Abstract
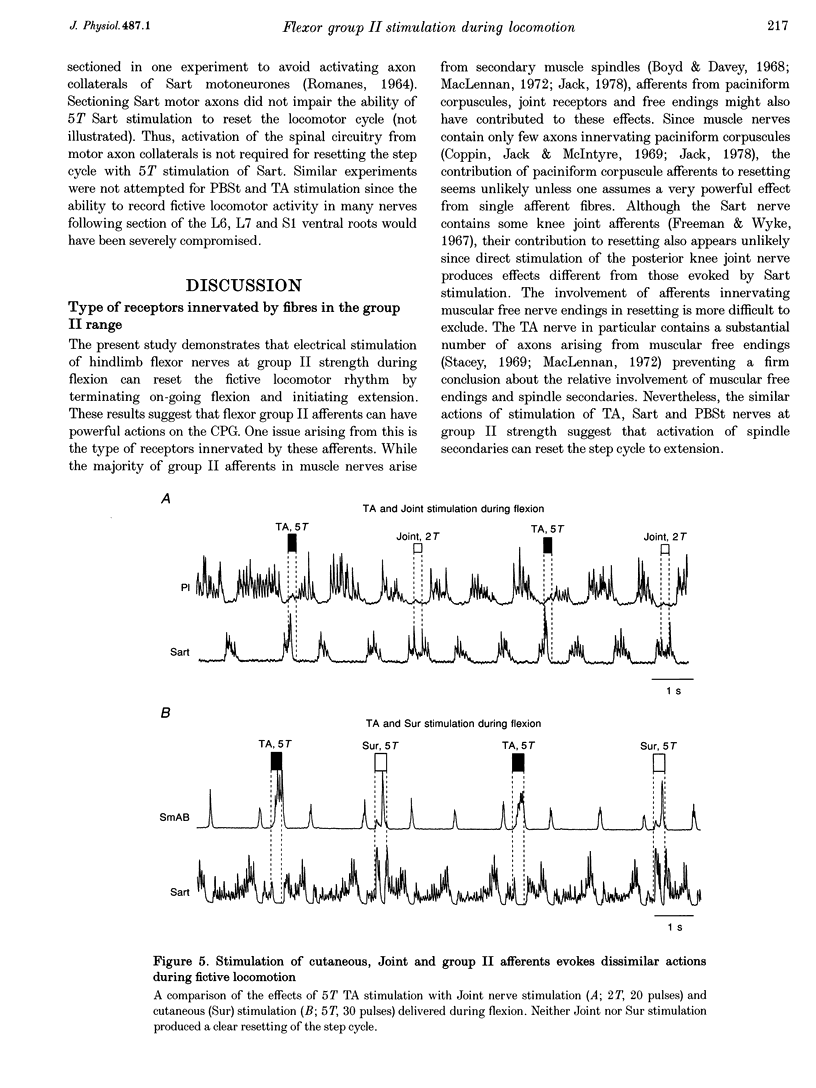
1. This study examines the effects of electrical stimulation of hindlimb flexor nerves on the fictive locomotion pattern. Locomotion was initiated by stimulation of the mesencephalic locomotor region in the decerebrate paralysed cat and monitored by recording the electroneurogram from selected hindlimb flexor and extensor muscle nerves. Flexor nerves were stimulated using short trains (20-50 stimuli at 100 Hz) during either the flexor or the extensor phase of the fictive locomotor cycle. 2. Stimulation of tibialis anterior (TA), posterior biceps and semitendinosus (PBSt) or sartorius (Sart) nerves at 5 times threshold (T) during the flexor phase of the fictive locomotor cycle terminated on-going activity in flexor nerves and initiated activity in extensors. Thus, flexor nerve stimulation during flexion shortened the locomotor cycle by resetting to extension. The failure of lower intensity (2T) stimulation of PBSt or Sart nerves to reset the step cycle to extension suggests that group II afferents are responsible for these actions. Resetting evoked by 2T stimulation of the TA nerve may be due to a high proportion of group II afferents with low electrical threshold. 3. During extension, stimulation of TA and PBSt nerves at 5T did not perturb the locomotor rhythm whereas Sart stimulation prolonged the locomotor cycle. 4. Stimulation of cutaneous or knee joint afferents failed to produce effects similar to those evoked by stimulation of flexor muscle nerves at group II strength. These findings are at odds with those obtained elsewhere in the acute spinal, DOPA fictive locomotion preparation. The possibility that group II resetting during fictive locomotion is not mediated by flexion reflex pathways but by previously unknown pathways released in the present preparation is discussed. 5. Since many of the flexor afferents recruited by 5T electrical stimulation are the length-sensitive group II fibres, spindle secondaries may act to regulate the duration and onset of flexor and extensor activity during real locomotion. The resetting from flexion to extension also suggests that unexpected or enhanced activity of flexor secondaries during swing would promote a switch of the step cycle to stance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway B. A., Hultborn H., Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68(3):643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Coppin C. M., Jack J. J., McIntyre A. K. Properties of group I afferent fibres from semitendinosus muscle in the cat. J Physiol. 1969 Jul;203(1):45P–46P. [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980 Oct 31;210(4469):492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967 Jun;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gossard J. P., Brownstone R. M., Barajon I., Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98(2):213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Guertin P., Angel M. J., Perreault M. C., McCrea D. A. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol. 1995 Aug 15;487(1):197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Pettersson L. G. Comments on group II excitation in hindlimb motoneurones in high and low spinal cats. Neurosci Res. 1988 Aug;5(6):563–566. doi: 10.1016/0168-0102(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967 Jul-Aug;70(3):389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Riddell J. S. A relay for input from group II muscle afferents in sacral segments of the cat spinal cord. J Physiol. 1993 Jun;465:561–580. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Hoffer J. A., Pratt C. A. Activity of spindle afferents from cat anterior thigh muscles. I. Identification and patterns during normal locomotion. J Neurophysiol. 1985 Sep;54(3):549–564. doi: 10.1152/jn.1985.54.3.549. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Exp Brain Res. 1987;65(2):282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987;65(2):294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- MacLennan C. R. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and the group II of the cat tibialis anterior muscle nerve. J Physiol. 1972 Apr;222(1):90P–91P. [PubMed] [Google Scholar]
- Noga B. R., Shefchyk S. J., Jamal J., Jordan L. M. The role of Renshaw cells in locomotion: antagonism of their excitation from motor axon collaterals with intravenous mecamylamine. Exp Brain Res. 1987;66(1):99–105. doi: 10.1007/BF00236206. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Collins D. F. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993 Sep;70(3):1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Ramirez J. M., Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res. 1992;90(3):557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
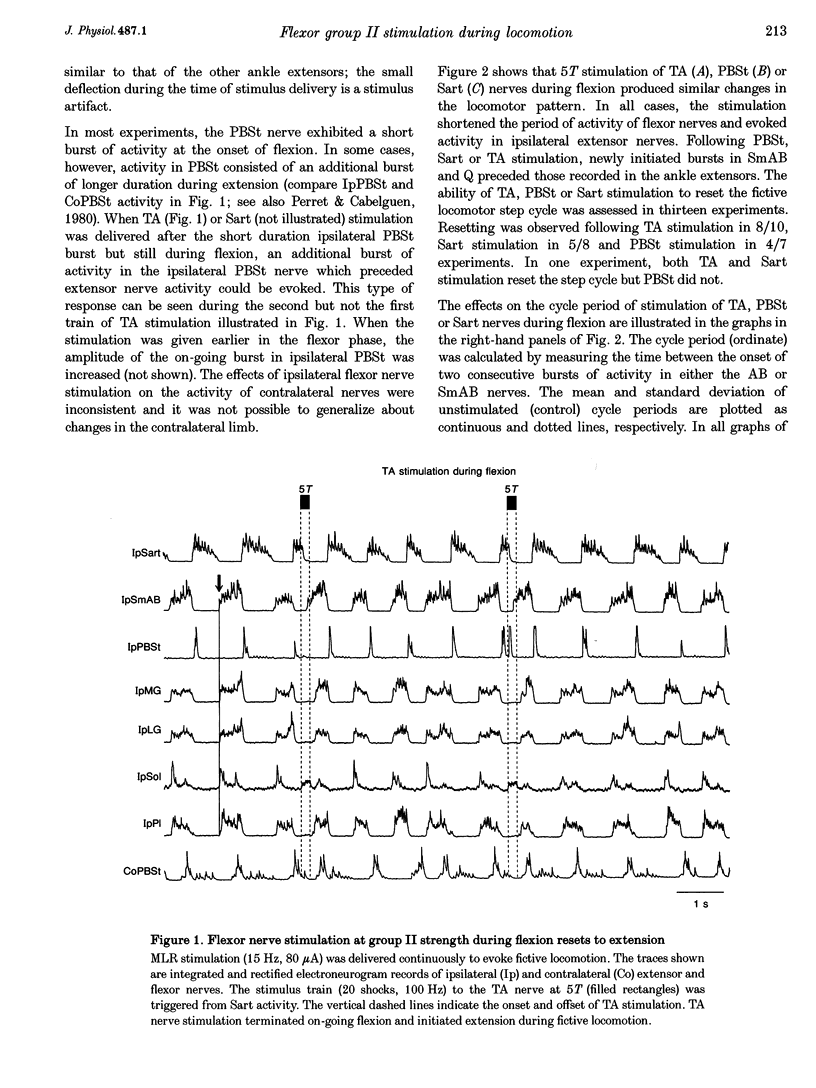
- Perret C., Cabelguen J. M. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res. 1980 Apr 14;187(2):333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Trend P., Hulliger M., Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res. 1989;80:61–60. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. THE MOTOR POOLS OF THE SPINAL CORD. Prog Brain Res. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- Shefchyk S., McCrea D., Kriellaars D., Fortier P., Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80(2):290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Stacey M. J. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231–254. [PMC free article] [PubMed] [Google Scholar]


