Abstract
Background
We previously reported in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) treated with immune checkpoint inhibitors (ICIs), pretreatment higher lactate dehydrogenase (LDH) and absolute (abx) neutrophils as well as lower percent (%) lymphocytes correlated with worse overall survival (OS). In this study we aimed to develop a prognostic signature for HNSCC treated with ICIs using these peripheral blood biomarkers (PBBMs).
Methods
Adults with R/M HNSCC treated with ICIs at our institution from 08/2012 to 03/2021 with pretreatment PBBMs were included. Follow-up continued until 02/15/2022. The cohort (n = 151) was randomly split into training (n = 100) and testing (n = 51) datasets. A prognostic score incorporating LDH, % lymphocytes, and abx neutrophils was developed from the training dataset using Cox proportional hazards regression. In the training dataset, a grid search identified the optimal cutpoints classifying patients into high, medium, and low-risk groups (trichotomized signature) as well as high vs. low-risk groups (dichotomized signature). The prognostic score, dichotomized and trichotomized signatures were then validated in the testing dataset.
Results
Training and testing datasets showed no clinically meaningful differences in clinicodemographic characteristics or PBBMs. An OS prognostic model was developed from the training dataset: Risk score = 1.24*log10(LDH) − 1.95*log10(% lymphocytes) + 0.47*log10(abx neutrophils). Optimal risk score cutpoints for the dichotomized and trichotomized signatures were defined in the training dataset, and Kaplan-Meier curves for both dichotomized and trichotomized signatures showed good separation between risk groups. Risk scores were calculated in the testing dataset, where the trichotomized signature demonstrated overlap between low and medium-risk groups but good separation from the high-risk group while the dichotomized signature showed clear separation between low and high-risk groups. Higher risk score correlated with worse OS (HR 2.08, [95%CI 1.17–3.68], p = 0.012). Progression-free survival Kaplan-Meier curves likewise showed excellent separation between dichotomized risk groups in the training and testing datasets.
Conclusions
We developed a prognostic signature for OS based on 3 previously identified PBBMs for HNSCC treated with ICIs and identified a high-risk group of patients least likely to have survival benefit from ICIs. This signature may improve ICI patient selection and warrants validation in an independent cohort as well as correlation with CPS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13051-6.
Keywords: Peripheral blood biomarkers, Head and neck cancer, Immunotherapy, Immune checkpoint inhibitors
Background
Better methods are needed to improve patient selection for immune checkpoint inhibitors (ICIs) in treatment of recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). We currently rely on combined proportion score (CPS), a measure of PD-L1 expression, to select patients to receive pembrolizumab. Although it correlates with improved outcomes in treatment with ICIs, CPS is an imperfect biomarker. Even in the PD-L1 enriched population of CPS ≥ 20, only a minority of patients respond to ICIs as demonstrated in the landmark KEYNOTE-048 phase III clinical trial [1].
In previously published work, we demonstrated that peripheral blood biomarkers (PBBMs) can provide valuable prognostic information among patients with R/M HNSCCs treated with ICIs [2, 3]. In contrast to CPS biomarker, PBBMs are cost-effective, easily obtainable without invasive procedures, and routinely measured in clinical practice. We observed that higher pretreatment lactate dehydrogenase (LDH) and absolute (abx) neutrophils as well as lower percent (%) lymphocytes correlated with worse overall survival (OS) and progression-free survival (PFS) [3]. Similarly, higher LDH and lower % lymphocytes correlated with worse objective response (ORR). Our findings in the immunotherapy setting are consistent with previously reported PBBMs in a variety of treatment modalities for HNSCC. Moreover, we showed for the first time that pretreatment elevated serum LDH is a negative prognostic marker for OS, PFS, and ORR to immunotherapy in R/M HNSCC [3–6].
In this study, we aimed to explore the clinical applicability of our prior results by developing a prognostic signature for pretreatment OS risk stratification in HNSCC treated with ICIs using the previously identified PBBMs.
Methods.
Study design and population
Patients with R/M HNSCC treated with ICIs between August 2012 and March 2021 at the University of Washington Medical Center/Fred Hutchinson Cancer Center were included. Those without pre-treatment peripheral blood data were excluded. Follow-up continued until February 15, 2022. Blinding and power analysis were not relevant to this study. This study was reviewed and approved by the Fred Hutchinson Cancer Research Institutional Review Board.
Demographics including age at initiation of ICI treatment, sex, and race were reported. Treatment regimens and line of treatment were recorded. Performance status (PS) was evaluated on the first day of ICI administration using the Eastern Cooperative Oncology Group (ECOG) scale as documented by the patient’s oncologist. Additional characteristics recorded included smoking history, alcohol use, primary site, p16 status, and Charlson comorbidity index (CCI). Smoking history was defined as current smoker, former smoker (quit ≥ 12 months prior to treatment initiation), or never smoker. Alcohol use was categorized according to CDC guidelines, with heavy drinking defined as ≥ 8 drinks/week for women or ≥ 15 drinks/week for men, moderate drinking defined as ≤ 1 drink/day for women or ≤ 2 drinks/day for men, and none/rare defined as ≤ 1 drink per week. CPS was not included as it was not routinely collected during the study time frame (the approval of pembrolizumab as monotherapy in the first-line setting based on the CPS biomarker occurred in October 2021).
Correlative blood sample collection
Correlative peripheral blood samples were collected within 24 h prior to starting ICI treatment. Based on our prior work demonstrating PBBMs that correlated with OS, the following PBBMs were evaluated: LDH, % lymphocytes, and abx neutrophils.
Statistical analysis
We randomly split the full cohort into training and testing datasets with a 2:1 ratio (n = 100 for the training dataset and n = 51 for the testing dataset). The prognostic signatures were developed using training data only and validated in the testing dataset. Descriptive statistics were provided, including median and range for continuous variables and count and percentage for categorical variables. We built an OS multivariable Cox regression model using the three previously identified PBBMs (LDH, % lymphocytes, abx neutrophils). CPS was not included as it was not routinely collected during this time. The continuous risk score = 1XLDH+2X%lymphocytes+3Xabx neutrophils was calculated based on estimated coefficients from the Cox model and 3 PBBMs. We stratified patients into discrete risk groups (dichotomized as high vs. low, or trichotomized as high vs. medium vs. low) using a grid search approach maximizing the score test statistics from the Cox model to identify the optimal cutpoints. For instance, for the dichotomized biomarker signature (cutoff θ), the high-risk group was defined as risk score ≥ θ and the low-risk group was defined as risk score < θ; for the trichotomized biomarker signature (cutoff θ1, θ2), the high-risk group was defined as risk score ≥ θ2, the medium-risk group was θ2 > risk score > θ1, and the low-risk group was risk score ≤ θ1. Kaplan-Meier curves were generated to show the survival difference by each risk group. We evaluated the biomarker signature (both dichotomized and trichotomized) association with both PFS and OS, adjusting for ECOG PS, p16 status, and smoking as potential confounding variables. Furthermore, in the validation analysis, we evaluated both the continuous risk score and biomarker signature in the testing dataset with Cox regression for PFS and OS. All analyses were conducted on R Version 4.3.2.
Results
We split our institutional cohort of R/M HNSCCs treated with ICIs (n = 151) into training (n = 100) and testing (n = 51) datasets. For the overall cohort (n = 151), the median age was 64.0 years (range 25.0–90.0), the majority were male (n = 115, 76.2%), and 64 (42.4%) were never smokers. Of the 59 (39%) patients with cancer of the oropharynx, 43 (72.9%) were p16 positive. Thirty-one (20.5%) of patients were treated with an ICI as a first-line therapy in the recurrent/metastatic setting, while 120 patients (79.5%) received an ICI as at least second line therapy. The majority (n = 118, 78.1%) were treated with PD-1 inhibitor monotherapy while the remaining (n = 33, 21.9%) were treated with a PD-1 inhibitor combination regimen. Training and testing datasets showed no clinically meaningful differences in age, sex, race, smoking, alcohol, Charlson comorbidity index, primary site, p16 status, ECOG PS, LDH, % lymphocytes, or abx neutrophils (Table 1).
Table 1.
Summary of patient characteristics and peripheral blood parameters by training and testing datasets
| Training Set | Testing Set | Total | |
|---|---|---|---|
| N = 100 | N = 51 | N = 151 | |
| Age | |||
| Median (range) | 64.0 (25.0–90.0) | 63.0 (30.0–80.0) | 64.0 (25.0–90.0) |
| Sex | |||
| F | 25 (25.0%) | 11 (21.6%) | 36 (23.8%) |
| M | 75 (75.0%) | 40 (78.4%) | 115 (76.2%) |
| Race | |||
| Asian | 7 (7.0%) | 4 (7.8%) | 11 (7.3%) |
| Black | 2 (2.0%) | 1 (2.0%) | 3 (2.0%) |
| Native American | 2 (2.0%) | 0 (0.0%) | 2 (1.3%) |
| Native Hawaiian/Pacific Islander | 3 (3.0%) | 0 (0.0%) | 3 (2.0%) |
| White | 82 (82.0%) | 43 (84.3%) | 125 (82.8%) |
| Declined/Unknown | 4 (4.0%) | 3 (5.9%) | 7 (4.6%) |
| Smoking | |||
| Never | 40 (40.0%) | 24 (47.1%) | 64 (42.4%) |
| Former | 50 (50.0%) | 17 (33.3%) | 67 (44.4%) |
| Current | 10 (10.0%) | 10 (19.6%) | 20 (13.2%) |
| Alcohol | |||
| Unknown | 4 | 0 | 4 |
| Heavy | 7 (7.3%) | 6 (11.8%) | 13 (8.8%) |
| Moderate | 32 (33.3%) | 16 (31.4%) | 48 (32.7%) |
| None/Rare | 57 (59.4%) | 29 (56.9%) | 86 (58.5%) |
| CCI | |||
| Median (range) | 7.0 (2.0–12.0) | 7.0 (2.0–12.0) | 7.0 (2.0–12.0) |
| ECOG PS | |||
| 0 | 27 (27.0%) | 15 (29.4%) | 42 (27.8%) |
| 1 | 56 (56.0%) | 29 (56.9%) | 85 (56.3%) |
| 2 | 16 (16.0%) | 5 (9.8%) | 21 (13.9%) |
| 3 | 1 (1.0%) | 2 (3.9%) | 3 (2.0%) |
| Number of Lines of Prior Systemic Therapy | |||
| ICI as First-Line | 20 (20.0%) | 11 (21.6%) | 31 (20.5%) |
| ICI as Second-Line or Greater | 80 (80.0%) | 40 (78.4%) | 120 (79.5%) |
| ICI Treatment Regimen | |||
| Single Agent | 79 (79.0%) | 38 (74.5%) | 117 (77.5%) |
| Combination Regimen | 21 (21.0%) | 13 (25.5%) | 34 (22.5%) |
| Primary Site | |||
| Common mucosal sites§ | 85 (85%) | 40 (78.4%) | 125 (82.8%) |
| Cutaneous | 8 (8.0%) | 3 (5.9%) | 11 (7.3%) |
| Nasopharynx | 4 (4.0%) | 3 (5.9%) | 7 (4.6%) |
| Sinonasal | 3 (3.0%) | 5 (9.8%) | 8 (5.3%) |
| P16 Status* | |||
| N | 9 (22.5%) | 7 (36.8%) | 16 (27.1%) |
| Y | 31 (77.5%) | 12 (63.2%) | 43 (72.9%) |
| LDH† | |||
| Median (range) | 2.2 (1.9–2.9) | 2.2 (1.9–3.2) | 2.2 (1.9–3.2) |
| Lymphocytes (%)† | |||
| Median (range) | 1.1 (0.3–1.8) | 1.1 (0.5–1.5) | 1.1 (0.3–1.8) |
| Neutrophils (abx)† | |||
| Median (range) | 0.7 (0.2–1.6) | 0.7 (0.1–1.3) | 0.7 (0.1–1.6) |
*Oropharynx subsite only
†Log10 transformed
§Oral cavity, oropharynx, hypopharynx, larynx, unknown primary
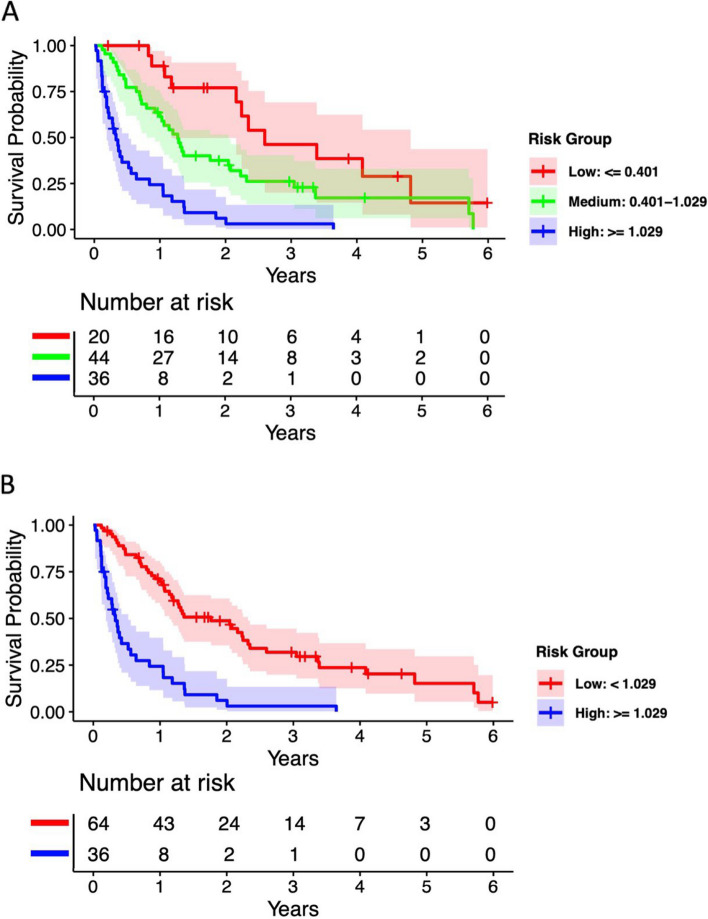
We used the training dataset to conduct an OS multivariable Cox regression model with the 3 previously identified biomarkers. Overall survival risk scores were calculated using coefficients from this model multiplied by the biomarker values for each patient, with log10 transformation used to normalize the biomarkers: OS risk score = 1.24*log10(LDH) − 1.95*log10(% lymphocytes) + 0.47*log10(abx neutrophils). We then used the grid search approach to identify the optimal cutpoints for the risk scores. For the trichotomized biomarker signature (high vs. medium vs. low risk), the optimal cutpoints were θ1 = 0.401 and θ2 = 1.029, resulting in 20 low-risk (risk score ≤ 0.401), 44 medium-risk (0.401 < risk score < 1.029), and 36 high-risk patients (risk score ≥ 1.029) in the training dataset. For the dichotomized biomarker signature (high vs. low risk), the optimal cutpoint was θ = 1.029, which was equivalent to combining low and medium groups in the trichotomized signature. Kaplan-Meier OS curves based on the training data are shown in Fig. 1A for the trichotomized biomarker signature and Fig. 1B for the dichotomized biomarker signature. In the Supplementary analysis, similar Kaplan-Meier curves were generated for PFS in the training datasets.
Fig. 1.
Kaplan-Meier estimates on OS by optimal cutpoints for (A) trichotomized (high, medium and low-risk) groups and (B) dichotomized (high and low-risk) in training dataset
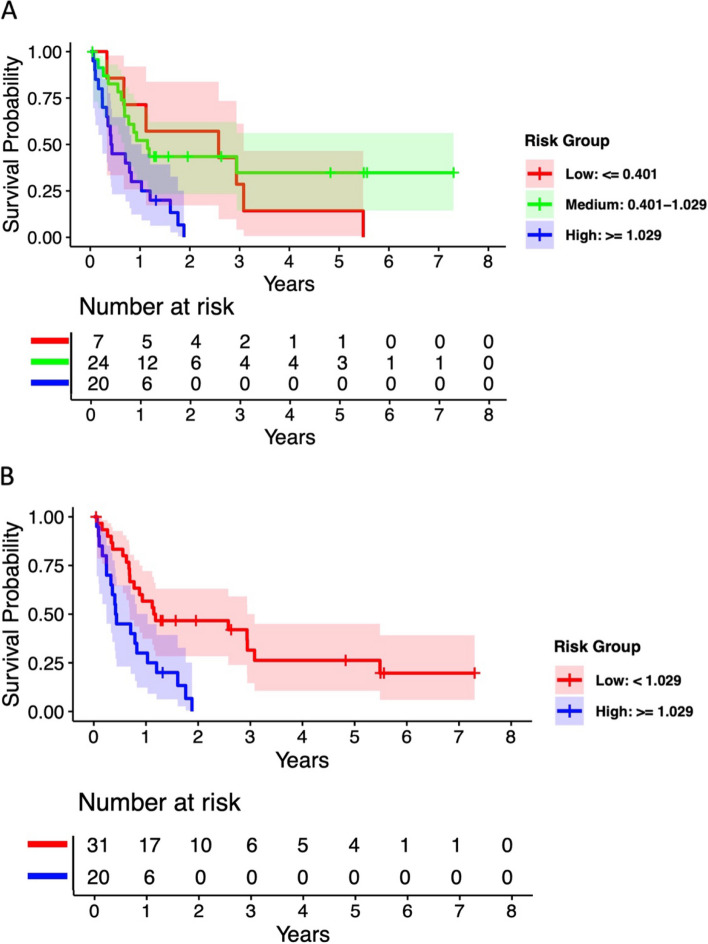
Using the OS risk score formula developed in the training dataset, we then calculated risk scores for patients in the testing data (n = 51) and trichotomized them into risk groups using the predetermined optimal cutpoints (θ1 = 0.401 and θ2 = 1.029), resulting in 7 low-risk, 24 medium-risk, and 20 high-risk patients. Kaplan-Meier OS curves for testing data showed some overlap between low and medium-risk groups, but good separation from the high-risk group with log-rank p-value = 0.005 (Fig. 2A). Similarly, Fig. 2B showed a clear survival difference by dichotomized risk groups (20 high vs. 31 low-risk patients, log-rank p-value = 0.001). We further evaluated the continuous risk score by Cox regression in the testing dataset and showed that a higher risk score is statistically significantly associated with worse OS (HR 2.08, [95%CI 1.17–3.68], p = 0.0125) after adjusting for ECOG PS, smoking, and p16 status. In the Supplementary analysis, similar Kaplan-Meier curves were generated for PFS in the testing datasets.
Fig. 2.
Kaplan-Meier estimates on OS by optimal cutpoints for (A) trichotomized (high, medium and low-risk) groups and (B) dichotomized (high and low-risk) in testing dataset
Discussion
In this study we developed a prognostic biomarker signature for OS based on 3 pretreatment peripheral blood biomarkers previously shown to correlate with oncologic outcomes for immunotherapy in R/M HNSCC: LDH, % lymphocytes, and abx neutrophils [3]. Using this biomarker signature, we stratified patients into high, medium, and low risk groups (trichotomized signature) as well as high versus low-risk groups (dichotomized signature). Currently the standard biomarker to select patients more likely to benefit from PD-1 inhibitors relies on PD-L1 expression of tumor cells, lymphocytes, and macrophages to calculate CPS. However, PD-L1-negative tumors occasionally respond to PD-1 inhibitors and even among tumors with high PD-L1 expression or favorable CPS, only a minority will respond to PD-1 inhibitors, indicating the importance of other mechanisms through with ICIs work [1, 7].
Robust literature exists demonstrating the importance of immune cells both in the tumor microenvironment and in the periphery in regards to immunotherapy response [7]. Lymphocytes, particularly NK cells and CD8 + cytotoxic T cells, play a fundamental role in antitumor immunity, with multiple studies in melanoma, non-small cell lung cancer, and more recently HNSCC showing elevated peripheral lymphocytes to be associated with improved response and survival in the ICI treatment setting [3, 7–9]. In fact, directly inhibiting egress of lymphocytes from lymph nodes, such as through administration of fingolimod, an immunomodulator used to treat multiple sclerosis, has been shown to reduce the efficacy of immunotherapy [7]. In contrast, neutrophils promote carcinogenesis through multiple mechanisms including production of various cytokines, growth factors, proteases, and reactive oxygen species [10]. Several studies in solid tumors, including head and neck, have demonstrated that elevated peripheral neutrophils are associated with poor survival and response to immunotherapy [3, 11–13]. LDH is likewise negatively correlated with oncologic outcomes in immunotherapy, most notably in melanoma, and recently shown in head and neck [3, 14–16]. It is a key enzyme in anaerobic glycolysis, which allows for proliferation of aggressive tumors under hypoxic conditions.
The utility of PBBMs in outcome prognostication in R/M HNSCC receiving ICI is a subject of ongoing scientific inquiry. To our knowledge, our data represents the only PBBM prognostic score relying on three routinely obtained laboratory results. Our observations complement early reported efforts at generating prognostic survival models in the immunotherapy treatment setting for HNSCC. Issa et al. recently developed and internally validated a nomogram to prognosticate survival using age and other variables they found to be associated with OS, including p16 status, neutrophils, lymphocytes, albumin, hemoglobin, and LDH [17]. While the PBBMs incorporated in our prognostic signature are consistent with those selected by Issa et al., our model relies on percent rather than absolute lymphocytes, as we previously found percent lymphocytes to be more strongly correlated with OS than absolute lymphocytes on our elastic net variable selection analysis [3]. Additionally, our signature allows for application of LDH as a continuous rather than dichotomous (high/low) variable used in the nomogram, allowing for increased information from this variable in the model.
The applicability and innovation of our prognostic signature lies in its low cost, ease of use and interpretation, as well as reliance on routinely-obtained bloodwork. Used as an adjunct or alternative to CPS, which requires an invasive procedure to obtain tissue for analysis, a prognostic signature based on peripheral blood may improve patient selection for expensive and potentially toxic immunotherapy without increasing morbidity. Although our trichotomized prognostic signature did not show good separation between the low and medium-risk groups in our relatively limited testing dataset, when we combined low and medium-risk groups in the dichotomized prognostic signature, we were crucially able to identify a high-risk group least likely to have a survival benefit from ICIs. This high-risk group may require more frequent monitoring and/or alternate or intensified therapies other than current standard ICI treatment regimens. With the significantly higher cost of ICIs compared to cytotoxic therapy, as well as the potential for severe immune-related adverse events, appropriate patient selection for these drugs is paramount [18]. The dichotomized prognostic signature has the potential to significantly improve patient selection for ICIs and warrants validation in an external, independent cohort.
Our study has several limitations, including the retrospective design and single institutional cohort, which likely reflects practice patterns at other tertiary academic centers but may not fully capture diversity in clinical practice throughout the field. Additionally, non-FDA-approved PD-1 inhibitor treatment regimens were included, although the vast majority (78.1%) of patients received an FDA-approved PD-1 inhibitor monotherapy. The majority of our patients underwent ICI treatment as second-line therapy, so our results may not extrapolate to the first-line ICI treatment setting. Our study did not include correlation with CPS biomarker, as most patients in our cohort began ICI treatment before routine use of CPS. Whether the high-risk score indicates an overall worse-performing group independent of ICI treatment is also not possible to conclude from our results. Studies evaluating the predictive potential of PBBMs for oncologic outcomes after treatment with ICIs as well as evaluating on-treatment or post-treatment PBBMs would add valuable insight into our understanding of PBBMs in the immunotherapy treatment setting.
Our work demonstrates a promising OS prognostic signature developed from previously identified PBBMs that can identify a subset of patients with R/M HNSCC at high risk for poor survival benefit from treatment with ICIs. The prognostic signature is also significant for PFS in both the training and testing datasets (Supplementary analysis). Ongoing work from our group aims to validate our prognostic signature in an external, independent cohort and compare with CPS biomarker.
Conclusions
In this study we developed a prognostic biomarker signature for OS based on LDH, % lymphocytes, and absolute neutrophils, which we previously demonstrated to correlate with OS in R/M HNSCC treated with ICIs. Using this prognostic signature, we were able to identify a high-risk group of patients least likely to have survival benefit from treatment with ICIs. This work represents an important step toward improving patient selection for immunotherapy in HNSCC and warrants validation in an independent, external cohort.
Supplementary Information
Acknowledgements
None.
Abbreviations
- ICI
Immune checkpoint inhibitor
- R/M
Recurrent/metastatic
- HNSCC
Head and neck squamous cell carcinoma
- CPS
Combined proportion score
- PBBM
Peripheral blood biomarker
- LDH
Lactate dehydrogenase
- Abx
Absolute
- %
Percent
- OS
Overall survival
- PFS
Progression-free survival
- ORR
Objective response rate
- PS
Performance status
- ECOG
Eastern Cooperative Oncology Group
- CCI
Charlson Comorbidity Index
Authors’ contributions
CP and CPR obtained and curated the data. KN, JV, and QVW led the statistical analysis. CP, CPR, and QVW were major contributors in writing the manuscript. CP, KN, JV, BB, ZHR, EM, RMF, NF, GEL, JJL, UP, NP, RGM, CPR, and QVW contributed to the conceptualization and writing of the manuscript. All authors read and approved the final manuscript.
Funding
National Institutes of Health T32 DC000018 (Dr. Pan).
Data availability
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Fred Hutchinson Cancer Research Institutional Review Board (IRB ID: STUDY00007717). The requirement to obtain informed consent was waived.
Consent for publication
Not applicable.
Competing interests
Conflict of Interest Disclosures: Dr. Futran reported educational consultancy role for Stryker Corporation. Dr. Rodriguez reported receipt of institutional research funding from AstraZeneca, Ayala, Bristol Myers Squibb, Ignyta, and Merck, and reported advisory board membership for Cue Biopharma. The other authors declare no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28. [DOI] [PubMed] [Google Scholar]
- 2.Pan C, Wu QV, Voutsinas J, Houlton JJ, Barber B, Futran N, et al. Neutrophil to lymphocyte ratio and peripheral blood biomarkers correlate with survival outcomes but not response among head and neck and salivary cancer treated with pembrolizumab and vorinostat. Head Neck. 2022;45:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan C, Wu QV, Voutsinas J, Houlton JJ, Barber B, Rizvi ZH, et al. Peripheral lymphocytes and lactate dehydrogenase correlate with response and survival in head and neck cancers treated with immune checkpoint inhibitors. Cancer Med. 2023;12:9384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luan CW, Tsai YT, Yang HY, Chen KY, Chen PH, Chou HH. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valero C, Pardo L, López M, García J, Camacho M, Quer M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39(2):219–26. [DOI] [PubMed] [Google Scholar]
- 6.Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- 8.Voutsadakis IA. Prediction of Immune checkpoint inhibitors benefit from routinely measurable peripheral blood parameters. Chin Clin Oncol. 2020;9(2):19–19. [DOI] [PubMed] [Google Scholar]
- 9.Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Saxena S, Awaji M, Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11(4):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusselier M, Deluche E, Delacourt N, Ballouhey J, Egenod T, Melloni B, et al. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS ONE. 2019;14(7):e0219060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh K, Kumar A, Ahmed J, Anwar A, Puccio C, Chun H, et al. Peripheral monocytes and neutrophils predict response to immune checkpoint inhibitors in patients with metastatic non-small cell lung cancer. Cancer Immunol Immunother. 2018;67(9):1365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Baseline neutrophil to lymphocyte ratio combined with serum lactate dehydrogenase level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol. 2018;179(1):213–5. [DOI] [PubMed] [Google Scholar]
- 14.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RRH, Hospers GAP, van den Eertwegh AJM, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63(5):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens A, Wistuba-Hamprecht K, Foppen MG, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issa M, Klamer BG, Mladkova N, Laliotis GI, Karivedu V, Bhateja P, et al. Update of a prognostic survival model in head and neck squamous cell carcinoma patients treated with immune checkpoint inhibitors using an expansion cohort. BMC Cancer. 2022;22(1):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.