Abstract
Background
Immune dysregulation has been identified as a contributing factor in the pathophysiology of schizophrenia. This study aimed to investigate variations in specific immune regulators and their correlation with psychopathology and cognitive functions in male patients with chronic schizophrenia.
Methods
Employing a cross-sectional design, this study included 72 male patients with chronic schizophrenia. The Positive and Negative Syndrome Scale (PANSS) and the Repeatable Battery for the Assessment of Neuropsychological Status were utilized to assess psychopathology and cognitive functions, respectively.
Results
Serum levels of interleukin (IL)-4, IL-10, IL-12p40, IL-13, and monocyte chemoattractant protein-1 (MCP-1) were measured. There were significantly increased levels of IL-4, IL-13, and MCP-1, alongside decreased levels of IL-10 in patients compared to controls (all P < 0.05). IL-4 levels showed a significant negative association with PANSS positive symptoms (beta=-0.222, P = 0.042). After controlling for antipsychotic medication, BMI, and smoking, this correlation was no longer significant (r=-0.232, P = 0.055). Additionally, positive correlations of IL-4 (beta = 0.297, P = 0.008), IL-13 (beta = 0.371, P = 0.001), and MCP-1 (beta = 0.280, P = 0.013) with language scores were observed. Increased levels of IL-4 (P = 0.044, OR = 1.994), IL-13 (P = 0.019, OR = 2.245), as well as IL-4 and MCP-1 interactions (P = 0.043, OR = 2.000) were positively associated with the risk of chronic schizophrenia, while lower levels of IL-10 (P = 0.003, OR = 0.2.867) were also linked to an increased risk.
Conclusion
The identified associations between specific immune markers and the clinical and cognitive features of chronic schizophrenia in males underscored the potential immune-mediated mechanisms underlying schizophrenia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06288-9.
Keywords: Schizophrenia, Immune dysregulation, Cognitive deficits, PANSS, RBANS
Introduction
Schizophrenia is a severe mental disorder with strong heterogeneity, primarily characterized by hallucinations, delusions, disorganized thinking, cognitive impairments, and abnormal affect or behavior. Schizophrenia typically manifests in early adulthood, imposing significant burdens on the individual, family, and society [1, 2]. The global prevalence of schizophrenia is approximately 1.0% [2, 3], with a lifetime prevalence in China ranging from 0.5 to 0.9% [4]. The course of schizophrenia is prolonged, and the prognosis is generally poor, significantly impacting the quality of life of patients and potentially reducing life expectancy [5]. Although the precise etiology remains elusive, inflammatory processes have been implicated as important contributory factors in the pathogenesis of schizophrenia [6, 7].
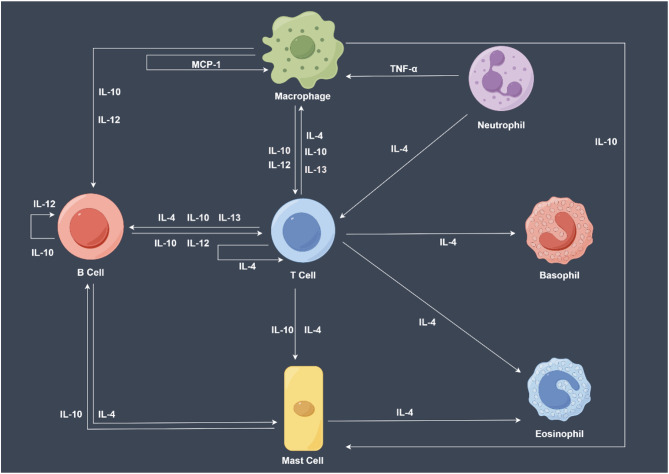
Inflammation plays a crucial role in the pathogenesis of schizophrenia. Cytokines interfere with normal brain function through various mechanisms, including disrupting the balance of neurotransmitters, damaging neurons, and causing synaptic dysfunction [8, 9]. Cytokines can directly interfere with the synthesis, release, and reuptake of neurotransmitters, such as dopamine and glutamate, leading to neurotransmitter dysfunction [10, 11]. Abnormalities in these neurotransmitter systems are closely associated with the positive and negative symptoms of schizophrenia [12]. Cytokines may inhibit the proliferation and differentiation of neural stem cells, affecting neuronal migration and synapse formation, resulting in neurodevelopmental disorders and brain structural abnormalities [13], which are prevalent in patients with schizophrenia. Furthermore, inflammation may also lead to white matter integrity impairment by affecting myelination and oligodendrocyte function, and white matter abnormalities are an important pathological feature of schizophrenia [14, 15]. Among the cytokines involved in regulating inflammatory responses, immune responses, and intercellular signaling, interleukin (IL)-4, -10, -12, -13, and the chemokine monocyte chemoattractant protein-1 (MCP-1) play key roles [16]. Figure 1 illustrates the production process of major cytokines.
Fig. 1.
Overview of the production of some cytokines. IL-4 is an adaptive cytokine primarily secreted by Th2 cells, mast cells, and eosinophils. IL-4 plays a pivotal role in regulating humoral immunity, promoting IgG and IgE production, enhancing the proliferation and differentiation of B cells, and suppressing Th1 cell-mediated inflammatory responses. IL-10 is a cytokine with extensive anti-inflammatory properties that is secreted by various cell types, including T cells, B cells, macrophages, and certain dendritic cells. IL-10 effectively inhibits the production of pro-inflammatory cytokines and limits autoimmune responses, thus playing a crucial role in maintaining immune tolerance and preventing immunopathological damage. IL-12 is produced by dendritic cells, macrophages, and certain B cells, and is essential for the differentiation and activation of Th1 cells. IL-12 is significant in anti-infective immune responses, particularly against intracellular pathogens, and promotes the recruitment and activation of natural killer cells and T cells. IL-13, with biological functions similar to IL-4, is predominantly produced by Th2 cells and contributes to regulating B cell function, enhancing antibody production, and balancing immune responses. (The figure was created using https://www.figdraw.com)
Recent studies have revealed significant abnormalities in the expression levels of immune regulators in patients with schizophrenia. For instance, comparisons have revealed elevated levels of IL-4, IL-10, IL-12, IL-13, and MCP-1 in the peripheral blood of drug-naïve patients with first-episode schizophrenia compared to healthy controls [17–20]. However, in long-term chronic schizophrenia patients, the levels of IL-4 and IL-12 showed a decreasing trend [18]. Additionally, although IL-12 levels are typically elevated in first-episode patients, they were significantly influenced by pharmacological treatment [21]. Conversely, IL-13 levels did not show significant changes in first-episode patients [19]. Despite some inconsistencies, these findings underscored a dysregulation of cytokine balance in schizophrenia.
Further research has explored the relationship between expression abnormalities of these immune regulators and the clinical symptoms and cognitive functions in schizophrenia. The levels of IL-4 and IL-10 have been significantly associated with negative symptoms [22, 23] and cognitive impairments in schizophrenia patients [24, 25]. On the contrary, levels of IL-12 and MCP-1 have been correlated with positive symptoms and cognitive dysfunction [21, 26–28]. These results suggest that the dysregulated expression of immune regulators may influence the neuroimmune processes, with a role in the pathophysiological mechanisms of schizophrenia and being closely related to the clinical manifestations of the patients.
Due to the chronic nature of the disease and prolonged treatment, long-term hospitalized patients with schizophrenia may exhibit special inflammatory characteristics. Additionally, the biological and hormonal differences of male patients may produce significant variations in disease manifestation and inflammatory responses compared to female patients. Therefore, studying the inflammatory traits of long-term hospitalized male schizophrenia patients can enhance the understanding of the pathophysiological mechanisms in this specific group.
We hypothesize that the levels of IL-4, IL-10, IL-12, IL-13, and MCP-1 in sera of these patients will vary and that these variations are associated with clinical pathological symptoms and cognitive functions. This study performed in long-term hospitalized schizophrenia patients assessed: (1) the variation in serum levels of IL-4, IL-10, IL-12, IL-13, and MCP-1; (2) the association of these cytokine levels with clinical symptoms and cognitive functions; and (3) the potential of these cytokines as biomarkers for predicting susceptibility to chronic schizophrenia.
Subjects and methods
Subjects
This cross-sectional, case-control study involved 72 male schizophrenia patients who were long-term inpatients at the Department of Psychiatry of the Fourth People’s Hospital of Lianyungang and its affiliated institutions. The inclusion criteria were males aged 18 to 60 years, Han ethnicity, meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia, with a minimum of two consecutive years of hospitalization and at least 12 months of stable medication, and ability to complete neuropsychological assessments. Additionally, participants had not received anti-inflammatory or antibiotic treatments in the four weeks prior to enrollment. All patients were evaluated by two senior psychiatrists. Exclusion criteria included any history of brain disorders (e.g., intellectual disability, dementia, neurodegenerative diseases), significant physical illnesses (e.g., endocrine or metabolic disorders), inability to complete neuropsychological assessments, or history of alcohol or drug dependence.
The control group consisted of 68 healthy individuals matched with the patients in terms of age, sex, education, body mass index (BMI), and smoking. These participants were recruited from the local community through advertisements. Members of the healthy control group had no history of any Axis I psychiatric disorders as defined by DSM-IV, no personal or familial history of psychiatric disorders, and no history of alcohol or drug dependence. They also had not received anti-inflammatory or antibiotic treatments in the four weeks prior to enrollment.
All participants underwent routine laboratory and physical examinations, and those with abnormal results were excluded. The purpose and procedures of the study were fully explained to all participants or their guardians, and participation was voluntary. All data were anonymized to protect participant privacy. The study protocol was approved by the Ethics Committee of the Fourth People’s Hospital of Lianyungang, and informed consent was obtained from all participants or their guardians.
Measures
All participants were required to complete a detailed questionnaire containing demographic and clinical data that included age, gender, educational level, smoking, BMI, age of onset, and disease duration. Additionally, doses of antipsychotic medications were converted to chlorpromazine equivalent doses for standardization [29]. The severity of psychotic symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS) [30–32], with an inter-rater correlation coefficient exceeding 0.8.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was employed to assess cognitive functions across all participants, encompassing five subscales: attention, language, visuospatial/constructional, immediate memory, and delayed memory. Initially, the total raw score for the subscales was calculated, which was then converted into a standardized score. Utilization of RBANS within the Chinese population has indicated favorable reliability and validity [33, 34].
Measurement of peripheral blood
Between 7:00 and 9:00 AM, fasting peripheral blood samples were collected from both healthy control subjects and patients, and stored using coagulant tubes. After collection, the samples were left to stand at 4 °C for 1 h, followed by centrifugation at 3,000 rpm for 15 min. Subsequently, the samples were stored in a -80 °C freezer until analysis. All blood samples were tested in duplicate by a technician who was blinded to the study sample and clinical status. The intra-assay coefficient of variation for IL-4, IL-10, IL-12p40, IL-13, and MCP-1 (all pg/mL) ranged from 2.6 to 6.3%, while the inter-assay coefficient of variation ranged from 2.5 to 9.6%. Serum levels of IL-4, IL-10, IL-12p40, IL-13, and MCP-1 were analyzed using the Luminex liquid suspension chip detection system, strictly following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of distribution for variables between the patient and healthy control groups. However, serum levels of IL-4, IL-10, IL-12p40, IL-13, and MCP-1 did not pass the normality test in both groups. Thus, the Mann-Whitney U test was utilized for these comparisons. Continuous variables that followed a normal distribution were analyzed using the independent samples or Student’s t-test, and are reported as mean ± standard deviation. For continuous variables that did not follow a normal distribution, the median along with the 25th and 75th percentiles were reported. Categorical variables were assessed using the chi-square test. Bonferroni correction was applied as a post-hoc test, with a corrected significance level of 0.01. Moreover, to evaluate effect sizes, Cohen’s d value was used, where 0.2, 0.5, and 0.8 respectively represented small, medium, and large effects. Differences were considered statistically significant at a P-value < 0.05 (two-tailed).
Relationships between nonparametric continuous variables were assessed using Spearman’s correlation analysis. Stepwise regression analysis was used to explore the relationship between psychopathological symptoms or cognitive functions and independent variables to analyze influencing factors. Histograms or Durbin-Watson tests were used to examine whether the residuals conformed to a normal distribution or were independent. Furthermore, we categorized IL-4, IL-10, IL-12p40, IL-13, and MCP-1 into high IL-4 (code 1), high IL-10 (code 1), high IL-12p40 (code 1), high IL-13 (code 1), and high MCP-1 (code 1) groups based on the median among all the participants. Due to the strong correlation between IL-4, IL-13, and MCP-1, we constructed IL-4*MCP-1 and IL-13*MCP-1 interaction terms, which were subsequently incorporated into binary logistic regression equations as independent variables. These models, with diagnosis (chronic schizophrenia vs. healthy control) as the dependent variable, included all measured parameters (except IL-12p40) as independent variables, adjusting for potential confounding factors including age, education, BMI, and smoking, to analyze variables that may predict susceptibility to long-term chronic schizophrenia. SPSS Windows statistical software version 22.0 was used for all statistical data (SPSS, Chicago, IL, USA). The G*power 3.1.9.7 were used to calculate the sample sizes (https://www.softpedia.com/get/Science-CAD/G-Power.shtml).
Results
Demographic and general clinical data in patients and healthy controls
Table 1 presents the demographic characteristics and general clinical data of patients with schizophrenia and healthy controls. There was no significant difference between the two groups in terms of age, education, BMI, and smoking (all P > 0.05). The age of onset of schizophrenia was 26.65 ± 8.29 years, the PANSS total score was 57.57 ± 15.19, with a positive symptom score of 11.18 ± 4.69, negative symptom score of 28.96 ± 6.15, general psychopathology score of 17.61 ± 7.05, and equivalent dose of chlorpromazine of 577.73 ± 186.23. Moreover, there was a significant difference in cognitive function between patients with schizophrenia and healthy controls (P < 0.05); the patients performed worse in RBANS total score and all subscales.
Table 1.
Demographic and clinical data of patients with schizophrenia and healthy controls
| Patients (n = 72) | HC (n = 68) | t/χ2/Z | P | |
|---|---|---|---|---|
| Age (years) | 40.40 ± 9.53 | 40.38 ± 9.50 | 0.013 a | 0.990 |
| Education (years) | 9 (6, 9) | 9 (6, 12) | -1.665 b | 0.096 |
| BMI (kg/m2) | 24.85 ± 3.57 | 25.38 ± 2.80 | -0.990 a | 0.324 |
| Smoking (n, %) | 35 (48.6) | 27 (39.7) | 1.124 c | 0.289 |
| Age of onset (years) | 26.65 ± 8.29 d | - | - | - |
| Duration of illness (years) | 12.79 ± 8.19 d | - | - | - |
| Equivalent dose of chlorpromazine (mg/d) | 577.73 ± 186.23 d | - | - | - |
| PANSS total score | 57.57 ± 15.19 d | - | - | - |
| P subscores | 11.18 ± 4.69 d | - | - | - |
| N subscores | 28.96 ± 6.15 d | - | - | - |
| G subscores | 17.61 ± 7.05 d | - | - | - |
| RBANS total score | 58.60 ± 11.13 | 88.32 ± 11.85 | -15.306 a | < 0.001 |
| Attention | 82.13 ± 13.78 | 106.54 ± 14.82 | -10.102 a | < 0.001 |
| Language | 72.07 ± 14.04 | 96.43 ± 11.61 | -11.150 a | < 0.001 |
| Visuospatial/constructional | 70.10 ± 14.70 | 88.63 ± 14.26 | -7.565 a | < 0.001 |
| Immediate memory | 50.81 ± 17.38 | 83.22 ± 16.97 | -11.157 a | < 0.001 |
| Delay memory | 54.35 ± 16.38 | 83.71 ± 17.54 | -10.240 a | < 0.001 |
a Independent samples t-test; b Mann-Whitney U test; c chi-square test; d Student’s t-test. Abbreviations are: HC, healthy controls; BMI, body mass index; PANSS, Positive and Negative Syndrome Scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
Comparison of immune regulators levels between schizophrenia patients and healthy controls
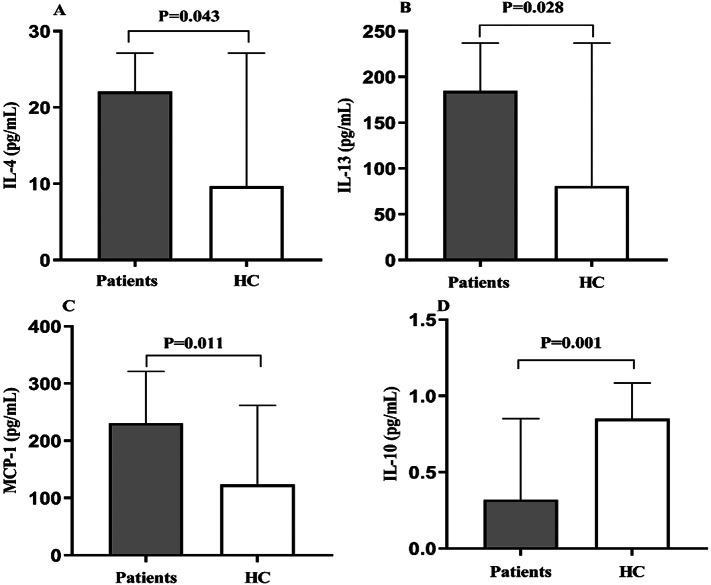
As shown in Table 2, serum IL-4, IL-13, and MCP-1 levels were significantly increased in patients with schizophrenia compared to the levels in healthy controls (P = 0.043, P = 0.028, and P = 0.011, respectively; Fig. 2A-C, respectively). Serum IL-10 levels were significantly lower in schizophrenia patients than the levels in healthy controls (P = 0.001, Fig. 2D). However, there was no significant difference in serum IL-12p40 levels between the patients and healthy controls (P = 0.263). After Bonferroni corrections, only serum IL-10 levels showed significant differences between groups (P = 0.005). No significant differences were found in MCP-1, IL-4, IL-12, or IL-13 levels (all P > 0.05).
Table 2.
Levels of immune regulators between patients with schizophrenia and healthy controls (HC)
| Patients (n = 72) | HC (n = 68) | Z | P | |
|---|---|---|---|---|
| IL-4 (pg/mL) | 22.05 (6.20, 27.15) | 9.66 (6.20, 27.15) | -2.023 | 0.043 |
| IL-10 (pg/mL) | 0.32 (0.19, 0.85) | 0.85 (0.38, 1.09) | -3.431 | 0.001 |
| IL-12p40 (pg/mL) | 76.37 (50.70, 109.10) | 84.25 (61.41, 113.83) | -1.119 | 0.263 |
| IL-13 (pg/mL) | 184.71 (71.12, 237.19) | 80.79 (61.25, 237.19) | -2.196 | 0.028 |
| MCP-1 (pg/mL) | 230.94 (102.71, 321.58) | 123.63 (86.54, 261.96) | -2.541 | 0.011 |
Comparisons between groups were performed using the Mann-Whitney U test
Fig. 2.
Comparison of IL-4 (A), IL-13 (B), MCP-1 (C), and IL-10 (D) levels between schizophrenia patients and healthy controls (HC)
Correlation of immune regulators with clinical symptoms in schizophrenia patients
Spearman’s correlation analysis revealed that IL-4 levels were significantly negatively associated with PANSN positive symptoms (r=-0.313, P = 0.007). No significant correlation was evident between IL-10, IL-13, MCP-1, and PANSS total scores and the PANSS subscales (all P > 0.05). Furthermore, stepwise regression analysis revealed independent associations with PANSS positive symptoms and IL-4 (beta=-0.222, t=-2.068, P = 0.042) and with the equivalent dose of chlorpromazine (beta = 0.412, t = 3.845, P < 0.001). Partial correlation analysis revealed that after controlling for antipsychotic medication, BMI, and smoking, the correlation between PANSS positive symptoms and IL-4 was not significant (r = -0.232, P = 0.055).
Correlation of immune regulators with cognitive functions in schizophrenia patients
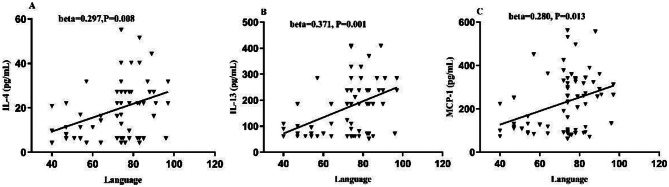
Spearman’s correlation analysis showed that levels of IL-4, IL-13, and MCP-1 were significantly positively associated with language (r = 0.318, P = 0.006; r = 0.379, P = 0.001; r = 0.286, P = 0.015, respectively). No significant correlation was found between IL-10 and RBANS total score and the subscales (all P > 0.05). Stepwise regression analysis revealed that IL-4 (beta = 0.297, t = 2.714, P = 0.008, Fig. 3A) and education (beta = 0.288, t = 2.626, P = 0.011) were independently correlated with language. IL-13 (beta = 0.371, t = 3.455, P = 0.001, Fig. 3B) and education (beta = 0.257, t = 2.397, P = 0.019), as well as MCP-1 (beta = 0.280, t = 2.539, P = 0.013, Fig. 3C) and education (beta = 0.290, t = 2.626, P = 0.011), were independently associated with language. After controlling for potential confounding factors including antipsychotic medication, BMI, smoking, and education, partial correlation analyses revealed that IL-4 (r = 0.307, P = 0.011), IL-13 (r = 0.376, P = 0.002), and MCP-1 (r = 0.293, P = 0.015) maintained significant positive associations with language scores.
Fig. 3.
Correlation of IL-4, IL-13, and MCP-1 with clinical symptoms and cognitive functions in schizophrenia patients. Positive correlations of serum IL-4, IL-13, and MCP-1 levels with language (A, B, C, respectively)
Independent variables predicting susceptibility to chronic schizophrenia
Because there was a strong correlation between IL-4 and IL-13 with MCP-1 (r = 0.690, P < 0.001; r = 0.619, P < 0.001), we constructed IL-4*MCP-1 and IL-13*MCP-1 interaction terms included in the logistic regression model. In the logistic regression model, we divided all participants into the high group (code 1) or low group (code 0) concerning the levels of IL-4, IL-10, IL-12p40, IL-13, and MCP-1 based on univariate regression analysis according to the median. All these parameters, except IL-12p40, were individually included in the binary logistic regression model adjusted for age, education, BMI, and smoking. Positive associations with the risk of chronic schizophrenia were evident for increased levels of IL-4 (B = 0.690, P = 0.044, OR = 1.994, 95% CI: 1.017−3.908), IL-13 (B = 0.809, P = 0.019, OR = 2.245, 95% CI: 1.141–4.416), and IL-4* MCP-1 (B = 0.693, P = 0.043, OR = 2.000, 95% CI: 1.020–3.920). Decreased levels of IL-10 (B = 1.053, P = 0.003, OR = 0.2.867, 95% CI: 1.444–5.693) were positively associated with the risk of chronic schizophrenia. However, no significant correlations were evident between IL-12p40, MCP-1 and IL-13*MCP-1 levels and the risk of chronic schizophrenia (all P > 0.05).
Discussion
There were four primary findings of this study. First, serum IL-4, IL-13, and MCP-1 levels were significantly elevated, while IL-10 levels were significantly decreased in male patients with chronic schizophrenia. Second, IL-4 levels showed a negative association with PANSS positive symptoms. However, this association was no longer statistically significant after controlling for potential confounding factors, including antipsychotic medication, BMI, and smoking. Third, IL-4, IL-13, and MCP-1 were significantly positively correlated with language. Fourth, increased levels of IL-4, IL-13, as well as IL-4*MCP-1 interaction were positively associated with the risk of chronic schizophrenia, whereas lower IL-10 levels were positively associated with the risk.
We observed elevated serum levels of IL-4, IL-13, and MCP-1, and decreased levels of IL-10 in male patients with chronic schizophrenia, suggesting that chronic schizophrenia may be associated with changes in specific patterns of immune responses. Increased levels of IL-4 and IL-13 generally indicate an increased Th2-type immune response [35]. Abnormalities in Th2-type immune responses may be associated with the onset and progression of schizophrenia [36, 37], consistent with our findings. Previous clinical studies have shown elevated levels of IL-4 and IL-13 in patients with schizophrenia [17, 20, 38]. However, there were some differences in the results between several studies [20, 39], which may be related to factors, such as sample size and stage of the disease. In addition, MCP-1, a chemokine and adaptive immune factor, can also observed be elevated in patients with chronic schizophrenia, suggesting a possible chronic inflammatory state in such patients. Mendelian data have suggested an association between MCP-1 and schizophrenia [16], and a meta-analysis reported that MCP-1 was elevated in different stages of schizophrenia [19], further supporting the hypothesized role for chronic inflammation in schizophrenia.
Serum IL-10 levels have been inconsistent in studies of different stages of schizophrenia; increased or no change in IL-10 levels have been described in patients with acute-phase schizophrenia [18, 40, 41]. As an important anti-inflammatory and immunomodulatory factor, IL-10 performs a key role in suppressing excessive immune responses [42]. Concerning chronic inflammation, the imbalance of the immune response as well as self-regulation of the immune system may suffer from disturbances that do not effectively increase IL-10 production to suppress inflammation [43, 44]. Our observation of reduced serum IL-10 levels in patients with chronic schizophrenia suggested that adaptive modifications of the immune system in response to the persistent inflammatory state may have contributed to the previously described exacerbation and persistence of the inflammatory response [42, 45], further highlighting the involvement of inflammation in the pathophysiological process of schizophrenia. In addition, the potential effects of antipsychotic medications administered to patients with long-term chronic schizophrenia on IL-4, IL-10, IL-13, and MCP-1 levels warrants further study.
Our findings revealed a significant negative correlation between serum IL-4 levels and positive symptoms in patients with chronic schizophrenia, although this association became non-significant after controlling for potential confounding factors. This observation presents a nuanced perspective that diverges from the traditional view that increased inflammatory factors are usually associated with worsening symptoms [34, 46–48]. However, IL-4, a major adaptive regulatory and anti-inflammatory cytokine, normally exerts an anti-inflammatory role in the immune system [49]. Thus, elevated IL-4 levels may reflect the initiation of self-regulation within the individual to reduce or resist the inflammatory state of psychiatric disorders by increasing anti-inflammatory factors [49–51]. Although the results of the present study may imply a protective effect of IL-4 on psychological symptoms in chronic schizophrenia, the results do not mean that IL-4 is the primary cause of symptom reduction. Rather, it is more likely that there was a regulatory response of IL-4 to chronic inflammation. Possible reasons also included more complex feedback of the immune network, stage or duration of the disease, and the intervention of other variables such as medication, age, and sex, which may explain the interesting result.
Our findings that elevated serum IL-4 and IL-13 levels were positively associated with cognitive deficits are consistent with previous studies [24, 52]. Other previous studies have indicated that IL-4 and IL-13 may exert neuroprotective effects by inhibiting microglia and astrocyte activation, and reducing the release of inflammatory factors [53, 54]. IL-4 and IL-13 affect learning and memory by influencing neuroplasticity, neurogenesis in the hippocampus, and modulation of the neurovascular unit [55]. However, elevated levels of IL-4 and IL-13 may lead to an immune system imbalance [56]. IL-4 directly mediates its effects through IL-4Rα expressed on gamma-aminobutyric acidergic neurons, and astrocytes respond to IL-4 to produce brain-derived neurotrophic factor, which is critical for learning and memory [57]. Elevated IL-4 acts on epithelial cells to produce C-C motif chemokine 11 that is associated with cognitive decline during aging [57–59]. An animal model study revealed that mice with severe immune deficiency exhibit amnesia [60], which can be reversed by supplementing with T cells. However, this reversal was reportedly not achieved with T cells from IL-4 knockout mice. The elimination of IL-4Rα in inhibitory neurons impairs context fear memory, and further single nucleus RNA-sequencing analyses from these mice have shown that IL-4 plays a crucial role in regulating synaptic function to promote memory formation. Another study demonstrated that hippocampal injections of exogenous IL-13 improve cognitive dysfunction in mice [54, 61].
The role of IL-4 in chronic schizophrenia patients appears to be multifaceted. On one hand, it may fluctuate in response to the exacerbation or amelioration of psychopathological symptoms [62]; on the other hand, alterations in IL-4 might potentially influence psychopathological symptoms or cognitive functions [63, 64]. Additionally, the relatively low PANSS positive symptom scores in our patient samples may also have made the positive correlation between IL-4 and cognitive functions more prominent. These complex mechanisms and interactions may help explain the observed differential associations of IL-4 with positive symptoms and cognitive domains.
In the present study, serum MCP-1 levels were positively correlated with cognitive deficits in patients with chronic schizophrenia. MCP-1 is an important regulator and chemokine that plays key roles in inflammatory response and immunomodulation. MCP-1 recruits inflammatory cells, such as monocytes and macrophages, which infiltrate and cause inflammation and neuronal damage, which in turn leads to cognitive dysfunction [65, 66]. Animal studies have revealed a correlation between Y-maze manifestations and serum MCP-1 levels [67], and the strong association between MCP-1 levels in cerebrospinal fluid and Alzheimer’s disease [68]. However, clinical findings have been inconsistent; a study that included 20 patients with schizophrenia found that elevated plasma MCP-1 levels were associated with worse cognitive flexibility [69]. A supervised machine learning study did not find MCP-1 involvement in cognitive deficits in deficit schizophrenia [70]. The differences in outcomes may be related to the duration of illness, severity of clinical symptoms, medications administered, gender, heterogeneity of schizophrenia, and varying degrees of cognitive deficits in different subtypes of patients. Nonetheless, at the clinical level, our findings establish an association between IL-4, IL-13, and MCP-1 levels and cognitive deficits. Although the mechanisms remain incompletely clear and may involve multiple aspects of inflammation-mediated neurological damage, the findings increase the understanding about the role of chronic inflammation in schizophrenia, including cognitive function.
Further analysis revealed that increased levels of IL-4 and IL-13 were associated with the risk of chronic schizophrenia, suggesting the involvements of inflammatory regulators and enhanced Th2 immune responses in the pathophysiology of chronic schizophrenia. The previous finding in patients with first-episode drug-naïve schizophrenia that the compensatory immune-regulatory system, including Th1 and Th2, was activated after risperidone treatment and associated with recovery from acute-phase schizophrenia [71] supports our findings from another perspective. We initially found an association of the interaction between IL-4 and MCP-1 and the risk of chronic schizophrenia.MCP-1. As a small molecule chemokine, MCP-1 regulates the inflammatory response by recruiting monocytes to the site of inflammation. The enhanced interaction between IL-4 and MCP-1 indicates that the recruitment and activation of inflammatory cells may be enhanced in the pathological setting of schizophrenia. This, in turn further promotes the establishment of an inflammatory environment, thereby increasing the risk of chronic schizophrenia.
IL-10 is usually a cytokine with strong anti-inflammatory effects, which inhibits the production of inflammatory cytokines. The positive correlation between reduced IL-10 levels and the risk of chronic schizophrenia susceptibility observed in the present study may suggest that impaired anti-inflammatory response may be a key factor in the chronic development of the disease. However, it is worth noting that there were still some inconsistent findings. For example, Facal et al. found that IL-6 polygenic score was associated with schizophrenia chronicity, explaining 1.51% of the variance, whereas IL-4 was not [72]. Laskaris et al. did not find an association between IL-4 and IL-13 and frontal thickness in patients with chronic schizophrenia, but did find an association in patients with first-episode schizophrenia between IL-4 and IL- 13 were associated with frontal thickness [73]. A systematic review and network meta-analysis demonstrated that IL-4 and IL-10 were decreased in chronic schizophrenia and that IL-4 was associated with age, smoking, BMI, medications, duration of illness, severity of psychopathic symptoms, and subtype [18]. Thus, the present study provided a preliminary result that IL-4, IL-10, IL-13, and IL-4 and MCP-1 interactions were correlated with the risk of chronic susceptibility to schizophrenia. Further investigation is warranted.
Furthermore, variations in regulatory cytokines may complement or interact with changes in pro-inflammatory cytokines such as IL-6, TNF-α, and these alterations may be important in schizophrenia pathology [34, 46]. Previous study has found that the trait marker IFN-γ is closely associated with multidimensional manifestations of psychosis, state markers (IL-6 and IL-1β) fluctuate across the psychosis spectrum, and lower levels of regulatory cytokines may lead to an uncontrolled inflammatory system [74]. Future studies need to further explore the dynamic balance between these regulatory and pro-inflammatory factors and their effects on schizophrenia. It is important to emphasize that the role of inflammatory factors in schizophrenia is complex, and their different forms and degrees of inflammatory response may play different roles in different stages or subtypes of the illness.
The present study had several limitations. First, the cross-sectional design limited our ability to establish causal relationships between variables, making it difficult to determine whether immune alterations precede or follow cognitive changes in schizophrenia. Second, as the study focused on chronic schizophrenia patients with relatively stable symptoms, our findings may not generalize to patients at different illness stages, particularly those in acute phases or early onset. Third, the inclusion of only male participants limited the generalizability of our results to female patients, which is particularly important given the known sex differences in both immune function and schizophrenia presentation. Fourth, the measurement of regulatory cytokines alone, while providing valuable insights, offered only a partial view of the immune system status in schizophrenia, as other immune markers might also play important roles in cognitive function and symptom presentation.
In conclusion, this study focused on the serum levels of immune modulatory factors and their association with disease characteristics in male patients with chronic schizophrenia. The levels of IL-4, IL-13, and MCP-1 were significantly elevated, whereas IL-10 was significantly reduced. IL-4, IL-13, and MCP-1 showed positive associations with cognitive functions, particularly language abilities. The findings suggested that these immune factors may play roles in the cognitive deficits and symptom manifestations of schizophrenia. Moreover, increased levels of IL-4 and IL-13. as well as the interaction between IL-4 and MCP-1 were associated with a higher risk of chronic schizophrenia, while reduced IL-10 levels were also positively related to disease risk. These findings further supported the involvement of the inflammatory hypothesis in the pathophysiology of schizophrenia, offering potential biomarkers and intervention points for future treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants in the study. We also thank Figdraw (www.figdraw.com) for the assistance in creating Fig. 1.
Author contributions
Tianming Guo and Haidong Yang wrote the manuscript; Xiaobin Zhang was responsible for study design; Haidong Yang and Xiaobin Zhang performed the statistical analysis; Haidong Yang, Lihua Chen, Lingshu Luan, and Man Yang were responsible for performing the clinical rating, recruiting the patients, and collecting the samples. All authors have contributed to and have approved the final manuscript.
Funding
The study was supported by the Suzhou Key Technologies Program (SKY2021063), Suzhou clinical Medical Center for mood disorders (No. Szlcyxzx202109), Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116), Suzhou Key Laboratory (SZS2024016), Guidance Project of Jiangsu Provincial Health Commission (Z2023074), Science foundation of Kangda college of Nanjing medical university (KD2023KYJJ130) and General Program of Lianyungang Health Committee (NO.202130). The funding sources of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Data availability
The data supporting the results of this study are available upon request from the corresponding author.
Declarations
Ethical approval and consent to participate
We declare that all experiments on human subjects were conducted in accordance with the Declaration of Helsinki and that all procedures were carried out with the adequate understanding and written consent of the subjects. All experimental protocols were approved by the Ethics Committee of Lian Yun Gang Fourth People’s Hospital. Informed consent was obtained from all the participants and/or their legal guardians. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaobin Zhang, Email: zhangxiaobim@163.com.
Haidong Yang, Email: yanghaidonglyg@163.com.
References
- 1.GBD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velligan DI, Rao S. The Epidemiology and Global Burden of Schizophrenia. J Clin Psychiatry 2023, 84(1). [DOI] [PubMed]
- 4.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–24. [DOI] [PubMed] [Google Scholar]
- 5.Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, Yenn Thoo H, Oliver D, Davies C, Morgan C, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry: Official J World Psychiatric Association (WPA). 2018;17(1):49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fišar Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2023, 120. [DOI] [PubMed]
- 7.Alexandros Lalousis P, Schmaal L, Wood SJ, Cropley RLEPR, Watson VL, Pantelis A, Suckling C, Barnes J, Pariante NM. Inflammatory subgroups of schizophrenia and their association with brain structure: a semi-supervised machine learning examination of heterogeneity. Brain Behav Immun. 2023;113:166–75. [DOI] [PubMed] [Google Scholar]
- 8.Pozzi D, Rasile M, Corradini I, Matteoli M. Environmental regulation of the chloride transporter KCC2: switching inflammation off to switch the GABA on? Transl Psychiatry. 2020;10(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann SM, Heider J, Wust R, Fallgatter AJ, Volkmer H. Microglia-neuron interactions in schizophrenia. Front Cell Neurosci. 2024;18:1345349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaylock RL, Faria M. New concepts in the development of schizophrenia, autism spectrum disorders, and degenerative brain diseases based on chronic inflammation: a working hypothesis from continued advances in neuroscience research. Surg Neurol Int. 2021;12:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotoyama H, Namba H, Tohmi M, Nawa H. Schizophrenia Animal modeling with epidermal growth factor and its homologs: their connections to the inflammatory pathway and the dopamine system. Biomolecules 2023, 13(2). [DOI] [PMC free article] [PubMed]
- 12.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399(10323):473–86. [DOI] [PubMed] [Google Scholar]
- 13.Howes OD, Onwordi EC. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol Psychiatry. 2023;28(5):1843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gou M, Chen W, Li Y, Chen S, Feng W, Pan S, Luo X, Tan S, Tian B, Li W, et al. Immune-Inflammatory Response and Compensatory Immune-Regulatory Reflex systems and White Matter Integrity in Schizophrenia. Schizophr Bull. 2024;50(1):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–12. [DOI] [PubMed] [Google Scholar]
- 16.Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: a bi-directional two-sample mendelian randomization study. Brain Behav Immun. 2021;97:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leon-Ortiz P, Rivera-Chavez LF, Torres-Ruiz J, Reyes-Madrigal F, Carrillo-Vazquez D, Moncada-Habib T, Cassiano-Quezada F, Cadenhead KS, de la Gomez-Martin D. Fuente-Sandoval C: systemic inflammation and cortical neurochemistry in never-medicated first episode-psychosis individuals. Brain Behav Immun. 2023;111:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, Walder K, Warren N. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2023;10(4):260–71. [DOI] [PubMed] [Google Scholar]
- 19.Frydecka D, Krzystek-Korpacka M, Lubeiro A, Stramecki F, Stanczykiewicz B, Beszlej JA, Piotrowski P, Kotowicz K, Szewczuk-Boguslawska M, Pawlak-Adamska E, et al. Profiling inflammatory signatures of schizophrenia: a cross-sectional and meta-analysis study. Brain Behav Immun. 2018;71:28–36. [DOI] [PubMed] [Google Scholar]
- 20.Yeh TC, Chu HT, Tsai CK, Chang HA, Yang FC, Huang SY, Liang CS. Distinct inflammation biomarkers in healthy individuals and patients with Schizophrenia: a reliability testing of Multiplex Cytokine Immunoassay by Bland-Altman Analysis. Psychiatry Investig. 2019;16(8):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crespo-Facorro B, Carrasco-Marin E, Perez-Iglesias R, Pelayo-Teran JM, Fernandez-Prieto L, Leyva-Cobian F, Vazquez-Barquero JL. Interleukin-12 plasma levels in drug-naive patients with a first episode of psychosis: effects of antipsychotic drugs. Psychiatry Res. 2008;158(2):206–16. [DOI] [PubMed] [Google Scholar]
- 22.Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S. Inflammation in first-episode psychosis: the contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;146(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simsek S, Yildirim V, Cim A, Kaya S. Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with First Episode, Early Onset Schizophrenia. J Child Adolesc Psychopharmacol. 2016;26(8):721–6. [DOI] [PubMed] [Google Scholar]
- 24.Ntouros E, Karanikas E, Floros G, Andreou C, Tsoura A, Garyfallos G, Bozikas VP. Social cognition in the course of psychosis and its correlation with biomarkers in a male cohort. Cogn Neuropsychiatry. 2018;23(2):103–15. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Xu H, Wang D, Wei G, Zhou H, Wang L, Zhou Y, Zhang X. The interactive effect of genetic polymorphisms of IL-10 and COMT on cognitive function in schizophrenia. J Psychiatr Res. 2021;136:501–7. [DOI] [PubMed] [Google Scholar]
- 26.Kristóf Z, Baranyi M, Tod P, Mut-Arbona P, Demeter K, Bitter I, Sperlágh B. Elevated serum purine levels in Schizophrenia: a reverse translational study to identify Novel inflammatory biomarkers. Int J Neuropsychopharmacol. 2022;25(8):645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baek SH, Kim H, Kim JW, Ryu S, Lee JY, Kim JM, Shin IS, Kim SW. Association between Peripheral Inflammatory cytokines and cognitive function in patients with first-episode Schizophrenia. J Pers Med 2022, 12(7). [DOI] [PMC free article] [PubMed]
- 28.Ma J, Yan L, Guo T, Yang S, Ni D, Liu Y, Wang J. A pilot study of biomarkers of oxidative stress in serum and schizophrenia. Psychiatry Res. 2020;284:112757. [DOI] [PubMed] [Google Scholar]
- 29.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–93. [DOI] [PubMed] [Google Scholar]
- 30.Lim K, Peh O-H, Yang Z, Rekhi G, Rapisarda A, See Y-M, Rashid NAA, Ang M-S, Lee S-A, Sim K et al. Large-scale evaluation of the positive and negative syndrome scale (PANSS) symptom architecture in schizophrenia. Asian J Psychiatry 2021, 62. [DOI] [PubMed]
- 31.Li L, Ma H, Wang X, Meng E. Validation of Chinese version of positive and negative syndrome Scale-6 in clinical setting: a preliminary study. Psychiatry Clin Psychopharmacol. 2021;31(4):386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Zhang C, Yang M, Liu J, Zhang Y, Liu D, Zhang X. Variations of plasma oxidative stress levels in male patients with chronic schizophrenia. Correlations with psychopathology and matrix metalloproteinase-9: a case-control study. BMC Psychiatry 2024, 24(1). [DOI] [PMC free article] [PubMed]
- 33.Cheng Y, Wu W, Wang J, Feng W, Wu X, Li C. Cinical research reliability and validity of the repeatable battery for the Assessment of Neuropsychological Status in community-dwelling elderly. Archives Med Sci. 2011;5:850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Peng R, Yang M, Zhang J, Shi Z, Zhang X. Association between elevated serum matrix metalloproteinase-2 and tumor necrosis factor-alpha, and clinical symptoms in male patients with treatment-resistant and chronic medicated schizophrenia. BMC Psychiatry. 2024;24(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwaszko M, Bialy S, Bogunia-Kubik K. Significance of Interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells 2021, 10(11). [DOI] [PMC free article] [PubMed]
- 36.Rose DR, Careaga M, Van de Water J, McAllister K, Bauman MD, Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun. 2017;63:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz MJ, Kronig H, Riedel M, Dehning S, Douhet A, Spellmann I, Ackenheil M, Moller HJ, Muller N. IL-2 and IL-4 polymorphisms as candidate genes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256(2):72–6. [DOI] [PubMed] [Google Scholar]
- 38.Hughes HK, Yang H, Lesh TA, Carter CS, Ashwood P. Evidence of innate immune dysfunction in first-episode psychosis patients with accompanying mood disorder. J Neuroinflamm 2022, 19(1). [DOI] [PMC free article] [PubMed]
- 39.O’Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160(3):256–62. [DOI] [PubMed] [Google Scholar]
- 40.Arabska J, Wysokinski A, Brzezinska-Blaszczyk E, Kozlowska E. Serum levels and in vitro CX3CL1 (Fractalkine), CXCL8, and IL-10 synthesis in phytohemaglutinin-stimulated and non-stimulated peripheral blood mononuclear cells in subjects with Schizophrenia. Front Psychiatry. 2022;13:845136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine alterations in Schizophrenia: an updated review. Front Psychiatry. 2019;10:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.York AG, Skadow MH, Oh J, Qu R, Zhou QD, Hsieh WY, Mowel WK, Brewer JR, Kaffe E, Williams KJ, et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature. 2024;627(8004):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahbaz C, Zibandey N, Kurtulmus A, Duran Y, Gokalp M, Kirpinar I, Sahin F, Guloksuz S, Akkoc T. Reduced regulatory T cells with increased proinflammatory response in patients with schizophrenia. Psychopharmacology. 2020;237(6):1861–71. [DOI] [PubMed] [Google Scholar]
- 44.Brockmann L, Tran A, Huang Y, Edwards M, Ronda C, Wang HH. Ivanov, II: intestinal microbiota-specific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56(12):2719–e27352717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagata K, Nishiyama C. IL-10 in mast cell-mediated Immune Responses: anti-inflammatory and proinflammatory roles. Int J Mol Sci 2021, 22(9). [DOI] [PMC free article] [PubMed]
- 46.Yang H, Zhang J, Yang M, Xu L, Chen W, Sun Y, Zhang X. Catalase and interleukin-6 serum elevation in a prediction of treatment-resistance in male schizophrenia patients. Asian J Psychiatr. 2023;79:103400. [DOI] [PubMed] [Google Scholar]
- 47.Feng T, McEvoy JP, Miller BJ. Longitudinal study of inflammatory markers and psychopathology in schizophrenia. Schizophr Res. 2020;224:58–66. [DOI] [PubMed] [Google Scholar]
- 48.He X, Ma Q, Fan Y, Zhao B, Wang W, Zhu F, Ma X, Zhou L. The role of cytokines in Predicting the efficacy of Acute Stage treatment in patients with Schizophrenia. Neuropsychiatr Dis Treat. 2020;16:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549(7671):282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Shi H, Yang G, Yang Y, Li W, Song M, Shao M, Su X, Lv L. Associations between expression of indoleamine 2, 3-dioxygenase enzyme and inflammatory cytokines in patients with first-episode drug-naive Schizophrenia. Transl Psychiatry. 2021;11(1):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou T, Yang Z, Ni B, Zhou H, Xu H, Lin X, Li Y, Liu C, Ju R, Ge J, et al. IL-4 induces reparative phenotype of RPE cells and protects against retinal neurodegeneration via Nrf2 activation. Cell Death Dis. 2022;13(12):1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai YL, Lee JH, Chong JR, Ballard C, Francis PT, Kennedy BK, Arumugam TV, Chen CP, Aarsland D, Lai MKP. Inflammatory panel cytokines are elevated in the neocortex of late-stage Alzheimer’s disease but not Lewy body dementias. J Neuroinflammation. 2023;20(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quarta A, Berneman Z, Ponsaerts P. Neuroprotective modulation of microglia effector functions following priming with interleukin 4 and 13: current limitations in understanding their mode-of-action. Brain Behav Immun. 2020;88:856–66. [DOI] [PubMed] [Google Scholar]
- 54.Brombacher TM, Nono JK, De Gouveia KS, Makena N, Darby M, Womersley J, Tamgue O, Brombacher F. IL-13-Mediated regulation of learning and memory. J Immunol. 2017;198(7):2681–8. [DOI] [PubMed] [Google Scholar]
- 55.Levin SG, Pershina EV, Bugaev-Makarovskiy NA, Chernomorets IY, Konakov MV, Arkhipov VI. Why do levels of anti-inflammatory cytokines increase during Memory Acquisition? Neuroscience. 2021;473:159–69. [DOI] [PubMed]
- 56.Ashwood P. Preliminary findings of elevated inflammatory plasma cytokines in children with Autism who have co-morbid gastrointestinal symptoms. Biomedicines 2023, 11(2). [DOI] [PMC free article] [PubMed]
- 57.Mamuladze T, Kipnis J. Type 2 immunity in the brain and brain borders. Cell Mol Immunol. 2023;20(11):1290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci USA. 2013;110(6):2264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herz J, Fu Z, Kim K, Dykstra T, Wall M, Li H, Salvador AF, Zou B, Yan N, Blackburn SM, et al. GABAergic neuronal IL-4R mediates T cell effect on memory. Neuron. 2021;109(22):3609–e36183609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawahara K, Suenobu M, Yoshida A, Koga K, Hyodo A, Ohtsuka H, Kuniyasu A, Tamamaki N, Sugimoto Y, Nakayama H. Intracerebral microinjection of interleukin-4/interleukin-13 reduces beta-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience. 2012;207:243–60. [DOI] [PubMed] [Google Scholar]
- 62.Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86. [DOI] [PubMed] [Google Scholar]
- 63.McGeer EG, McGeer PL, Lovell MA. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19(1):355–61. [DOI] [PubMed] [Google Scholar]
- 64.Guedes JR, Ferreira PA, Costa J, Laranjo M, Pinto MJ, Reis T, Cardoso AM, Lebre C, Casquinha M, Gomes M, et al. IL-4 shapes microglia-dependent pruning of the cerebellum during postnatal development. Neuron. 2023;111(21):3435–e34493438. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Yehuda H, Arad M, Peralta Ramos JM, Sharon E, Castellani G, Ferrera S, Cahalon L, Colaiuta SP, Salame T-M, Schwartz M. Key role of the CCR2-CCL2 axis in disease modification in a mouse model of tauopathy. Mol Neurodegeneration 2021, 16(1). [DOI] [PMC free article] [PubMed]
- 66.Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol 2021, 101. [DOI] [PMC free article] [PubMed]
- 67.Tfilin M, Gobshtis N, Fozailoff D, Fraifeld VE, Turgeman G. Polarized anti-inflammatory mesenchymal stem cells increase hippocampal neurogenesis and improve cognitive function in aged mice. Int J Mol Sci 2023, 24(5). [DOI] [PMC free article] [PubMed]
- 68.Zhou F, Sun Y, Xie X, Zhao Y. Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Res Ther. 2023;15(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klaus F, Mitchell K, Liou SC, Eyler LT, Nguyen TT. Chemokine MCP1 is associated with cognitive flexibility in schizophrenia: a preliminary analysis. J Psychiatr Res. 2021;138:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Hakeim HK, Almulla AF, Maes M. The Neuroimmune and Neurotoxic Fingerprint of Major Neurocognitive psychosis or deficit Schizophrenia: a supervised machine learning study. Neurotox Res. 2020;37(3):753–71. [DOI] [PubMed] [Google Scholar]
- 71.Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA, Cordeiro Q, Belangero SI, Gadelha A, Bressan RA, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29(3):416–31. [DOI] [PubMed] [Google Scholar]
- 72.Facal F, Arrojo M, Paramo M, Costas J. Association between psychiatric admissions in patients with schizophrenia and IL-6 plasma levels polygenic score. Eur Arch Psychiatry Clin Neurosci 2024. [DOI] [PubMed]
- 73.Laskaris L, Mancuso S, Shannon Weickert C, Zalesky A, Chana G, Wannan C, Bousman C, Baune BT, McGorry P, Pantelis C, et al. Brain morphology is differentially impacted by peripheral cytokines in schizophrenia-spectrum disorder. Brain Behav Immun. 2021;95:299–309. [DOI] [PubMed] [Google Scholar]
- 74.Corsi-Zuelli F, Quattrone D, Ragazzi TCC, Loureiro CM, Shuhama R, Menezes PR, Louzada-Junior P, Del-Ben CM. Transdiagnostic dimensions of symptoms and experiences associated with immune proteins in the continuity of psychosis. Psychol Med. 2024;54(9):2099–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results of this study are available upon request from the corresponding author.