Abstract
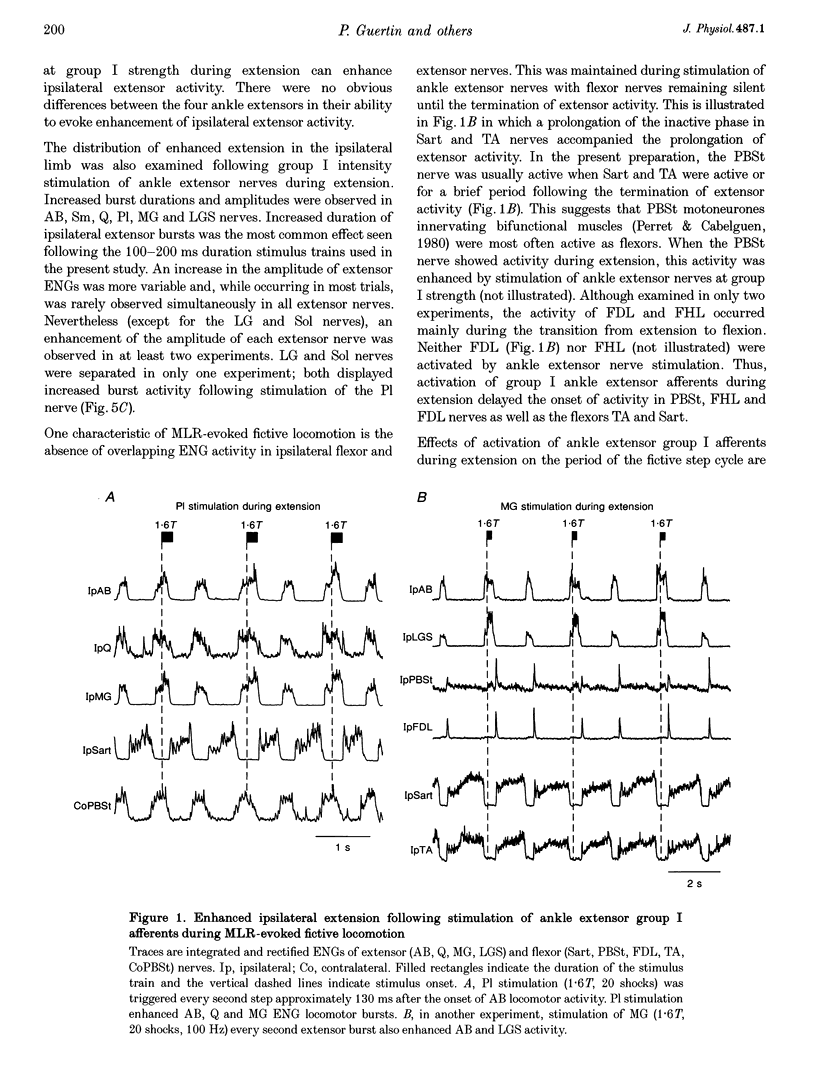
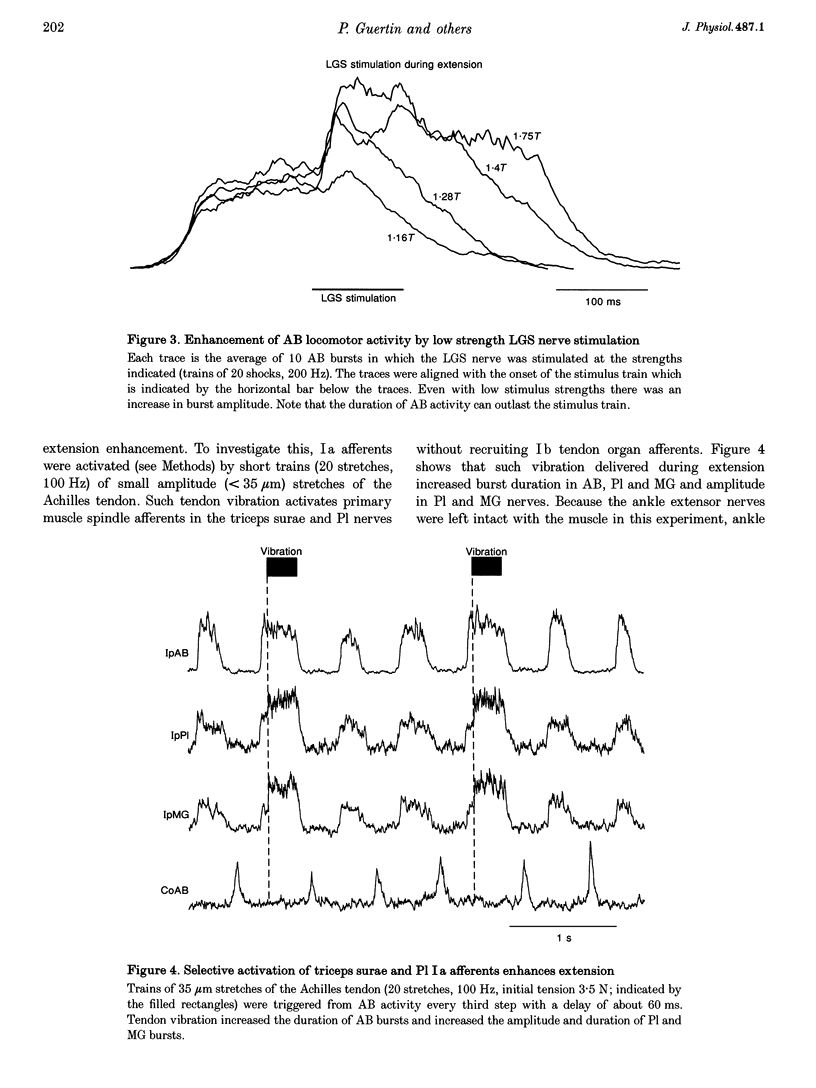
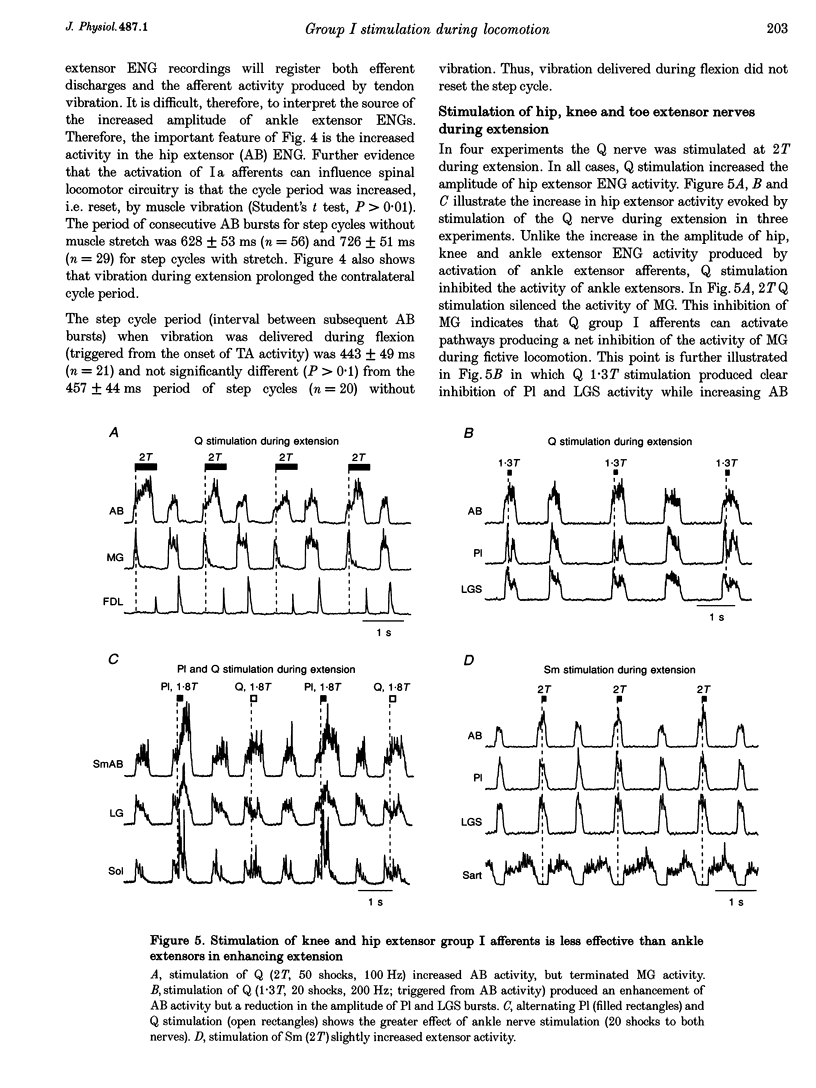
1. The effects of stimulating hindlimb extensor nerves (100-200 ms trains, 100 Hz, < or = 2 times threshold) during the flexor and extensor phases of the locomotor step cycle were analysed in the decerebrate, paralysed cat during fictive locomotion evoked by stimulation of the mesencephalic locomotor region. 2. Stimulation during extension of either the medial gastrocnemius (MG), lateral gastrocnemius-soleus (LGS) or plantaris (Pl) nerves was equally effective in increasing the duration and amplitude of electroneurogram (ENG) activity recorded in ipsilateral ankle, knee and hip extensor nerves. Enhancement of extensor ENG activity could be evoked with near threshold stimulation intensity and appeared within 10-40 ms of the onset of ankle extensor nerve stimulation. Stimulation of anterior biceps during extension occasionally evoked a modest increase in the duration of activity of hip, knee and ankle extensors. Stimulation of quadriceps during extension enhanced the activity of proximal extensors and soleus, but inhibited other ankle extensors. 3. Selective activation of ankle extensor Ia spindle afferents by muscle stretch also enhanced ipsilateral extension. It is argued that both muscle spindle and tendon organ afferents can contribute to the increase in extensor nerve activity evoked by group I stimulation intensity during fictive locomotion. 4. During flexion, stimulation of either the MG, Pl or LGS nerves at group I strength terminated on-going activity in ipsilateral flexors and initiated a burst of activity in ipsilateral hip, knee and ankle extensors, i.e. reset the step cycle to extension. 5. Low strength stimulation of the mixed muscle and cutaneous nerve innervating the plantar aspect of the foot produced extension enhancement and resetting similar to that evoked by group I muscle afferent stimulation. Stimulation of the cutaneous nerve supplying the dorsal aspect of the foot during extension enhanced extensor activity, and during flexion, enhanced the activity of flexors. 6. The effects reported here during fictive locomotion may also occur during overground locomotion with natural activation of group I muscle spindle and tendon organ afferents. Extensor spindle and tendon organ afferents may thus serve as an excitatory reflex system helping to shape the amplitude, duration and timing of ipsilateral extensor activity. Increased or unexpected activation of group I ankle extensor afferents or plantar foot afferents during locomotion could also compensate for increased loading of the limb.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway B. A., Hultborn H., Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68(3):643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S., Jankowska E., McCrea D. The heteronymous monosynaptic actions of triceps surae group Ia afferents on hip and knee extensor motoneurones in the cat. Exp Brain Res. 1986;61(2):443–446. doi: 10.1007/BF00239533. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard J. P., Brownstone R. M., Barajon I., Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98(2):213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992 Jul;72(3):623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981 Jul;316:411–425. doi: 10.1113/jphysiol.1981.sp013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Mackel R. Pattern of 'non-reciprocal' inhibition of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981 Jul;316:393–409. doi: 10.1113/jphysiol.1981.sp013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O., Iizuka M., Kudo N. Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci Lett. 1992 Dec 14;148(1-2):43–46. doi: 10.1016/0304-3940(92)90800-m. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Collins D. F. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993 Sep;70(3):1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Ramirez J. M., Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res. 1992;90(3):557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
- Perreault M. C., Angel M. J., Guertin P., McCrea D. A. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol. 1995 Aug 15;487(1):211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret C., Cabelguen J. M. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res. 1980 Apr 14;187(2):333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Trend P., Hulliger M., Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res. 1989;80:61–60. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Shik M. L., Severin F. V., Orlovskii G. N. Upravlenie khod'boi i begom posredstvom elektricheskoi stimulatsii srednego mozga. Biofizika. 1966;11(4):659–666. [PubMed] [Google Scholar]
- Whelan P. J., Hiebert G. W., Pearson K. G. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res. 1995;103(1):20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]


