Abstract
Background
Epidemiological studies investigating the association between flavonoid intake and bone mineral density (BMD) draw inconsistent conclusions. Our study aims to investigate the association between flavonoid intake and BMD and osteoporosis and the mediating role of composite dietary antioxidant index (CDAI) in their relationship using data from the National Health and Nutrition Examination Survey (NHANES).
Methods
The study assessed the relationship between flavonoid intake and femur BMD and osteoporosis in 10,225 individuals from NHANES 2007–2010 and 2017–2018. Multivariable linear regression analyses were used to detect the association between flavonoid intake and femur BMD in adult Americans. Restricted cubic splines (RCS) were used to examine the nonlinear relationship between flavonoid intake and their subclasses and osteoporosis risk in individuals 20 years or older. We explored the mediating role of CDAI in the association between flavonoid intake and BMD.
Results
In fully adjusted multivariable regression analyses, compared with people in the first quartile, people in the fourth quartile of total flavonoid intake have a higher BMD at total femur (0.013, 95% CI: 0.004, 0.022, P = 0.001), femur neck (0.010, 95% CI: 0.004, 0.017, P = 0.001), trochanter (0.010, 95% CI: 0.004, 0.017, P = 0.001), and intertrochanter (0.012, 95% CI: 0.003, 0.020, P = 0.006). The positive relationship between flavonoid intake and femur BMD was present in both sexes. Furthermore, we found that people in the fourth quartile of total flavonoid intake have a lower risk of osteoporosis compared with the first quartile (OR = 0.686, 95% CI: 0.528–0.890, P = 0.005). RCS found a linear inverse relationship between total flavonoid intake and osteoporosis in individuals ≥ 20 years (Overall P = 0.015, nonlinear P = 0.086). Moreover, CDAI partially mediates the association of total flavonoid intake with femur BMD.
Conclusions
Our findings suggest that higher flavonoid intake is associated with higher BMD and lower risk of osteoporosis in Americans. Furthermore, we found distinct associations between different flavonoid subclasses and osteoporosis risk. More studies with stronger evidence are needed to explore the causal association between flavonoid intake and bone health and their underlying mechanisms.
Keywords: Flavonoids, Osteoporosis, Bone mineral density, Americans, NHANES
Introduction
Osteoporosis is a common bone disease characterized by low bone mass that leads to a higher risk of fragility fractures and mortality in the elderly [1]. Based on the World Health Organization (WHO) diagnostic criteria, over one-fifth of the world's population over 50 has osteoporosis [2]. In the United States, an estimated 10.2 million older adults had osteoporosis, and 43.4 million had low bone mass in 2010 [3]. As the population ages, this number still increases dramatically, placing a significant health and economic burden on society [4]. The development of osteoporosis could be attributed to various factors, including genetic, environmental, and dietary factors, and others [5, 6].
Flavonoids are bioactive compounds that widely exist in various plant-based foods, such as vegetables, soybeans, fruits, and others [7]. Flavonoids can be categorized into six subclasses, including isoflavones, anthocyanins, flavan-3-ols, flavanones, flavones, and flavonols, which have been proven to be associated with many health conditions in humans [8–10]. Evidence has shown the potential of flavonoids in reducing bone loss and preventing osteoporosis as their anti-inflammatory or antioxidant ability [11, 12]. However, some clinical studies investigating the association between flavonoid intake and osteoporosis draw inconsistent conclusions [13, 14]. In an early meta-analysis involving ten randomized controlled trials (RCTs), Ma et al. [13] found that supplementation with 90 mg/d isoflavones for six months could improve spinal bone mineral density (BMD). In another meta-analysis of ten RCTs, Liu et al. [14] found no significant change in BMD in women after supplementing with 87 mg/d soy isoflavones for at least one year. To date, only a few studies have investigated the relationship between dietary intake of total flavonoids and their subclasses and BMD [15–17]. Zhang et al. [15] found that dietary flavonoid intake was positively associated with the lumbar, femur, and whole-body BMD in women but not men. Similar results were observed in two other studies exploring the association between flavonoid intake and bone health in British women [16, 17]. However, to our knowledge, no studies have examined the relationship between flavonoid intake and bone health in the U.S. population. Whether flavonoid intake is positively associated with BMD in men remains to be demonstrated. Furthermore, many previous basic studies have shown that flavonoids may play an osteogenic role by reducing oxidative stress [18–20], but few clinical studies have validated this conclusion.
Thus, this study aimed to investigate the association between flavonoids intake (isoflavones, anthocyanins, flavan-3-ols, flavanones, flavones, flavonols, and total flavonoids) and femur BMD and osteoporosis based on the National Health and Nutrition Examination Survey (NHANES) 2007–2010 and 2017–2018. To investigate whether dietary oxidative stress mediated the association between flavonoid intake and BMD, we explored the mediating role of the composite dietary antioxidant index (CDAI) in the association between flavonoid intake and BMD.
Methods
Study design
The NHANES is a national nutrition and health program on the U.S. population that collects and publicly releases data biennially. The National Center for Health Statistics Ethics Review Committee gives consent for the NHANES program. Each participant or their guardians signed informed consent forms for the NHANES programs. We combined the NHANES data from cycles 2007–2010 and 2017–2018 because there was no flavonoid intake information from 2011 to 2016. We enrolled subjects aged 20 years or older. At first, 29,940 individuals from NHANES 2007–2010 and 2017–2018 were enrolled. Then, 12,218 participants were excluded because they were younger than 20 years, 5,658 participants were excluded because of missing BMD data, and 1,839 participants were excluded because of missing flavonoid intake data. Finally, 10,225 individuals were enrolled in this study. The participant selection flowchart was displayed in Fig. 1.
Fig. 1.
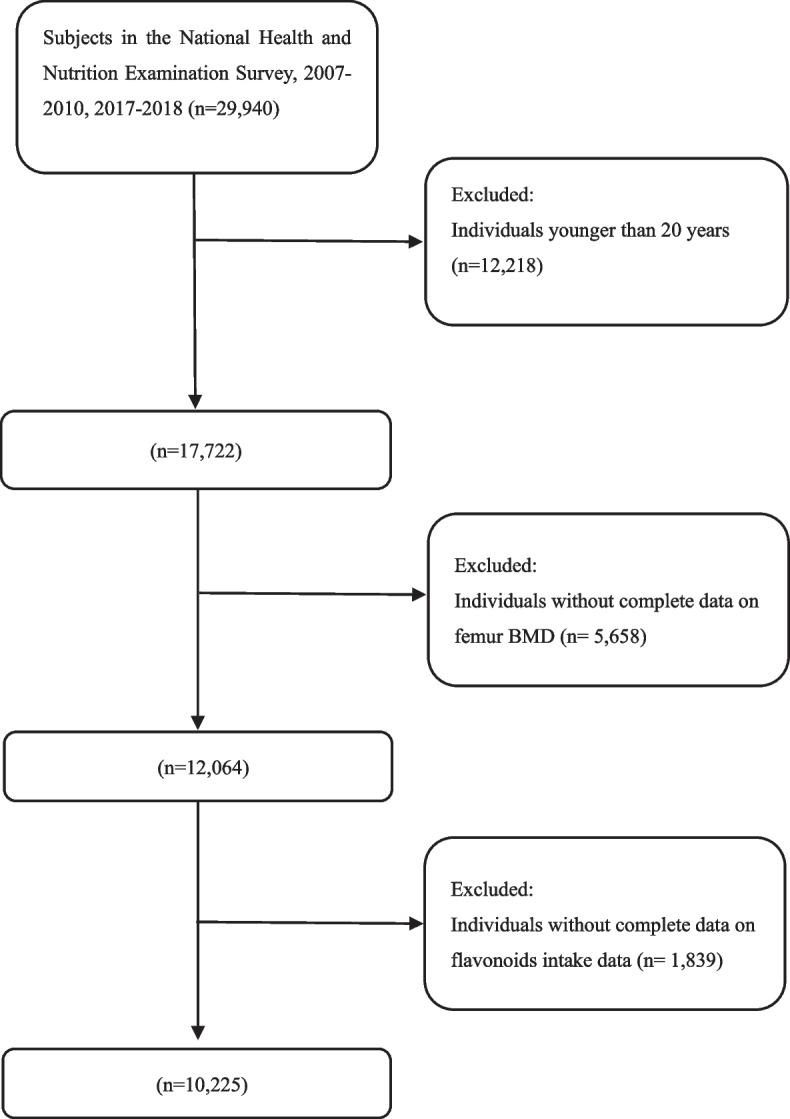
Flowchart of participants selection
BMD measurement and osteoporosis
BMD as a continuous variable is the outcome indicator for the present study, including total femur, femur neck, total femur, trochanter, and intertrochanter BMD. All BMD data was acquired by dual-energy X-ray absorptiometry (DXA) using Hologic densitometers. Professionals collected and standardized BMD data. Detailed BMD data can be accessed in DXXOFBMD, DXXNKBMD, DXXTRBMD, and DXXINBMD datasets on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Osteoporosis as a categorical variable is another outcome indicator in this study. According to the diagnostic criteria by the WHO, osteoporosis was determined by any femur BMD values less than 2.5 standard deviations (T-score < 2.5) from the reference group [21]. The BMD thresholds for total femur, femoral neck, trochanter, and intertrochanter were 0.68 g/cm2, 0.59 g/cm2, 0.49 g/cm2, and 0.78 g/cm2, respectively [22]. Participants were defined as having osteoporosis if their BMD at any of the four sites was below the threshold values.
Dietary flavonoid intake assessment
The USDA Dietary Research Food and Nutrient Database (FNDDS) is a food/beverage database used mainly to calculate nutrient intakes for NHANES, what We Eat in the United States (WWEIA), and meal recalls [23]. The USDA Automated Multiple-Pass Method was used to calculate intake information of flavonoids [24]. All collected foods were coded using the USDA FNDDS database and then correlated to specific flavonoid values using the USDA Survey Food Code Flavonoid Values Database (Flavonoid Database). The flavonoids comprise six subclasses, including isoflavones, anthocyanins, flavonols, flavan-3-ols, flavanones, and flavones, encompassing 29 different bioactive compounds [25]. The average dietary flavonoid intakes were calculated through two 24-h dietary recall interviews. The initial dietary recall interview was performed at the Mobile Examination Center (MEC), followed by a second interview 3 to 10 days later via a return phone call. The final intake of flavonoids was estimated by averaging two 24-h dietary recalls.
Covariates measurements
Covariates were chosen based on the published studies to eliminate potential effects on the final results [26–28]. Sociodemographic characteristics such as race/ethnicity, age, sex, poverty income ratio (PIR), and education level were collected through self-reported questionnaires. Race/ethnicity was divided into five groups (Non-Hispanic White, Black, Mexican American, Other Hispanic, and Other Race). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Education level was classified into three groups (College degree or above, High school graduate, and Under high school). Questionnaires determined smoking behavior, drinking behavior, and physical activity: Smoke at least 100 cigarettes in life? Have you ever had 5 or more drinks every day? Do any vigorous-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate in a typical week? In addition, total protein, serum calcium, serum phosphorus, blood urea nitrogen, cholesterol, and serum uric acid were collected by laboratory measurements. Healthy Eating Index-2015 (HEI-2015) is a density-based index calculated based on dietary nutrient intake per 1,000 kcal rather than absolute amounts [29]. The total score ranges from 0 to 100, with higher scores indicating better dietary quality [29]. Multiple interpolation was performed using the MICE package in R for missing covariates. Detailed data on covariates can be seen at the NHANES website (http://www.cdc.gov/nchs/nhanes/).
Statistical analysis
Mean ± standard deviation and percentages were used to represent continuous and categorical variables. To compare differences between the characteristics of participants, we used linear regression models and χ2 tests for continuous and categorical variables, respectively. In the primary analysis, multivariable linear regression models were performed to determine the association of flavonoid intake and their subclasses with BMD, with Mobile Examination Center (MEC) weight adjusted. When the continuous variable (femur BMD) and the dichotomous variable (osteoporosis) were used as outcome variables, the effect values were beta values and odds ratios, respectively. We first built an unadjusted model (Model 1). Then, Model 2 was created by adjusting race/ethnicity, age, and sex. Finally, Model 3 was created by adjusting all variables of race/ethnicity, age, sex, BMI, PIR, education level, smoking behavior, drinking behavior, physical activity, total protein, serum calcium, serum phosphorus, blood urea nitrogen, cholesterol, serum uric acid, and HEI-2015. Then, we explored the association between flavonoid intake and osteoporosis in individuals aged 20 years or older. Then, logistic regression models with restricted cubic splines (RCS) of three knots (5th, 50th, and 95th percentiles) were used to examine the nonlinear association between flavonoid intake and the risk of osteoporosis in individuals ≥ 20 years. The potential mediated effect of CDAI on the association between total flavonoid intake and BMD was estimated by parallel mediator analysis. The direct effect (DE) is the effect of total flavonoid intake on BMD without mediators. Indirect effects (IE) are the consequences of total flavonoid intake on BMD that are mediated by mediators. The fraction of mediators was estimated by dividing IE by TE (total effect). All analyses were performed by R software (4.3.1) and EmpowerStats (4.0), with P values < 0.05 regarded as statistically significant.
Results
Baseline characteristics
The participants' baseline characteristics were listed in Table 1. A sample of 10,225 subjects ≥ 20 were recruited in our analyses, of which 5,120 (50.07%) were men and 5,105 (49.93%) were women, with a mean age of 52.86 ± 17.19 years. The mean participants' intake of isoflavones, anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, and total flavonoids was 1.73 ± 10.10, 12.48 ± 30.06, 158.06 ± 346.14, 14.24 ± 26.50, 0.87 ± 1.55, 17.58 ± 16.52, 204.95 ± 363.32 mg/d. Compared to the first quartile of flavonoid intake, individuals in the higher quartile of flavonoid intake are more likely to be older, Non-Hispanic White, well educated, and have higher values of PIR, blood urea nitrogen, serum phosphorus, HEI-2015, and more vigorous recreational activities. They have fewer smoking and drinking behaviors and lower BMI, serum total protein, and serum uric acid. (Table 1).
Table 1.
Characteristics of enrolled participants based on total flavonoid intake quartiles
| Total | Q1 | Q2 | Q3 | Q4 | P value | |
|---|---|---|---|---|---|---|
| N | 10225 | 2556 | 2556 | 2556 | 2557 | |
| Age (years) | 52.86 ± 17.19 | 51.35 ± 17.67 | 52.12 ± 17.29 | 54.42 ± 17.09 | 53.54 ± 16.55 | < 0.001 |
| Gender (%) | 0.451 | |||||
| Men | 5120 (50.07%) | 1275 (49.88%) | 1310 (51.25%) | 1282 (50.16%) | 1253 (49.00%) | |
| Women | 5105 (49.93%) | 1281 (50.12%) | 1246 (48.75%) | 1274 (49.84%) | 1304 (51.00%) | |
| Race/ethnicity (%) | < 0.001 | |||||
| Mexican American | 1638 (16.02%) | 422 (16.51%) | 512 (20.03%) | 441 (17.25%) | 263 (10.29%) | |
| Other Hispanic | 1092 (10.68%) | 259 (10.13%) | 310 (12.13%) | 332 (12.99%) | 191 (7.47%) | |
| Non-Hispanic White | 4927 (48.19%) | 1226 (47.97%) | 1143 (44.72%) | 1168 (45.70%) | 1390 (54.36%) | |
| Non-Hispanic Black | 1950 (19.07%) | 549 (21.48%) | 468 (18.31%) | 470 (18.39%) | 463 (18.11%) | |
| Other Race | 618 (6.04%) | 100 (3.91%) | 123 (4.81%) | 145 (5.67%) | 250 (9.78%) | |
| Education level (%) | < 0.001 | |||||
| Under High school | 2609 (25.52%) | 839 (32.82%) | 717 (28.05%) | 592 (23.16%) | 461 (18.03%) | |
| High school graduate | 2439 (23.85%) | 715 (27.97%) | 602 (23.55%) | 536 (20.97%) | 586 (22.92%) | |
| College degree or above | 5177 (50.63%) | 1002 (39.20%) | 1237 (48.40%) | 1428 (55.87%) | 1510 (59.05%) | |
| PIR | 2.59 ± 1.54 | 2.24 ± 1.45 | 2.51 ± 1.52 | 2.73 ± 1.57 | 2.89 ± 1.57 | < 0.001 |
| BMI | 28.53 ± 5.71 | 29.03 ± 6.04 | 28.50 ± 5.75 | 28.17 ± 5.39 | 28.41 ± 5.61 | < 0.001 |
| Blood urea nitrogen (mg/dl) | 13.92 ± 5.83 | 13.61 ± 6.12 | 13.94 ± 5.92 | 14.20 ± 5.50 | 13.93 ± 5.75 | 0.004 |
| Serum total calcium (mg/dl) | 9.42 ± 0.36 | 9.41 ± 0.36 | 9.41 ± 0.35 | 9.42 ± 0.37 | 9.42 ± 0.36 | 0.717 |
| Cholesterol (mg/dl) | 195.80 ± 41.20 | 196.32 ± 42.48 | 195.43 ± 41.21 | 195.49 ± 40.97 | 195.98 ± 40.13 | 0.827 |
| Serum phosphorus (mg/dl) | 3.71 ± 0.55 | 3.68 ± 0.57 | 3.73 ± 0.56 | 3.70 ± 0.53 | 3.74 ± 0.53 | < 0.001 |
| Total protein (mg/dl) | 7.16 ± 0.45 | 7.17 ± 0.44 | 7.18 ± 0.46 | 7.17 ± 0.46 | 7.13 ± 0.45 | < 0.001 |
| Serum uric acid (mg/dl) | 5.49 ± 1.41 | 5.57 ± 1.44 | 5.48 ± 1.43 | 5.43 ± 1.38 | 5.49 ± 1.37 | 0.005 |
| HEI-2015 | 51.82 ± 11.89 | 45.01 ± 9.88 | 52.64 ± 10.56 | 56.53 ± 11.71 | 53.08 ± 12.21 | < 0.001 |
| Vigorous recreational activities | < 0.001 | |||||
| Yes | 1921 (18.79%) | 346 (13.54%) | 494 (19.33%) | 562 (21.99%) | 519 (20.30%) | |
| No | 8304 (81.21%) | 2210 (86.46%) | 2062 (80.67%) | 1994 (78.01%) | 2038 (79.70%) | |
| Have you ever had 5 or more drinks every day (%) | < 0.001 | |||||
| Yes | 2435 (23.81%) | 711 (27.81%) | 685 (26.80%) | 587 (22.97%) | 452 (17.68%) | |
| No | 7790 (76.19%) | 1846 (72.19%) | 1871 (73.20%) | 1969 (77.03%) | 2104 (82.32%) | |
| Smoked at least 100 cigarettes in life (%) | < 0.001 | |||||
| Yes | 4795 (46.89%) | 1377 (53.87%) | 1201 (46.99%) | 1074 (42.02%) | 1143 (44.70%) | |
| No | 5430 (53.11%) | 1179 (46.13%) | 1355 (53.01%) | 1482 (57.98%) | 1414 (55.30%) | |
| Total femur BMD (g/cm2) | 0.96 ± 0.16 | 0.96 ± 0.17 | 0.97 ± 0.16 | 0.96 ± 0.16 | 0.96 ± 0.16 | 0.155 |
| Femur neck BMD(g/cm2) | 0.82 ± 0.15 | 0.82 ± 0.16 | 0.82 ± 0.16 | 0.81 ± 0.15 | 0.82 ± 0.15 | 0.136 |
| Trochanter BMD (g/cm2) | 0.72 ± 0.14 | 0.72 ± 0.14 | 0.73 ± 0.13 | 0.72 ± 0.14 | 0.73 ± 0.13 | 0.055 |
| Intertrochanter BMD(g/cm2) | 1.14 ± 0.19 | 1.13 ± 0.19 | 1.14 ± 0.19 | 1.14 ± 0.19 | 1.14 ± 0.19 | 0.145 |
| Isoflavones (mg/d) | 1.73 ± 10.10 | 0.25 ± 0.97 | 1.20 ± 4.64 | 3.06 ± 12.47 | 2.40 ± 15.02 | < 0.001 |
| Anthocyanidins (mg/d) | 12.48 ± 30.06 | 1.34 ± 2.48 | 6.95 ± 8.89 | 21.28 ± 28.89 | 20.35 ± 48.99 | < 0.001 |
| Flavan-3-ols (mg/d) | 158.06 ± 346.14 | 4.29 ± 3.90 | 12.67 ± 9.24 | 55.11 ± 56.27 | 560.00 ± 508.92 | < 0.001 |
| Flavanones (mg/d) | 14.24 ± 26.50 | 1.02 ± 2.73 | 9.88 ± 13.88 | 28.44 ± 32.68 | 17.61 ± 33.68 | < 0.001 |
| Flavones (mg/d) | 0.87 ± 1.55 | 0.39 ± 0.57 | 0.78 ± 1.06 | 1.05 ± 1.83 | 1.27 ± 2.11 | < 0.001 |
| Flavonols (mg/d) | 17.58 ± 16.52 | 6.49 ± 4.19 | 12.29 ± 7.57 | 16.60 ± 10.10 | 34.92 ± 21.48 | < 0.001 |
| Total flavonoids (mg/d) | 204.95 ± 363.32 | 13.77 ± 6.80 | 43.78 ± 11.83 | 125.53 ± 46.12 | 636.55 ± 520.09 | < 0.001 |
Mean ± SD for continuous variables: the P value was calculated by the linear regression model. (%) for categorical variables: the P value was calculated by the chi-square test
Abbreviations: PIR poverty income ratio, BMD bone mineral density, BMI, body mass index, HEI-2015 Healthy Eating Index-2015
Associations between total flavonoid intake and femur BMD
Table 2 showed the associations between total flavonoid intake and femur BMD at four interest sites. The unadjusted models found no association between total flavonoid intake and femur BMD. However, after adjusting for covariates in models 2 and 3, we found a positive association between total flavonoid intake and femur BMD at four interest sites. When compared to the first quarter of flavonoid intake, the fully adjusted beta (β) and 95% CIs for those in the second to fourth quarters were as follows: for total femur: 0.009 (95% CI: 0.002, 0.016, P = 0.004), 0.010 (95% CI: 0.001, 0.019, P = 0.011), 0.013 (95% CI: 0.004, 0.022, P = 0.001), respectively; for femur neck: 0.003 (95% CI: −0.002, 0.008, P = 0.102), 0.007 (95% CI: 0.002, 0.012, P = 0.044), 0.010 (95% CI: 0.004, 0.017, P = 0.001), respectively; for trochanter: 0.006 (95% CI: −0.001, 0.012, P = 0.086), 0.007 (95% CI: 0.001, 0.014, P = 0.034), 0.010 (95% CI: 0.004, 0.017, P = 0.001), respectively; for intertrochanter 0.008 (95% CI: 0.002, 0.014, P = 0.031), 0.010 (95% CI: 0.001, 0.018, P = 0.020), 0.012 (95% CI: 0.003, 0.020, P = 0.006), respectively. Then, we conducted a sex-stratified analysis, as shown in Table 3. The positive correlation between total flavonoid intake and femoral BMD remains significant in men and women.
Table 2.
Associations between total flavonoid intake and femur BMD in individuals ≥ 20 years
| Exposure: Total flavonoids quartiles |
Model 1 β (95% CI) P value |
Model 2 β (95% CI) P value |
Model 3 β (95% CI) P value |
|---|---|---|---|
| Total femur BMD | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.007 (−0.002, 0.016) 0.103 | 0.010 (0.002, 0.017) 0.013 | 0.009 (0.002, 0.016) 0.004 |
| Q3 | 0.001 (−0.008, 0.010) 0.769 | 0.012 (0.005, 0.020) 0.001 | 0.010 (0.001, 0.019) < 0.011 |
| Q4 | 0.004 (−0.005, 0.013) 0.379 | 0.017 (0.009, 0.025) < 0.001 | 0.013 (0.004, 0.022) 0.001 |
| P for trend | 0.680 | < 0.001 | 0.003 |
| Femoral neck BMD | |||
| Q1 | Reference | Reference | Reference |
| Q2 | −0.000 (−0.009, 0.008) 0.985 | 0.004 (−0.003, 0.011) 0.242 | 0.003 (−0.002, 0.008) 0.102 |
| Q3 | −0.009 (−0.017, −0.000) 0.047 | 0.006 (−0.001, 0.013) 0.08247 | 0.007 (0.002, 0.012) 0.044 |
| Q4 | −0.003 (−0.011, 0.006) 0.496 | 0.012 (0.005, 0.019) < 0.001 | 0.010 (0.004, 0.017) 0.001 |
| P for trend | 0.205 | < 0.001 | 0.006 |
| Trochanter BMD | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.007 (−0.000, 0.014) 0.065 | 0.009 (0.002, 0.016) 0.008 | 0.006 (−0.001, 0.012) 0.086 |
| Q3 | 0.004 (−0.003, 0.012) 0.244 | 0.012 (0.005, 0.019) < 0.001 |
0.007 (0.001, 0.014) 0.034 |
| Q4 | 0.009 (0.001, 0.016) 0.024 | 0.016 (0.010, 0.023) < 0.001 | 0.010 (0.004, 0.017) 0.001 |
| P for trend | 0.054 | < 0.001 | 0.002 |
| Intertrochanter BMD | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.009 (−0.002, 0.019) 0.093 | 0.011 (0.002, 0.020) 0.019 | 0.008 (0.002, 0.014) 0.031 |
| Q3 | 0.002 (−0.008, 0.012) 0.702 | 0.014 (0.005, 0.023) 0.003 | 0.010 (0.001, 0.018) 0.020 |
| Q4 | 0.003 (−0.007, 0.014) 0.513 | 0.018 (0.009, 0.028) < 0.001 | 0.012 (0.003, 0.020) 0.006 |
| P for trend | 0.833 | < 0.001 | 0.008 |
MEC weight was adjusted. Model 1: no covariates were adjusted. Model 2: age gender, and race/ethnicity were adjusted
Model 3: age, gender, race/ethnicity, BMI, PIR, education level, drinking behavior, smoking behavior, physical activities, total protein, serum calcium, serum uric acid, cholesterol, serum phosphorus, blood urea nitrogen, and HEI-2015
Abbreviations: BMD bone mineral density, BMI body mass index, PIR poverty and income ratio, HEI-2015 Healthy Eating Index-2015, MEC Mobile Examination Center
Table 3.
Associations between total flavonoid intake and femur BMD in men and women ≥ 20 years
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Total femur | ||||||
|
Model 1 β (95% CI) P value |
Model 2 β (95% CI) P value |
Model 3 β (95% CI) P value |
Model 1 β (95% CI) P value |
Model 2 β (95% CI) P value |
Model 3 β (95% CI) P value |
|
| Q1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 | 0.013 (0.002, 0.025) 0.024 | 0.014 (0.003, 0.025) 0.016 | 0.011 (0.001, 0.021) 0.033 | −0.002 (−0.014, 0.010) 0.740 | 0.007 (−0.004, 0.017) 0.216 | 0.003 (−0.006, 0.013) 0.0473 |
| Q3 | 0.011 (−0.000, 0.023) 0.055 | 0.017 (0.006, 0.028) 0.002 | 0.012 (0.002, 0.023) 0.022 | −0.009 (−0.021, 0.002) 0.114 | 0.009 (−0.002, 0.019) 0.102 | 0.010 (0.003, 0.017) 0.008 |
| Q4 | 0.011 (−0.001, 0.023) 0.073 | 0.017 (0.006, 0.028) 0.003 | 0.009 (0.001, 0.017) 0.042 | −0.001 (−0.012, 0.011) 0.914 |
0.018 (0.007, 0.028) < 0.001 |
0.014 (0.004, 0.023) 0.005 |
| P for trend | 0.110 | 0.002 | 0.018 | 0.628 | < 0.001 | 0.005 |
| Femoral neck | ||||||
| Q1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 | 0.007 (−0.004, 0.019) 0.225 | 0.007 (−0.003, 0.018) 0.150 | 0.006 (−0.004, 0.016) 0.224 | −0.009 (−0.021, 0.003) 0.125 | 0.002 (−0.008, 0.011) 0.759 | −0.001 (−0.010, 0.009) 0.242 |
| Q3 | 0.002 (−0.009, 0.014) 0.689 | 0.011 (0.001, 0.022) 0.029 | 0.009 (−0.001, 0.019) 0.074 | −0.020 (−0.032, −0.008) < 0.001 | 0.002 (−0.008, 0.012) 0.704 | 0.003 (−0.009, 0.021) 0.965 |
| Q4 | 0.005 (−0.007, 0.016) 0.435 | 0.013 (0.003, 0.023) 0.014 | 0.009 (0.002, 0.018) 0.011 | −0.009 (−0.021, 0.002) 0.117 | 0.012 (0.002, 0.022) 0.015 | 0.010 (0.001, 0.019) 0.030 |
| P for trend | 0.629 | 0.010 | 0.041 | 0.042 | 0.020 | 0.033 |
| Trochanter | ||||||
| Q1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 | 0.012 (0.002, 0.022) 0.016 | 0.013 (0.003, 0.023) 0.008 | 0.009 (0.001, 0.019) 0.042 | −0.001 (−0.011, 0.009) 0.843 | 0.006 (−0.003, 0.014) 0.213 | 0.002 (−0.006, 0.011) 0.591 |
| Q3 | 0.014 (0.004, 0.024) 0.008 | 0.017 (0.008, 0.027) < 0.001 | 0.010 (0.001, 0.020) 0.035 | −0.005 (−0.015, 0.004) 0.281 | 0.008 (−0.001, 0.016) 0.087 | 0.004 (−0.005, 0.012) 0.404 |
| Q4 | 0.014 (0.004, 0.024) 0.0088 | 0.017 (0.007, 0.027) < 0.001 | 0.010 (0.002, 0.019) 0.030 | 0.005 (−0.004, 0.015) 0.297 | 0.017 (0.008, 0.025) < 0.001 | 0.011 (0.004, 0.019) 0.004 |
| P for trend | 0.009 | < 0.001 | 0.024 | 0.470 | < 0.001 | 0.004 |
| Intertrochanter | ||||||
| Q1 | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 | 0.014 (0.000, 0.028) 0.046 | 0.014 (0.001, 0.026) 0.041 | 0.010 (−0.002, 0.023) 0.090 | 0.000 (−0.014, 0.014) 0.982 | 0.009 (−0.004, 0.021) 0.161 | 0.006 (−0.006, 0.017) 0.343 |
| Q3 | 0.011 (−0.002, 0.025) 0.108 | 0.017 (0.004, 0.030) 0.009 | 0.012 (0.001, 0.024) 0.040 | −0.008 (−0.022, 0.006) 0.258 | 0.011 (−0.001, 0.024) 0.074 | 0.008 (−0.004, 0.020) 0.172 |
| Q4 | 0.010 (−0.004, 0.024) 0.155 | 0.017 (0.004, 0.031) 0.001 | 0.009 (0.001, 0.018) 0.047 | −0.001 (−0.014, 0.013) 0.931 | 0.020 (0.008, 0.033) 0.001 | 0.016 (0.004, 0.027) 0.007 |
| P for trend | 0.218 | 0.008 | 0.222 | 0.662 | 0.002 | 0.007 |
MEC weight was adjusted. Model 1: no covariates were adjusted. Model 2: age gender, and race/ethnicity were adjusted. Model 3: age, gender, race/ethnicity, BMI, PIR, education level, drinking behavior, smoking behavior, physical activities, total protein, serum calcium, serum uric acid, cholesterol, serum phosphorus, blood urea nitrogen, and HEI-2015
Abbreviations: BMD bone mineral density, BMI body mass index, PIR poverty and income ratio, HEI-2015 Healthy Eating Index-2015, MEC Mobile Examination Center
Associations between flavonoid intake and osteoporosis individuals ≥ 20 years
The relationship between flavonoid intake and osteoporosis in individuals ≥ 20 years was shown in Table 4. In fully adjusted models, compared with people in the first quartile, people in the fourth quartile of total flavonoids intake (OR = 0.686, 95% CI: 0.528–0.890, P = 0.005), anthocyanidins intake (OR = 0.705, 95% CI: 0.584–0.839, P = 0.009), flavan-3-ols intake (OR = 0.662, 0.511, 0.856, P = 0.002), flavones intake (OR = 0.544, 95% CI: 0.414–0.715, P < 0.001), flavonols intake OR = 0.768, 0.588, 0.968, P = 0.045) have a lower risk of osteoporosis. However, multivariable analyses showed that isoflavones (OR = 0.874, 95% CI: 0.670–1.140, P = 0.321) and flavanones intake (OR = 0.994, 95% CI: 0.773–1.277, P = 0.959) have no positive effects on osteoporosis in the model 3.
Table 4.
Associations between flavonoid intake and osteoporosis ≥ 20 years
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
|---|---|---|---|---|---|
| Total flavonoid intake | |||||
| Model 1 OR (95% CI) P value | Reference | 0.880 (0.712, 1.087) 0.235 | 0.929 (0.753, 1.145) 0.488 | 0.782 (0.629, 0.973) 0.027 | 0.053 |
| Model 2 OR (95% CI) P value | Reference | 0.778 (0.617, 0.981) 0.034 | 0.689 (0.548, 0.866) 0.001 | 0.604 (0.476, 0.765) < 0.001 | < 0.001 |
| Model 3 OR (95% CI) P value | Reference | 0.890 (0.692, 1.144) 0.362 | 0.811 (0.627, 1.049) 0.111 | 0.686 (0.528, 0.890) 0.005 | 0.004 |
| Isoflavones intake | |||||
| Model 1 OR (95% CI) P value | Reference | 1.018 (0.823, 1.261) 0.867 | 0.843 (0.678, 1.048) 0.123 | 0.736 (0.585, 0.926) 0.009 | 0.002 |
| Model 2 OR (95% CI) P value | Reference | 0.941 (0.747, 1.186) 0.607 | 0.895 (0.707, 1.134) 0.358 | 0.841 (0.655, 1.080) 0.175 | 0.154 |
| Model 3 OR (95% CI) P value | Reference | 0.949 (0.743, 1.211) 0.672 | 0.944 (0.734, 1.213) 0.651 | 0.874 (0.670, 1.140) 0.321 | 0.346 |
| Anthocyanidins intake | |||||
| Model 1 OR (95% CI) P value | Reference | 1.151 (0.925, 1.433) 0.20807 | 1.112 (0.892, 1.387) 0.34516 | 1.166 (0.937, 1.450) 0.169 | 0.234 |
| Model 2 OR (95% CI) P value | Reference | 0.884 (0.695, 1.124) 0.315 | 0.730 (0.573, 0.929) 0.011 | 0.650 (0.512, 0.826) < 0.001 | < 0.001 |
| Model 3 OR (95% CI) P value | Reference | 0.946 (0.730, 1.225) 0.672 | 0.868 (0.663, 1.037) 0.104 | 0.705 (0.584, 0.839) 0.009 | 0.020 |
| Flavan-3-ols intake | |||||
| Model 1 OR (95% CI) P value | Reference | 0.880 (0.712, 1.088) 0.237 | 0.923 (0.748, 1.138) 0.452 | 0.788 (0.634, 0.979) 0.031 | 0.057 |
| Model 2 OR (95% CI) P value | Reference | 0.718 (0.569, 0.905) 0.005 | 0.707 (0.562, 0.888) 0.003 | 0.594 (0.468, 0.753) < 0.001 | < 0.001 |
| Model 3 OR (95% CI) P value | Reference | 0.817 (0.636, 1.050) 0.114 | 0.794 (0.615, 1.024) 0.076 | 0.662 (0.511, 0.856) 0.002 | 0.002 |
| Flavanones intake | |||||
| Model 1 OR (95% CI) P value | Reference | 0.744 (0.599, 0.926) 0.008 | 0.796 (0.641, 0.988) 0.039 | 0.967 (0.786, 1.190) 0.752 | 0.940 |
| Model 2 OR (95% CI) P value | Reference | 0.655 (0.517, 0.830) 0.00045 | 0.643 (0.508, 0.815) < 0.001 | 0.802 (0.639, 1.006) 0.056 | 0.083 |
| Model 3 OR (95% CI) P value | Reference | 0.736 (0.573, 0.946) 0.017 | 0.760 (0.589, 0.982) 0.036 | 0.994 (0.773, 1.277) 0.959 | 0.925 |
| Flavones intake | |||||
| Model 1 OR (95% CI) P value | Reference | 0.791 (0.645, 0.969) 0.023 | 0.730 (0.593, 0.899) 0.003 | 0.576 (0.462, 0.719) < 0.001 | < 0.001 |
| Model 2 OR (95% CI) P value | Reference | 0.624 (0.498, 0.781) < 0.001 | 0.554 (0.440, 0.696) < 0.001 | 0.439 (0.345, 0.559) < 0.001 | < 0.001 |
| Model 3 OR (95% CI) P value | Reference | 0.681 (0.536, 0.866) 0.002 | 0.624 (0.485, 0.803) < 0.001 | 0.544 (0.414, 0.715) < 0.001 | < 0.001 |
| Flavonols intake | |||||
| Model 1 OR (95% CI) P value | Reference | 0.869 (0.710, 1.064) 0.173 | 0.758 (0.615, 0.934) 0.009 | 0.574 (0.458, 0.718) < 0.001 | < 0.001 |
| Model 2 OR (95% CI) P value | Reference | 0.903 (0.725, 1.126) 0.365 | 0.798 (0.636, 1.002) 0.052 | 0.653 (0.512, 0.833) < 0.001 | < 0.001 |
| Model 3 OR (95% CI) P value | Reference | 1.026 (0.810, 1.298) 0.833 | 0.918 (0.717, 1.176) 0.498 | 0.768 (0.588, 0.968) 0.045 | 0.033 |
MEC weight was adjusted. Model 1: no covariates were adjusted. Model 2: age gender, and race/ethnicity were adjusted. Model 3: age, gender, race/ethnicity
BMI, PIR, education level, drinking behavior, smoking behavior, physical activities, total protein, serum calcium, serum uric acid, cholesterol, serum phosphorus, blood urea nitrogen, and HEI-2015
Abbreviations: BMI body mass index, PIR poverty and income ratio, HEI-2015 Healthy Eating Index-2015. MEC Mobile Examination Center
Dose–response associations between flavonoid intake and osteoporosis in individuals ≥ 20 years
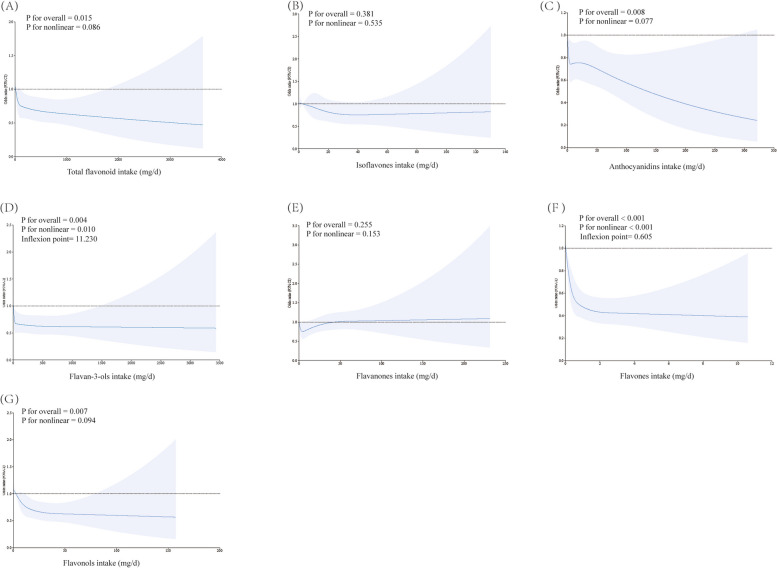
Figure 2 showed the results of RCS. The dose–response association found no linear or non-linear relationship of isoflavones (Overall P = 0.381, non-linear P = 0.535) and flavanones intake (Overall P = 0.255, non-linear P = 0.153) with osteoporosis. We found a linear inverse relationship between total flavonoids (Overall P = 0.015, non-linear P = 0.086), anthocyanins (Overall P = 0.008, non-linear P = 0.077), flavonols intake (Overall P = 0.007, non-linear P = 0.094) and risk of osteoporosis. The RCS model revealed the non-linear negative relationship between flavan-3-ols (non-linear P = 0.010, inflexion point = 11.230), and flavones (non-linear P < 0.001, inflexion point = 0.605) and osteoporosis risk.
Fig. 2.
Restricted cubic spline (RCS) analysis with associations between flavonoids intake ((A) Total flavonoid (B) Isoflavones (C) Anthocyanidins (D) Flavan-3-ols (E) Flavanones (F) Flavones (G) Flavonols) and osteoporosis. Covariates included age, gender, race/ethnicity, BMI, PIR, education level, drinking behavior, smoking behavior, physical activities, total protein, serum calcium, serum uric acid, cholesterol, serum phosphorus, blood urea nitrogen, and HEI-2015 were adjusted. Abbreviation: BMI: body mass index. PIR: poverty and income ratio. HEI-2015: Healthy Eating Index-2015
Mediation analysis
The result of mediation analysis indicates that CDAI partially mediates the association of total flavonoid intake with total femur, femoral neck, trochanter, and intertrochanter BMD. The proportion of CDAI mediating the positive association between total flavonoid intake and total femur, femoral neck, trochanter, and intertrochanter BMD was 12.20%, 12.90%, 13.51%, and 10.87%, respectively (Table 5).
Table 5.
CDAI as a mediator in the associations of total flavonoid intake with BMD (g/cm2)
| Mediation effect (Total flavonoid intake – CDAI – BMD) | Total femur BMD | Femoral neck BMD | Trochanter BMD | Intertrochanter BMD |
|---|---|---|---|---|
| Total effect | 0.0041 (0.0019–0.0063) | 0.0031 (0.0009–0.0051) | 0.0037 (0.0008–0.0057) | 0.0046 (0.0019–0.0072) |
| Direct effect | 0.0036 (0.0013–0.0058) | 0.0027 (0.006–0.0049) | 0.0032 (0.0012–0.0052) | 0.0041 (0.0014–0.0068) |
| Indirect effect | 0.0005 (0.0002–0.0009) | 0.0004 (0.0001–0.0007) | 0.0005 (0.0002–0.0009) | 0.0005 (0.0001–0.0010) |
| Mediated (%) | 12.20% | 12.90% | 13.51% | 10.87% |
Model was adjusted for age, gender, race/ethnicity, BMI, PIR, education levels, drinking behavior, smoking behavior, physical activities, total protein, serum calcium, serum uric acid, cholesterol, serum phosphorus, blood urea nitrogen, and HEI-2015
Abbreviations: BMD bone mineral density, BMI body mass index, PIR poverty and income ratio, CDAI Dietary Antioxidant Composite Index, HEI-2015 Healthy Eating Index-2015
Discussion
Using a large population from nationally representative sample, this study is the first to reveal the positive association between total flavonoid intake and femur BMD in U.S. adults. This association was significant in both men and women. Furthermore, different subclasses of flavonoids may have different effects. A higher intake of anthocyanidins, flavan-3-ols, flavone, flavonols, and total flavonoid intake is associated with a lower risk of osteoporosis in adult Americans. These findings emphasize the need for flavonoid intake in bone health and provide important dietary recommendations for the prevention of osteoporosis. However, no causal inferences between flavonoid intake and BMD can be concluded due to the nature of cross-sectional studies.
Many clinical studies have examined the relationship between flavonoid intake and bone health but reached inconsistent conclusions [30–32]. In an early study in Japan, Nagata et al. [33] found no association between serum isoflavones and BMD in postmenopausal Japanese women. However, they did not adjust confounders like physical activity, calcium status, and others, which may influence the final results. In addition, the small sample (only 87 postmenopausal women) was another limitation of the study. In contrast, our study included a large sample of 10,225 people and adjusted for a larger confounder, which may produce a more convincing result. Two earlier studies of British women came to similar conclusions to ours. In a study of 3,326 Scottish women, Hardcastle et al. [16] found a positive effect of flavonoid intake on bone health. Every milligram of flavonoid intake was associated with a 0.009 g/cm2 increase in femur neck BMD. In another study in the UK, Welch et al. [17] explored the relationship between habitual intake of flavonoid subclasses and BMD in a cohort of 3160 females. Compared to the lowest quartile of anthocyanin intake, people in the highest had an increased hip and spine BMD by 0.029 and 0.034 g/cm2, respectively.
A few explanations that could clarify the positive effects of flavonoid intake on bone health. Oxidative stress is an imbalance of oxidative and antioxidant effects in our body, which is an important contributor to aging and disease, including osteoporosis [34]. Excess ROS generated by oxidative stress imbalance could inhibit the expression of osterix and Runx2, thereby reducing osteogenic activity [35]. Studies showed that flavonoids may prevent osteoporosis by scavenging ROS in the body [36]. Evidence suggested that flavonoid intake could reduce inflammatory cytokines IL-6 and TNF-α in human circulation [37]. Fruits and flavonoid phytochemicals could decrease osteoclast activity by decreasing MMP-2, MMP-9, and NFATc1 [38]. They also increase Osterix, osteocalcin, and Runx2 (Cbfa1) pathways to promote bone formation [38]. Quercetin is a main dietary flavonoid found in vegetables. Quercetin has been found to inhibit receptor activators of nuclear factor-kappa B ligand (RANKL) and RANKL-induced osteoclast genes to prevent bone loss in ovariectomized mice [39, 40]. CDAI is a standardized indicator that estimates the total dietary antioxidant capacity in our diet [41–43], which has been proven to influence BMD in Americans [44, 45]. The present study found that CDAI partially mediates the association of total flavonoid intake with femur BMD. Thus, the positive association between flavonoid intake and BMD could be attributed to the fact that they change the antioxidant capacity in our diet. However, the proportion of CDAI mediating the effect of total flavonoid intake on BMD is relatively low (Less than 20%). On the one hand, CDAI may not fully reflect the antioxidant index in human bodies. Future studies could analyze some serum inflammatory biomarkers, like IL-6 and TNF-α, as mediators of flavonoid intake and BMD. On the other, flavonoid intake may promote bone health in other ways. Studies have found that flavonoids may act as phytoestrogens to exert an anti-osteoporotic effect [46, 47]. In total, further studies with stronger evidence are required to verify our results and find other factors that mediate the effect of total flavonoid intake on bone health.
Consumption of different flavonoid subclasses varies widely among Americans. A previous study indicated that flavan-3-ols account for over 80 percent of total flavonoid consumption, whereas flavones account for about 0.3 percent [48]. In our study, different flavonoid subclasses have different effects on osteoporosis risk. We found that isoflavones and flavanones intakes were not associated with osteoporosis risk. In contrast, we found that anthocyanins, flavonols, flavan-3-ols, and flavones intake were negatively associated with osteoporosis risk. Soy isoflavones have been widely investigated for their anti-osteoporosis effects among flavonoids. Isoflavones are compounds structurally similar to estrogen and are thought to exert estrogen-like anti-osteoporotic effects [49]. In a study with a 4-year follow-up, Zhang et al. [49] found that soy food consumption may reduce the risk of fracture in postmenopausal women. However, in an RCT study involving 403 postmenopausal women, Wong et al. [50] found that a daily supplement with 120 mg/day of soy isoflavones could not reduce bone loss in the spine and femur. The conclusion was similar to our study that isoflavone intake could not reduce the risk of osteoporosis in American adults. In fact, our study showed that the U.S. population has a very low intake of isoflavones, with a mean value of 1.73 mg/day, compared to 158.06 mg/day for flavan-3-ols, which comprise the majority of isoflavone intake. Flavan-3-ols are mainly present in green tea and fruits. The subclass of flavan-3-ol, like catechin and epigallocatechin, has been proven to be associated with bone health. Studies have demonstrated that epigallocatechin inhibits osteoclast differentiation and promotes osteoblast activity [51, 52]. These findings align with our results, which showed that the people in the highest quartile of flavan-3-ols intake have a significantly reduced osteoporosis risk compared with the first quartile (OR = 0.662).
Interestingly, the RCS model in our studies revealed the non-linear negative relationship between flavan-3-ols and flavones and osteoporosis risk. Within a certain range, flavan-3-ols and flavones intake are negatively associated with the osteoporosis risk. However, the relationship was not significant after reaching specific limits. Although no previous studies have explained their specific mechanisms, we hypothesize that these two flavonoid subclasses have saturating effects on bone health. However, further studies are needed to explain the mechanisms in the future.
Our results have several advantages. First, this is the first study to explore the relationship between flavonoid intake and their subclasses and osteoporosis in a U.S. population using the most recent cycles from the NHANES database, which is representative of the general U.S. population. We included a large sample of 10,225 people, which gives our study more reliability. Second, our study uses multiple regression analysis, adjusting for a large number of confounders, thereby reducing the error in the results. Third, previous research on the relationship between flavonoids and BMD or osteoporosis has mostly focused on women, and our study demonstrates that flavonoids also have a positive impact on men's bone health. Fourth, our study performed dose–response analyses to assess the association between total flavonoid intake and osteoporosis risk. Our study also has some limitations. First, we adjusted for many potential confounders, including socioeconomic status, lifestyle, and other health factors. However, some confounders are not included, such as vitamin D and calcium intake, because they cannot be acquired in relevant NHANES cycles. In addition, most factors were collected through questionnaires and recall, which may be subject to recall bias and inaccuracies. Second, we cannot make causal inferences due to the nature of cross-sectional studies. Third, dietary intake was estimated from the mean of two 24-h recalls and may be subject to recall bias. Further studies are required to explore the relationship between biomarkers of flavonoid [33] (like serum isoflavonoid) and BMD. Fourth, there was a linear negative correlation between total flavonoid intake and the risk of osteoporosis. However, some subclasses of flavonoids may have nonlinear relationships with osteoporosis risk, and further studies are needed to explore them and their underlying mechanisms. Fifth, we performed numerous subgroup analyses based on different flavonoid subclasses and BMD sites, which increases the risk of type I errors and false positives. Sixth, we did not adjust the examination weights when we conducted the RCS, which may limit the generalization of the results.
Conclusions
Our findings suggest that flavonoid intake is associated with the BMD and risk of osteoporosis, and the relationship exists in both men and women. The finding provides important dietary recommendations for the prevention of osteoporosis. However, more prospective studies with stronger evidence are needed to explore the causal association between flavonoid intake and BMD and the underlying mechanisms.
Acknowledgements
We acknowledge the data from the National Health and Nutrition Examination Survey (NHANES).
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- RCS
Restricted cubic splines
- BMI
Body mass index
- PIR
Poverty income ratio
- WHO
World Health Organization
- RCTs
Randomized controlled trials
- RANKL
Receptor activators of nuclear factor-kappa B ligand
- DXA
Dual-energy X-ray absorptiometry
- FNDDS
Food and Nutrient Database
- WWEIA
We Eat in the United States
- MEC
Mobile Examination Center
- CDAI
Composite dietary antioxidant index
- HEI-2015
Healthy Eating Index-2015
Authors’ contributions
Conceptualization, Peilun Xiao, Ye Tian; Data curation, Peilun Xiao, Zhihang Wang, Zeyao Lu; Formal analysis, Peilun Xiao, Zhihang Wang, Zeyao Lu; Investigation, Peilun Xiao, Ying Xu, Ye Tian; Methodology, Peilun Xiao, Zhihang Wang, Zeyao Lu; Project administration, Peilun Xiao, Ye Tian; Software, Peilun Xiao, Shijia Liu, Chongjun Huang, Ye Tian; Visualization, Peilun Xiao, Ye Tian; Writing – original draft, Peilun Xiao, Zhihang Wang, Zeyao Lu; Writing – review & editing, Peilun Xiao, Ye Tian. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This work was supported in part by the following grants: the National Natural Science Foundation of China (Grant No. 82470923) to YT, the National Natural Science Foundation of China (Grant No. 81970760) to YT, the National Natural Science Foundation of China (Grant No. 82271294) to YX, the Natural Science Foundation of Liaoning Province (Grant No. 2021-MS-201) to YX.
Data availability
The datasets generated and/or analysed during the current study are available in the [NHANES] repository,[https://www.cdc.gov/nchs/nhanes/].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ying Xu, Email: xuy5@sj-hospital.org.
Ye Tian, Email: tiany3@sj-hospital.org.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Xiao PL, Cui AY, Hsu CJ, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. 2022;33:2137–53. 10.1007/s00198-022-06454-3. [DOI] [PubMed] [Google Scholar]
- 3.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28:1979–88. 10.1007/s00198-017-3996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606–21. 10.1016/s2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 6.Lo JC, Yang W, Park-Sigal JJ, Ott SM. Osteoporosis and Fracture Risk among Older US Asian Adults. Curr Osteoporos Rep. 2023;21:592–608. 10.1007/s11914-023-00805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed M, Eun JB. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit Rev Food Sci Nutr. 2018;58:3159–88. 10.1080/10408398.2017.1353480. [DOI] [PubMed] [Google Scholar]
- 8.Xing W, Gao W, Zhao Z, et al. Dietary flavonoids intake contributes to delay biological aging process: analysis from NHANES dataset. J Transl Med. 2023;21:492. 10.1186/s12967-023-04321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Gao Y, Shen Q, Li J, Zhou Z, Shen L. Dietary flavonoid intake is associated with a lower risk of depressive symptoms in US adults: Data from NHANES 2007–2008, 2009–2010, and 2017–2018. J Affect Disord. 2024;345:293–9. 10.1016/j.jad.2023.10.128. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Nie J, Deng MG, et al. Dietary flavonoid intake is associated with a lower risk of diabetic nephropathy in US adults: data from NHANES 2007–2008, 2009–2010, and 2017–2018. Food Funct. 2023;14:4183–90. 10.1039/d3fo00242j. [DOI] [PubMed] [Google Scholar]
- 11.Sehmisch S, Erren M, Kolios L, et al. Effects of isoflavones equol and genistein on bone quality in a rat osteopenia model. Phytother Res. 2010;24(Suppl 2):S168–74. 10.1002/ptr.3060. [DOI] [PubMed] [Google Scholar]
- 12.Santos MA, Florencio-Silva R, Medeiros VP, et al. Effects of different doses of soy isoflavones on bone tissue of ovariectomized rats. Climacteric. 2014;17:393–401. 10.3109/13697137.2013.830606. [DOI] [PubMed] [Google Scholar]
- 13.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57–64. 10.1016/j.clnu.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009;44:948–53. 10.1016/j.bone.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZQ, He LP, Liu YH, Liu J, Su YX, Chen YM. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporos Int. 2014;25:2417–25. 10.1007/s00198-014-2763-9. [DOI] [PubMed] [Google Scholar]
- 16.Hardcastle AC, Aucott L, Reid DM, Macdonald HM. Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res. 2011;26:941–7. 10.1002/jbmr.285. [DOI] [PubMed] [Google Scholar]
- 17.Welch A, MacGregor A, Jennings A, Fairweather-Tait S, Spector T, Cassidy A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J Bone Miner Res. 2012;27:1872–8. 10.1002/jbmr.1649. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Song D, Lin X, et al. Corylifol A protects against ovariectomized-induced bone loss and attenuates RANKL-induced osteoclastogenesis via ROS reduction, ERK inhibition, and NFATc1 activation. Free Radic Biol Med. 2023;196:121–32. 10.1016/j.freeradbiomed.2023.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Chai S, Yang Y, Wei L, et al. Luteolin rescues postmenopausal osteoporosis elicited by OVX through alleviating osteoblast pyroptosis via activating PI3K-AKT signaling. Phytomedicine. 2024;128: 155516. 10.1016/j.phymed.2024.155516. [DOI] [PubMed] [Google Scholar]
- 20.Si Y, Li Y, Gu K, Yin H, Ma Y. Icariin ameliorates osteoporosis in ovariectomized rats by targeting Cullin 3/Nrf2/OH pathway for osteoclast inhibition. Biomed Pharmacother. 2024;173: 116422. 10.1016/j.biopha.2024.116422. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group Osteoporos Int. 1994;4:368–81. 10.1007/bf01622200. [DOI] [PubMed] [Google Scholar]
- 22.Looker AC, Orwoll ES, Johnston CC, Jr. et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761–8. 10.1359/jbmr.1997.12.11.1761 [DOI] [PubMed]
- 23.Sebastian RS, Wilkinson Enns C, Goldman JD, et al. A New Database Facilitates Characterization of Flavonoid Intake, Sources, and Positive Associations with Diet Quality among US Adults. J Nutr. 2015;145:1239–48. 10.3945/jn.115.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor S, Korpusik M, Das S, et al. Use of Natural Spoken Language With Automated Mapping of Self-reported Food Intake to Food Composition Data for Low-Burden Real-time Dietary Assessment: Method Comparison Study. J Med Internet Res. 2021;23: e26988. 10.2196/26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian RS, Fanelli Kuczmarski MT, Goldman JD, Moshfegh AJ, Zonderman AB, Evans MK. Usual Intake of Flavonoids Is Inversely Associated with Metabolic Syndrome in African American and White Males but Not Females in Baltimore City, Maryland, USA. Nutrients 2022;14. 10.3390/nu14091924 [DOI] [PMC free article] [PubMed]
- 26.Xiao PL, Fuerwa C, Hsu CJ, et al. Socioeconomic status influences on bone mineral density in American men: findings from NHANES 2011–2020. Osteoporos Int. 2022;33:2347–55. 10.1007/s00198-022-06498-5. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Peng B, Liu J, Liu Z, Xia Y, Geng B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front Immunol. 2022;13: 975400. 10.3389/fimmu.2022.975400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Z, Shi G, Liao X, et al. Effect of pulmonary function on bone mineral density in the United States: results from the NHANES 2007–2010 study. Osteoporos Int. 2023;34:955–63. 10.1007/s00198-023-06727-5. [DOI] [PubMed] [Google Scholar]
- 29.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin X, Gibson AA, Gale J, et al. Does weight loss reduce the incidence of total knee and hip replacement for osteoarthritis?-A prospective cohort study among middle-aged and older adults with overweight or obesity. Int J Obes (Lond). 2021;45:1696–704. 10.1038/s41366-021-00832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Wluka AE, Simpson JA, et al. Body weight at early and middle adulthood, weight gain and persistent overweight from early adulthood are predictors of the risk of total knee and hip replacement for osteoarthritis. Rheumatology (Oxford). 2013;52:1033–41. 10.1093/rheumatology/kes419. [DOI] [PubMed] [Google Scholar]
- 32.Colbert CJ, Almagor O, Chmiel JS, et al. Excess body weight and four-year function outcomes: comparison of African Americans and whites in a prospective study of osteoarthritis. Arthritis Care Res (Hoboken). 2013;65:5–14. 10.1002/acr.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Soy product intake and serum isoflavonoid and estradiol concentrations in relation to bone mineral density in postmenopausal Japanese women. Osteoporos Int. 2002;13:200–4. 10.1007/s001980200014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, Li H, Li J, Hu J, Yang K, Tao L. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed Pharmacother. 2023;163: 114834. 10.1016/j.biopha.2023.114834. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Kim MJ, Lee SH et al. Metallothionein 3 Promotes Osteoblast Differentiation in C2C12 Cells via Reduction of Oxidative Stress. Int J Mol Sci 2021;22. 10.3390/ijms22094312 [DOI] [PMC free article] [PubMed]
- 36.Weaver CM, Alekel DL, Ward WE, Ronis MJ. Flavonoid intake and bone health. J Nutr Gerontol Geriatr. 2012;31:239–53. 10.1080/21551197.2012.698220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peluso I, Raguzzini A, Serafini M. Effect of flavonoids on circulating levels of TNF-α and IL-6 in humans: a systematic review and meta-analysis. Mol Nutr Food Res. 2013;57:784–801. 10.1002/mnfr.201200721. [DOI] [PubMed] [Google Scholar]
- 38.Horcajada MN, Offord E. Naturally plant-derived compounds: role in bone anabolism. Curr Mol Pharmacol. 2012;5:205–18. 10.2174/1874467211205020205. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji M, Yamamoto H, Sato T, et al. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J Bone Miner Metab. 2009;27:673–81. 10.1007/s00774-009-0088-0. [DOI] [PubMed] [Google Scholar]
- 40.Wattel A, Kamel S, Prouillet C, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;92:285–95. 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Lai W, Zhao M, Zhang Y, Hu Y. Association between the Composite Dietary Antioxidant Index and Atherosclerotic Cardiovascular Disease in Postmenopausal Women: A Cross-Sectional Study of NHANES Data, 2013–2018. Antioxidants (Basel) 2023;12. 10.3390/antiox12091740 [DOI] [PMC free article] [PubMed]
- 42.Wang M, Huang ZH, Zhu YH, He P, Fan QL. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011–2018. Food Funct. 2023. 10.1039/d3fo01157g. [DOI] [PubMed] [Google Scholar]
- 43.Maugeri A, Hruskova J, Jakubik J, et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. 2019;131:274–81. 10.1016/j.freeradbiomed.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Tang Y, Peng B, Tian C, Geng B. Bone mineral density is associated with composite dietary antioxidant index among US adults: results from NHANES. Osteoporos Int. 2023;34:2101–10. 10.1007/s00198-023-06901-9. [DOI] [PubMed] [Google Scholar]
- 45.Cui A, Yan J, Zeng Y, et al. Association between composite dietary antioxidant and bone mineral density in children and adolescents aged 8–19 years: findings from NHANES. Sci Rep. 2024;14:15849. 10.1038/s41598-024-66859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miksicek RJ. Estrogenic flavonoids: structural requirements for biological activity. Proc Soc Exp Biol Med. 1995;208:44–50. 10.3181/00379727-208-43830. [DOI] [PubMed] [Google Scholar]
- 47.Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol Sci. 2002;69:354–61. 10.1093/toxsci/69.2.354. [DOI] [PubMed] [Google Scholar]
- 48.Fanelli Kuczmarski M, Sebastian RS, Goldman JD et al. Dietary Flavonoid Intakes Are Associated with Race but Not Income in an Urban Population. Nutrients 2018;10. 10.3390/nu10111749 [DOI] [PMC free article] [PubMed]
- 49.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165:1890–5. 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 50.Wong WW, Lewis RD, Steinberg FM, et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr. 2009;90:1433–9. 10.3945/ajcn.2009.28001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko CH, Lau KM, Choy WY, Leung PC. Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism. J Agric Food Chem. 2009;57:7293–7. 10.1021/jf901545u. [DOI] [PubMed] [Google Scholar]
- 52.Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res. 2009;29:437–56. 10.1016/j.nutres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the [NHANES] repository,[https://www.cdc.gov/nchs/nhanes/].