUM AND POTASSIUM PERMEABILITY CHANGES IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:431–437. doi: 10.1113/jphysiol.1963.sp007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. P., Walton K. Electrophysiological properties of neonatal rat motoneurones studied in vitro. J Physiol. 1986 Jan;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SHORTESS G. K. QUANTITATIVE ASPECTS OF REPETITIVE FIRING OF MAMMALIAN MOTONEURONES, CAUSED BY INJECTED CURRENTS. J Physiol. 1963 Oct;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Bräu M. E., Hermsteiner M., Vogel W. Single-channel recording in myelinated nerve fibers reveals one type of Na channel but different K channels. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7238–7242. doi: 10.1073/pnas.86.18.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Kameyama M., Yamaguchi K., Fukuda J. Single transient K channels in mammalian sensory neurons. Biophys J. 1986 Jun;49(6):1243–1247. doi: 10.1016/S0006-3495(86)83754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniffki K. D., Siemen D., Vogel W. Development of sodium permeability inactivation in nodal membranes. J Physiol. 1981;313:37–48. doi: 10.1113/jphysiol.1981.sp013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Swenson R. P., Jr External monovalent cations that impede the closing of K channels. J Gen Physiol. 1986 May;87(5):795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S., Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991 Oct;66(4):1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Kinetic analysis of two types of Na+ channels in rat dorsal root ganglia. J Physiol. 1993 Jul;466:9–37. [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Safronov B. V., Kampe K., Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol. 1993 Jan;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A., Reid G., Vogel W., Bostock H. Ion channels in human axons. J Neurophysiol. 1993 Sep;70(3):1274–1279. doi: 10.1152/jn.1993.70.3.1274. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Stansfeld C., Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988 Oct 31;93(1):49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Stocker M., Sakmann B., Seeburg P., Baumann A., Grupe A., Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988 Dec 19;242(1):199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995 Mar;15(3 Pt 2):2218–2224. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]