Abstract
1. The effects of immunoglobulin G (IgG) from patients with Lambert-Eaton myasthenic syndrome on Ca2+ currents in mammalian motor nerve terminals are unknown. Therefore, we recorded these currents in phrenic nerves of mice injected with serum from either LEMS patients, myasthenia gravis patients, or healthy control individuals. 2. In control preparations, the endplate currents induced by repetitive stimulation at > or = 20 Hz were depressed as expected. However, in the LEMS animals quantal content decreased and either depression did not occur or synaptic facilitation occurred. 3. Ca2+ currents were smaller in LEMS animals. At 0.5 Hz stimulation frequency, normalized Ca2+ currents in LEMS animals were 57 +/- 14% of those in control. At higher frequencies, Ca2+ currents become smaller in control but not in LEMS animals. 4. Ca2+ currents in controls were unaffected by addition of nifedipine but were reduced by 37% upon addition of omega-conotoxin GVIA. In LEMS animals, however, the currents were depressed by 43% by nifedipine but were unaffected by omega-conotoxin GVIA. Thus, LEMS is associated with reduced Ca2+ currents and a shift to dihydropyridine sensitivity.
Full text
PDF

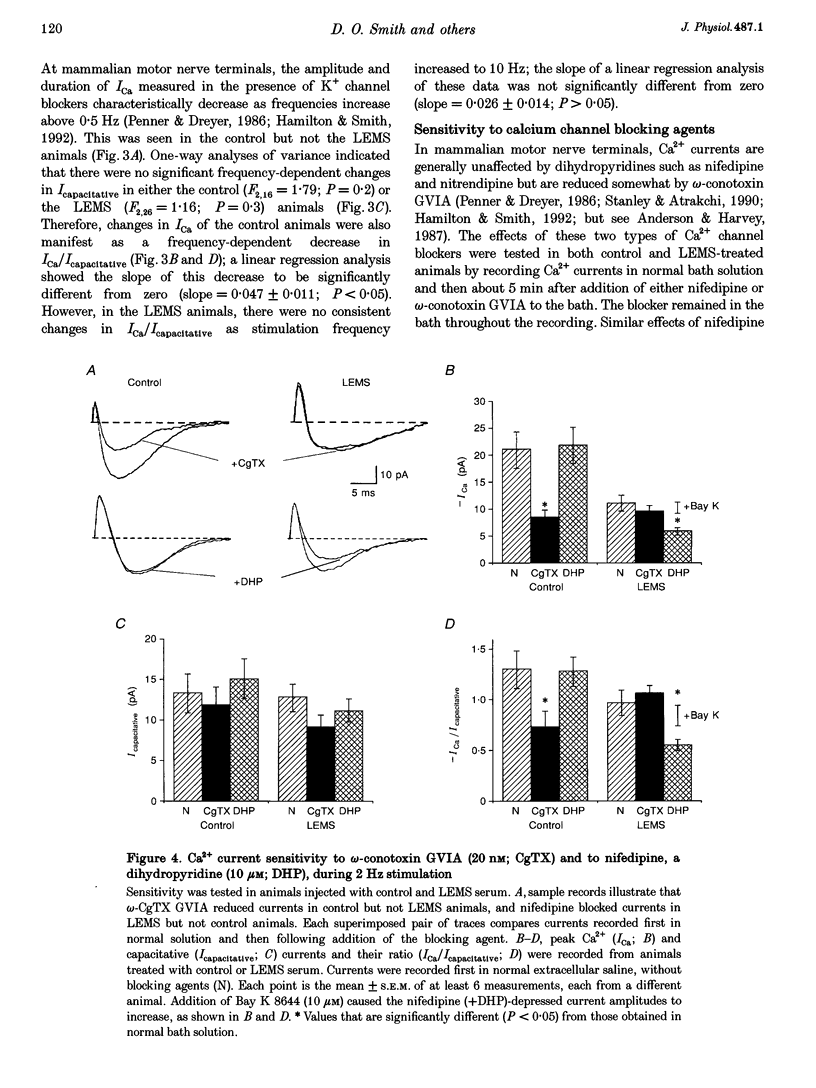
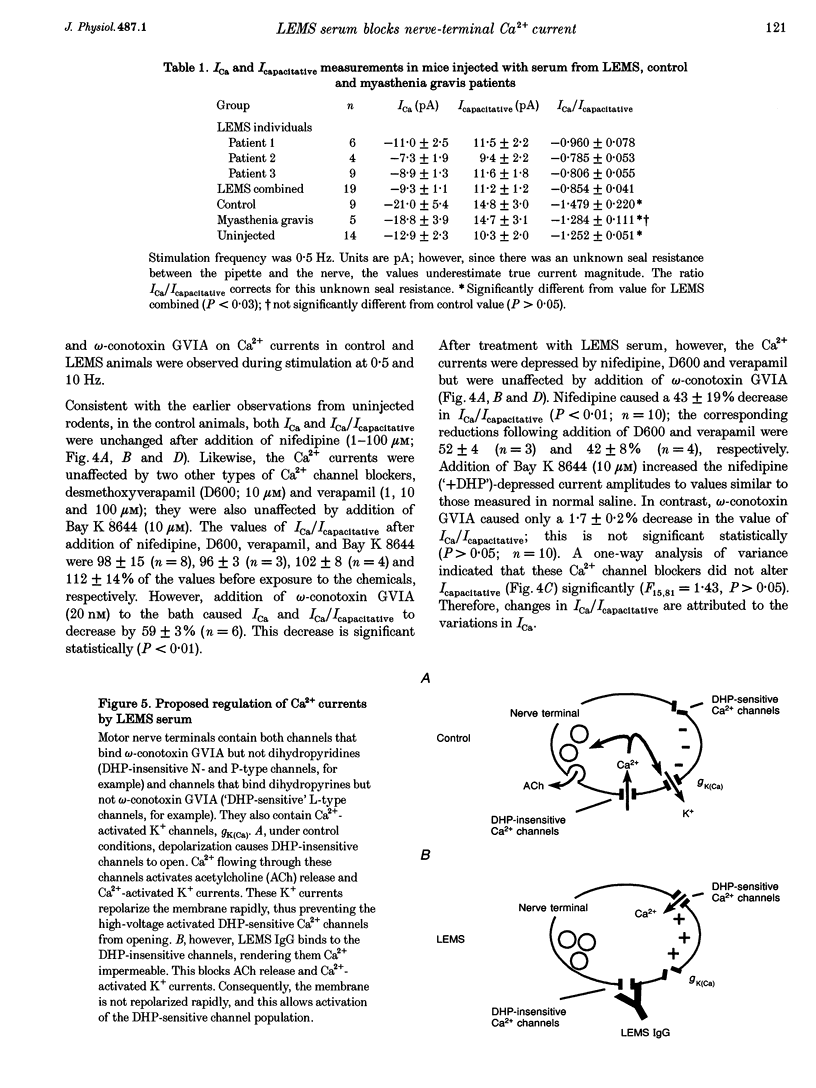


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L. Omega-conotoxin does not block the verapamil-sensitive calcium channels at mouse motor nerve terminals. Neurosci Lett. 1987 Nov 23;82(2):177–180. doi: 10.1016/0304-3940(87)90125-x. [DOI] [PubMed] [Google Scholar]
- Atchison W. D. Dihydropyridine-sensitive and -insensitive components of acetylcholine release from rat motor nerve terminals. J Pharmacol Exp Ther. 1989 Nov;251(2):672–678. [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartschat D. K., Blaustein M. P. Calcium-activated potassium channels in isolated presynaptic nerve terminals from rat brain. J Physiol. 1985 Apr;361:441–457. doi: 10.1113/jphysiol.1985.sp015654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Lambert E. H. Detailed analysis of neuromuscular transmission in a patient with the myasthenic syndrome sometimes associated with bronchogenic carcinoma. Mayo Clin Proc. 1968 Oct;43(10):689–713. [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H., Engel A. G., Lang B., Newsom-Davis J., Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajela R. K., Atchison W. D. The proteins synaptotagmin and syntaxin are not general targets of Lambert-Eaton myasthenic syndrome autoantibody. J Neurochem. 1995 Mar;64(3):1245–1251. doi: 10.1046/j.1471-4159.1995.64031245.x. [DOI] [PubMed] [Google Scholar]
- Hamilton B. R., Smith D. O. Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J Physiol. 1991 Jan;432:327–341. doi: 10.1113/jphysiol.1991.sp018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. R., Smith D. O. Calcium currents in rat motor nerve terminals. Brain Res. 1992 Jul 3;584(1-2):123–131. doi: 10.1016/0006-8993(92)90885-d. [DOI] [PubMed] [Google Scholar]
- Hewett S. J., Atchison W. D. Specificity of Lambert-Eaton myasthenic syndrome immunoglobulin for nerve terminal calcium channels. Brain Res. 1992 Dec 25;599(2):324–332. doi: 10.1016/0006-8993(92)90408-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Kim Y. I. Passive transfer of the Lambert-Eaton myasthenic syndrome: neuromuscular transmission in mice injected with plasma. Muscle Nerve. 1985 Feb;8(2):162–172. doi: 10.1002/mus.880080213. [DOI] [PubMed] [Google Scholar]
- Lambert E. H., Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- Lang B., Newsom-Davis J., Peers C., Prior C., Wray D. W. The effect of myasthenic syndrome antibody on presynaptic calcium channels in the mouse. J Physiol. 1987 Sep;390:257–270. doi: 10.1113/jphysiol.1987.sp016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B., Newsom-Davis J., Prior C., Wray D. Antibodies to motor nerve terminals: an electrophysiological study of a human myasthenic syndrome transferred to mouse. J Physiol. 1983 Nov;344:335–345. doi: 10.1113/jphysiol.1983.sp014943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H. Autoantibodies bind solubilized calcium channel-omega-conotoxin complexes from small cell lung carcinoma: a diagnostic aid for Lambert-Eaton myasthenic syndrome. Mayo Clin Proc. 1989 Dec;64(12):1498–1504. doi: 10.1016/s0025-6196(12)65705-x. [DOI] [PubMed] [Google Scholar]
- Leveque C., Hoshino T., David P., Shoji-Kasai Y., Leys K., Omori A., Lang B., el Far O., Sato K., Martin-Moutot N. The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert-Eaton myasthenic syndrome antigen. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3625–3629. doi: 10.1073/pnas.89.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Barrett E. F. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990 Aug;10(8):2614–2625. doi: 10.1523/JNEUROSCI.10-08-02614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Uchitel O. D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993 Dec 13;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Charlton M. P. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992 Jan;12(1):297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Atrakchi A. H. Calcium currents recorded from a vertebrate presynaptic nerve terminal are resistant to the dihydropyridine nifedipine. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9683–9687. doi: 10.1073/pnas.87.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]