Abstract
Background
It has been suggested that the association between body mass index and breast cancer risk differs between Asian women and Western women. We aimed to assess the associations between body mass index and breast cancer incidence in East Asian women.
Methods
Pooled analyses were performed using individual participant data of 319,189 women from 13 cohort studies in Japan, Korea, and China. Participants’ height and weight were obtained by measurement or self-reports at cohort baseline. Breast cancer was defined as code C50.0-C50.9 according to the International Classification. Using a Cox proportional hazards model, hazard ratios of breast cancer were estimated for each body mass index category, with the reference group set as the group with a body mass index of 21 to < 23 kg/m2. The hazard ratio for a 5 kg/m2 increase in body mass index was also calculated.
Results
During a mean 16.6 years of follow-up, 4819 women developed breast cancer. Similar to Westerners, a steady increase in breast cancer risk with increasing body mass index was observed in postmenopausal women, but the slope of the risk increase appeared to slow at a body mass index of 26–28 kg/m2. In premenopausal women, the inverse association seen in Westerners was not observed. The risk of developing breast cancer after 50 years of age increased slightly with increasing body mass index, which was more pronounced in the older birth cohort. There was no significant association between body mass index and the risk of developing breast cancer before 50 years of age, but the risk estimates changed from positive to negative as the birth cohort got younger.
Conclusions
In East Asia, the role of body mass index in breast cancer in premenopausal women may be changing along with the increase in obesity and breast cancer. The increased risk of postmenopausal breast cancer with a higher body mass index was as robust as that of Western women.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01907-5.
Keywords: Body mass index, Breast cancer, Pooled analysis, Asians
Background
Breast cancer is the most common female cancer worldwide [1]. The incidence of breast cancer has been increasing in Asian countries, especially China, Korea, and Japan, although it remains lower than that in Western countries [2, 3]. The prevalence of overweight and obesity has also increased over the same period, but the burden of postmenopausal breast cancer attributable to overweight is not thought to be high, with estimates of 0.4–2.9% in Japan, 8.2% in Korea, and 5.9–8.8% in China [4–7]. The burden of premenopausal breast cancer is unknown.
Obesity is known to be a main risk factor for postmenopausal breast cancer. Numerous cohort studies have universally and consistently revealed a positive association between body mass index (BMI) and breast cancer risk in postmenopausal women [8–24], which has been confirmed in several meta-analyses [25–28]. This is presumed to be due primarily to the estrogenic effects of adipose tissue rather than the ovaries in postmenopausal women [29]. A nonlinear positive association with a plateau in increasing breast cancer risk at higher BMIs has been suggested in Western women [28], whereas a linear association has been observed among Asian women, for whom severe obesity is less common [26].
It has been suggested that the effect of BMI on premenopausal breast cancer differs between Asian women and Western women [27, 30, 31]; BMI has been inversely associated with breast cancer incidence in premenopausal women in most Western cohort studies [21, 27, 28, 32–34], but such an association has scarcely been observed in Asian studies [9–11, 15, 16, 18–20, 26]. A 2014 pooled analysis of 0.2 million women from eight population-based prospective cohort studies in Japan suggested a possible positive association between BMI and breast cancer risk in premenopausal women [26]. However, a 2022 Japanese cohort study of 0.8 million women and a 2021 Korean cohort study of 6.6 million women based on data from a health checkup database managed by the National Health Insurance Service retrospectively revealed an inverse association between BMI and breast cancer incidence in premenopausal women [14, 35].
Thus, it is necessary to determine whether the positive association between BMI and breast cancer in postmenopausal Asian women is linear or nonlinear and whether BMI is positively or inversely associated with breast cancer in premenopausal women. The aim of this study was to investigate the role of BMI in the incidence of breast cancer in premenopausal and postmenopausal East Asian women using more than 300,000 individual-level data pooled through the collaboration of several cohort studies.
Methods
Study population
This project was conducted by the Asia Cohort Consortium (ACC), an international collaboration involving more than a million participants across Asia aimed at elucidating the etiology of various diseases [36, 37]. The present pooled analysis included twelve population-based cohorts and one hospital-based cohort, all from Japan, Korea, and China (Supplementary Table 1). To pool the data, the relevant cohort investigators provided the following individual participant data: age, sex, height, weight, and other confounders such as smoking status and alcohol consumption at baseline as well as breast cancer incidence during the follow-up period. The ACC’s coordinating center harmonized the data.
Women who reported no history of any cancers and/or had data on height, weight, and menopausal status were included in the analysis (Supplementary Fig. 1). After women with a BMI of < 14 kg/m2 or > 50 kg/m2 were excluded, a total of 319 189 participants were included in the analysis.
Assessment of exposure
Participants’ height and weight were obtained by either measurement or self-administered questionnaires at baseline in each study. BMI was calculated as (weight in kg)/(height in m)2. In some cohorts with self-reports, the correlation coefficients between the responses reported on the questionnaire and the actual measurements were 0.93–0.97 for height, 0.85–0.97 for weight, and 0.90–0.91 for BMI [38–40]. Other studies for which validation was unavailable used questions similar to those for cohorts for which validation was available. Identical cutoff points for BMI were used for the pooled data. To examine the association with breast cancer in finer BMI categories, BMI was divided into the following seven categories: < 18.5 kg/m2, 18.5 to < 21 kg/m2, 21 to < 23 kg/m2, 23 to < 25 kg/m2, 25 to < 27.5 kg/m2, 27.5 to < 30 kg/m2, and ≥ 30 kg/m2, using the cutoff points recommended by the World Health Organization [41].
Menopausal status data were obtained from the questionnaire at baseline. Smoking status, alcohol consumption, age at menarche and menopause, parity number, and age at first delivery were also obtained from the baseline questionnaire. Details of the definitions of reproductive factors were described previously [42].
Outcomes and follow-up
Information on migration and death was obtained from the residential registry and death certificates. The incidence of cancer was confirmed mainly with reference to local cancer registries and/or through active patient notification from major local hospitals. The causes of cancer were coded according to the International Classification of Diseases for Oncology (ICD-O-2 or -3) or the International Classification of Diseases and Health Related Problems, 10th Revision (ICD-10). Breast cancer was defined as code C50, and the first primary cancer was included in this study.
Statistical analyses
Hazard ratios (HRs) and 95% confidence intervals (CIs) of breast cancer incidence were estimated for each BMI category using a Cox proportional hazards model. Follow-up periods were calculated as the time from baseline to the date of breast cancer diagnosis, date of death, date of moving out of the study area, or the end of follow-up, whichever came first. The reference group was defined as the group with a BMI ranging from 21 to < 23 kg/m2. Tests for a linear trend were performed using the Cox model with BMIs treated as continuous variables, thereby providing HRs for every 5-kg/m2 increase in BMI.
Because some premenopausal women undergo menopause during the follow-up period, additional analyses focused on breast cancer that developed before or after menopause among premenopausal women at baseline. Because no study collected information on menopausal status after the start of follow-up, 50 years of age, when approximately 50% of the participants were reported to be postmenopausal, was used as a proxy cutoff point; breast cancer was divided into breast cancer that developed before 50 years of age (early-onset) and breast cancer that developed after 50 years of age (later-onset). Then, the HRs for early-onset breast cancer were estimated by censoring the years of observation when participants reached 50 years of age. The HRs for later-onset breast cancer were estimated by using the years of observation thereafter. Sensitivity analysis was performed when 45 years of age, at which 16% of the participants were reported to be postmenopausal, was used as a cutoff point.
In addition, restricted cubic splines [43] with four knots placed at the 5th, 35th, 65th, and 95th percentiles of the BMI were used to delineate the dose‒response relationship between BMI and breast cancer and to test the nonlinear associations. A BMI of 22 kg/m2 was used as the spline reference.
After potential risk factors for breast cancer were identified through a literature review, the confounding factors included cohort (13 cohorts), age at enrollment (years, continuous), smoking status (never, former, or current smoker), alcohol consumption (non-drinkers or current drinkers), age at menarche (≤ 10 years, 11–12 years, 13–14 years, 15–16 years, or ≥ 17 years), nulliparity (yes or no), age at first delivery (≤ 20 years, 21–25 years, 26–30 years, or ≥ 31 years), and age at menopause (≤ 44 years, 45–49 years, 50–54 years, or ≥ 55 years; postmenopausal women only). All analyses were adjusted for these confounders. Dummy variables were created for missing categorical covariate data.
To evaluate the cohort effect by birth year on the associations, the analysis was repeated with the participants divided into three groups by birth year tertile. Country-specific associations were also evaluated.
Sensitivity analysis was performed after excluding patients who were diagnosed with breast cancer in the first three years because they might have had latent cancer at baseline, as well as after excluding one hospital-based study (Korean National Cancer Center cohort).
All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). P values were calculated by a two-sided test.
Results
Among 118,786 premenopausal and 200,403 postmenopausal women at baseline, 2,202 and 2,617 had developed breast cancer, respectively, during the mean 16.6 years of follow-up (Supplementary Table 1).
The mean age and BMI at baseline were 43.7 years and 23.0 kg/m2, respectively, for premenopausal women and 60.6 years and 23.5 kg/m2, respectively, for postmenopausal women (Table 1). More than 90% of the premenopausal and postmenopausal women were never smokers and/or had ever given birth.
Table 1.
Characteristics of women at baseline by menopausal status
| n | Premenopausal women | Postmenopausal women | ||
|---|---|---|---|---|
| 118,786 | 200,403 | |||
| Age at enrollment, mean, s.d | 43.7 | 5.1 | 60.6 | 8.1 |
| Body mass index, mean, s.d | 23.0 | 3.1 | 23.5 | 3.5 |
| Body mass index, n, % | ||||
| < 18.5 | 5,862 | 4.9% | 11,885 | 5.9% |
| 18.5- < 21 | 27,243 | 22.9% | 34,326 | 17.1% |
| 21- < 23 | 32,295 | 27.2% | 46,852 | 23.4% |
| 23- < 25 | 26,556 | 22.4% | 45,963 | 22.9% |
| 25- < 27.5 | 17,294 | 14.6% | 37,651 | 18.8% |
| 27.5- < 30 | 6,566 | 5.5% | 16,091 | 8.0% |
| 30- | 2,970 | 2.5% | 7,635 | 3.8% |
| Smoking status, n, % | ||||
| Never | 104,385 | 91.9% | 164,467 | 91.2% |
| Former | 1567 | 1.4% | 3678 | 2.0% |
| Current | 7598 | 6.7% | 12,198 | 6.8% |
| Alcohol consumption, n, % | ||||
| Non-drinkers | 87,553 | 76.1% | 150,976 | 81.5% |
| Current drinkers | 27,444 | 23.9% | 34,300 | 18.5% |
| Age at menarche (years), n, % | ||||
| ≤ 10 | 257 | 0.2% | 157 | 0.1% |
| 11–12 | 12,330 | 11.1% | 7778 | 4.4% |
| 13–14 | 52,410 | 47.3% | 52,241 | 29.5% |
| 15–16 | 35,427 | 32.0% | 71,843 | 40.5% |
| ≥ 17 | 10,330 | 9.3% | 45,165 | 25.5% |
| Nulliparity, n, % | ||||
| No | 108,760 | 94.0% | 174,919 | 92.3% |
| Yes | 6961 | 6.0% | 14,623 | 7.7% |
| Age at first delivery (years), n, % | ||||
| ≤ 20 | 4992 | 4.7% | 23,160 | 13.6% |
| 21–25 | 49,110 | 45.8% | 93,919 | 55.3% |
| 26–30 | 43,940 | 41.0% | 43,971 | 25.9% |
| ≥ 31 | 9134 | 8.5% | 8786 | 5.2% |
| Age at menopause (years), n, % | ||||
| ≤ 44 | 27,861 | 16.0% | ||
| 45–49 | 62,059 | 35.5% | ||
| 50–54 | 75,850 | 43.4% | ||
| ≥ 55 | 8818 | 5.1% | ||
s.d. standard deviation
A higher BMI was associated with an increased risk for breast cancer among both premenopausal and postmenopausal women at baseline (Table 2). In postmenopausal women, spline regression analysis revealed a steady increase in breast cancer risk with increasing BMI, but the slope of the increase in risk appeared to decrease as BMI reached approximately 26–28 kg/m2 (p for nonlinearity = 0.01) (Fig. 1b). In premenopausal women, BMI at baseline was not associated with the risk of developing breast cancer before 50 years of age, whereas a slight increase in the risk of developing breast cancer after 50 years of age was observed with increasing BMI (Table 3 and Fig. 1a). The results were essentially unaltered when 45 years of age was used as a cutoff point; the fully multivariate adjusted HRs per 5-kg/m2 increase in BMI were 1.00 (95% CI 0.80–1.25) and 1.11 (95% CI 1.03–1.19) for breast cancer developed before and after 45 years of age, respectively.
Table 2.
The association between body mass index and breast cancer risk in Asian women by menopausal status at baseline
| Body mass index at baseline (kg/m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 18.5 | 18.5– < 21 | 21– < 23 | 23– < 25 | 25– < 27.5 | 27.5– < 30 | 30- | Trend (per 5 kg/m2) | ||
| HR (95% CI) | HR (95% CI) | HR | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | p | |
| Premenopausal women | |||||||||
| Number of participants | 5,862 | 27,243 | 32,295 | 26,556 | 17,294 | 6,566 | 2,970 | ||
| Person-years | 115,078 | 509,956 | 589,871 | 483,623 | 315,679 | 119,619 | 53,686 | ||
| Number of cases | 92 | 470 | 578 | 496 | 370 | 135 | 61 | ||
| Crude rate (per 100,000) | 79.95 | 92.16 | 97.99 | 102.56 | 117.21 | 112.86 | 113.62 | ||
| Age- and study-adjusted (HR1) | 0.86 (0.69–1.07) | 0.97 (0.86–1.10) | 1 (ref.) | 1.01 (0.89–1.14) | 1.12 (0.99–1.28) | 1.07 (0.89–1.29) | 1.11 (0.85–1.44) | 1.08 (1.01–1.16) | 0.02 |
| Multivariate-adjusted (HR2)a | 0.86 (0.69–1.07) | 0.97 (0.86–1.10) | 1 (ref.) | 1.01 (0.90–1.14) | 1.13 (0.99–1.28) | 1.07 (0.89–1.29) | 1.11 (0.85–1.44) | 1.08 (1.01–1.16) | 0.02 |
| Multivariate-adjusted (HR3)b | 0.83 (0.67–1.04) | 0.96 (0.85–1.08) | 1 (ref.) | 1.01 (0.90–1.14) | 1.13 (0.99–1.29) | 1.08 (0.89–1.30) | 1.11 (0.85–1.44) | 1.09 (1.02–1.17) | 0.01 |
| (Excluding cases within 3 years) | |||||||||
| Multivariate-adjusted (HR2)a | 0.85 (0.66–1.08) | 0.93 (0.81–1.07) | 1 (ref.) | 1.01 (0.89–1.16) | 1.13 (0.98–1.30) | 1.13 (0.93–1.38) | 1.03 (0.77–1.39) | 1.10 (1.02–1.18) | 0.01 |
| Multivariate-adjusted (HR3)b | 0.82 (0.64–1.05) | 0.92 (0.80–1.05) | 1 (ref.) | 1.01 (0.89–1.16) | 1.14 (0.99–1.31) | 1.15 (0.94–1.40) | 1.03 (0.77–1.39) | 1.11 (1.03–1.20) | 0.005 |
| Postmenopausal women | |||||||||
| Number of participants | 11,885 | 34,326 | 46,852 | 45,963 | 37,651 | 16,091 | 7,635 | ||
| Person-years | 167,878 | 526,765 | 734,224 | 727,835 | 595,491 | 254,248 | 117,526 | ||
| Number of cases | 98 | 331 | 526 | 669 | 564 | 276 | 153 | ||
| Crude rate (per 100,000) | 58.38 | 62.84 | 71.64 | 91.92 | 94.71 | 108.56 | 130.18 | ||
| Age- and study-adjusted (HR1) | 0.83 (0.67–1.03) | 0.89 (0.77–1.02) | 1 (ref.) | 1.26 (1.12–1.41) | 1.27 (1.13–1.43) | 1.40 (1.21–1.62) | 1.64 (1.37–1.96) | 1.28 (1.21–1.35) | < 0.001 |
| Multivariate-adjusted (HR2)a | 0.83 (0.67–1.03) | 0.89 (0.77–1.02) | 1 (ref.) | 1.26 (1.12–1.41) | 1.27 (1.13–1.44) | 1.40 (1.21–1.62) | 1.64 (1.37–1.97) | 1.28 (1.22–1.35) | < 0.001 |
| Multivariate-adjusted (HR4)c | 0.80 (0.64–1.00) | 0.87 (0.76–1.00) | 1 (ref.) | 1.27 (1.13–1.42) | 1.30 (1.15–1.46) | 1.43 (1.24–1.66) | 1.72 (1.43–2.06) | 1.32 (1.25–1.39) | < 0.001 |
| (Excluding cases within 3 years) | |||||||||
| Multivariate-adjusted (HR2)a | 0.80 (0.63–1.02) | 0.87 (0.75–1.02) | 1 (ref.) | 1.22 (1.08–1.39) | 1.28 (1.12–1.46) | 1.46 (1.24–1.71) | 1.72 (1.42–2.10) | 1.31 (1.24–1.39) | < 0.001 |
| Multivariate-adjusted (HR4)c | 0.77 (0.60–0.99) | 0.85 (0.73–1.00) | 1 (ref.) | 1.23 (1.08–1.40) | 1.31 (1.14–1.49) | 1.50 (1.28–1.75) | 1.80 (1.48–2.19) | 1.35 (1.27–1.43) | < 0.001 |
HR hazard ratio, CI confident interval, ref reference
aEstimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), and alcohol consumption (non-drinkers or current drinkers)
bEstimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), alcohol consumption (non-drinkers or current drinkers), age at menarche (≤ 10 y, 11–12 y, 13–14 y, 15–16 y, ≥ 17 y), nulliparity (yes, no), and age at first delivery (≤ 20 y, 21–25 y, 26–30 y, ≥ 31 y)
cEstimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), alcohol consumption (non-drinkers or current drinkers), age at menarche (≤ 10 y, 11–12 y, 13–14 y, 15–16 y, ≥ 17 y), nulliparity (yes, no), age at first delivery (≤ 20 y, 21–25 y, 26–30 y, ≥ 31 y), and age at menopause (≤ 44 y, 45–49 y, 50–54 y, ≥ 55 y)
Fig. 1.
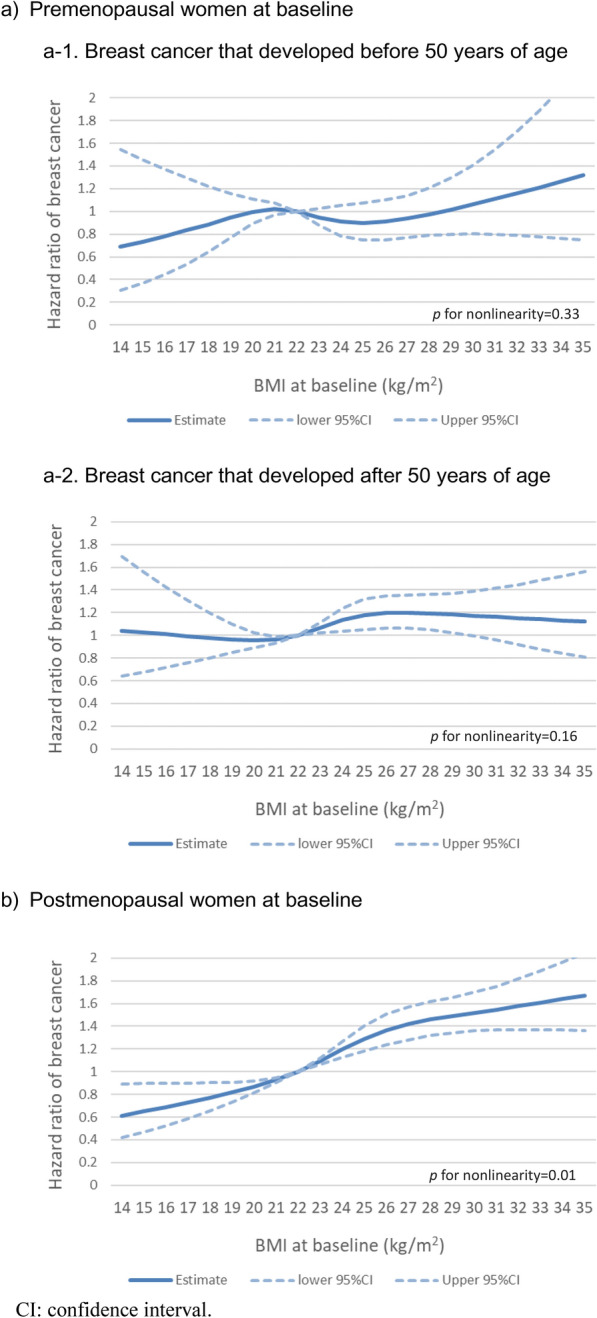
Spline regression curves for the association between body mass index and breast cancer risk by menopausal status at baseline and age at diagnosis.
Table 3.
The association between body mass index and breast cancer by age at diagnosis in premenopausal women at baseline
| Body mass index at baseline (kg/m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 18.5 | 18.5– < 21 | 21– < 23 | 23– < 25 | 25– < 27.5 | 27.5- < 30 | 30- | Trend (per 5 kg/m2) | ||
| HR (95% CI) | HR (95% CI) | HR | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | p | |
| Breast cancer that developed before 50 years of age | |||||||||
| Number of participants | 5,571 | 25,518 | 29,294 | 23,556 | 14,804 | 5,566 | 2,484 | ||
| Person-years | 48,597 | 194,531 | 197,523 | 146,036 | 86,777 | 31,975 | 14,699 | ||
| Number of cases | 24 | 160 | 161 | 122 | 91 | 26 | 15 | ||
| Crude rate (per 100,000) | 49.39 | 82.25 | 81.51 | 83.54 | 104.87 | 81.31 | 102.05 | ||
| Age- and study-adjusted (HR1) | 0.85 (0.55–1.31) | 1.15 (0.92–1.43) | 1 (ref.) | 0.97 (0.77–1.23) | 1.19 (0.92–1.54) | 0.92 (0.61–1.39) | 1.19 (0.70–2.02) | 1.02 (0.89–1.17) | 0.80 |
| Multivariate-adjusted (HR2)a | 0.84 (0.55–1.29) | 1.14 (0.92–1.42) | 1 (ref.) | 0.97 (0.77–1.23) | 1.19 (0.92–1.54) | 0.91 (0.60–1.38) | 1.18 (0.69–2.00) | 1.02 (0.89–1.17) | 0.78 |
| Multivariate-adjusted (HR3)b | 0.84 (0.55–1.30) | 1.15 (0.92–1.43) | 1 (ref.) | 0.97 (0.77–1.23) | 1.19 (0.92–1.54) | 0.91 (0.60–1.37) | 1.18 (0.69–2.00) | 1.02 (0.89–1.16) | 0.81 |
| (Excluding cases within 3 years) | |||||||||
| Multivariate-adjusted (HR2)a | 0.80 (0.44–1.48) | 1.02 (0.75–1.40) | 1 (ref.) | 0.93 (0.67–1.30) | 1.09 (0.75–1.59) | 1.13 (0.66–1.93) | 0.65 (0.24–1.77) | 1.02 (0.84–1.23) | 0.86 |
| Multivariate-adjusted (HR3)b | 0.80 (0.44–1.48) | 1.02 (0.75–1.40) | 1 (ref.) | 0.93 (0.67–1.30) | 1.10 (0.76–1.60) | 1.13 (0.66–1.93) | 0.66 (0.24–1.80) | 1.02 (0.84–1.24) | 0.84 |
| Breast cancer that developed after 50 years of age | |||||||||
| Number of participants | 5147 | 24,698 | 29,973 | 25,109 | 16,544 | 6263 | 2806 | ||
| Person-yearsc | 66,481 | 315,425 | 392,348 | 337,587 | 228,903 | 87,644 | 38,987 | ||
| Number of cases | 68 | 310 | 417 | 374 | 279 | 109 | 46 | ||
| Crude rate (per 100,000) | 102.28 | 98.28 | 106.28 | 110.79 | 121.89 | 124.37 | 117.99 | ||
| Age- and study-adjusted (HR1) | 0.94 (0.72–1.22) | 0.91 (0.79–1.06) | 1 (ref.) | 1.03 (0.90–1.19) | 1.14 (0.98–1.32) | 1.15 (0.93–1.42) | 1.09 (0.80–1.48) | 1.10 (1.02–1.19) | 0.02 |
| Multivariate-adjusted (HR2)a | 0.94 (0.73–1.23) | 0.92 (0.79–1.06) | 1 (ref.) | 1.03 (0.90–1.19) | 1.14 (0.98–1.33) | 1.15 (0.93–1.42) | 1.10 (0.81–1.49) | 1.10 (1.02–1.19) | 0.02 |
| Multivariate-adjusted (HR3)b | 0.90 (0.69–1.16) | 0.90 (0.78–1.04) | 1 (ref.) | 1.04 (0.91–1.20) | 1.16 (0.99–1.35) | 1.17 (0.94–1.44) | 1.10 (0.81–1.49) | 1.12 (1.04–1.21) | 0.004 |
| (Excluding cases within 3 years) | |||||||||
| Multivariate-adjusted (HR2)a | 0.94 (0.72–1.22) | 0.93 (0.80–1.08) | 1 (ref.) | 1.04 (0.90–1.20) | 1.17 (1.01–1.37) | 1.18 (0.95–1.46) | 1.11 (0.81–1.52) | 1.11 (1.03–1.20) | 0.009 |
| Multivariate-adjusted (HR3)b | 0.89 (0.68–1.16) | 0.91 (0.79–1.06) | 1 (ref.) | 1.05 (0.91–1.21) | 1.19 (1.02–1.39) | 1.19 (0.96–1.48) | 1.11 (0.81–1.52) | 1.13 (1.05–1.22) | 0.002 |
HR hazard ratio, CI confident interval
aEstimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), and alcohol consumption (non-drinkers or current drinkers)
bEstimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), alcohol consumption (non-drinkers or current drinkers), age at menarche (≤ 10 y, 11–12 y, 13–14 y, 15–16 y, ≥ 17 y), nulliparity (yes, no), and age at first delivery (≤ 20 y, 21–25 y, 26–30 y, ≥ 31 y)
cFollow-up period after 50 years of age
When patients diagnosed with breast cancer during the first 3 years of follow-up were excluded, none of the results were substantially altered (Tables 2 and 3). The exclusion of one hospital-based study also did not change the associations; the fully multivariate adjusted HRs of breast cancer for the lowest (< 18.5 kg/m2) and highest (≥ 30 kg/m2) BMIs were 0.80 (95% CI 0.64–1.00) and 1.71 (95% CI 1.42–2.05), respectively, in postmenopausal women. The corresponding HRs were 0.90 (95% CI 0.57–1.43) and 1.23 (95% CI 0.71–2.13), respectively, for breast cancer before 50 years of age, and 0.91 (95% CI 0.70–1.18) and 1.12 (95% CI 0.82–1.52), respectively, for breast cancer after 50 years of age in premenopausal women.
In premenopausal women, a significant positive association between BMI at baseline and the risk of developing breast cancer after 50 years of age was observed in the oldest birth cohort, but not in the younger birth cohorts (Table 4). There was no significant association between BMI at baseline and the risk of developing breast cancer before 50 years of age in all birth cohorts, but the risk estimates changed from positive to negative as the birth cohort got younger; the HR per 5-kg/m2 increase in BMI was 1.25 (95% CI 0.85–1.84) in women with a birth year of 1944 or earlier and 0.92 (95% CI 0.76–1.12) in women with a birth year of 1953 or after. BMI at baseline was positively associated with the risk of developing breast cancer after 50 years of age in the Japanese cohorts, while an inverse association was observed between BMI at baseline and the risk of developing breast cancer before 50 years of age in the Korean cohorts. A positive association between BMI and breast cancer in postmenopausal women was observed across all birth cohorts and countries, although the association was weaker in the Korean cohorts. The oldest cohort was predominantly Japanese, while the youngest birth cohort consisted mainly of Korean and Chinese participants.
Table 4.
The association between body mass index and breast cancer risk according to birth year and country
| n | Body mass index at baseline (kg/m2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 18.5 | 18.5– < 21 | 21– < 23 | 23– < 25 | 25– < 27.5 | 27.5– < 30 | 30- | Trend (per 5 kg/m2) | p for interaction | |||
| HR (95% CI) | HR (95% CI) | HR | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | p | |||
| Birth year | |||||||||||
| Premenopausal women at baseline | |||||||||||
| Breast cancer that developed before 50 years of age | |||||||||||
| 1944 or before | 32,654 | 1.03 (0.29–3.66) | 1.35 (0.66–2.76) | 1 (ref.) | 1.22 (0.57–2.60) | 1.96 (0.92–4.19) | 0.43 (0.06–3.31) | 4.53 (1.47–13.96) | 1.25 (0.85–1.84) | 0.27 | 0.69 |
| 1945–1952 | 39,693 | 0.88 (0.44–1.76) | 0.99 (0.69–1.42) | 1 (ref.) | 0.84 (0.57–1.23) | 1.16 (0.77–1.75) | 1.13 (0.62–2.05) | 1.18 (0.51–2.73) | 1.12 (0.90–1.38) | 0.32 | |
| 1953 or after | 34,446 | 0.82 (0.44–1.51) | 1.23 (0.91–1.67) | 1 (ref.) | 1.07 (0.77–1.49) | 1.11 (0.76–1.62) | 0.85 (0.46–1.56) | 0.75 (0.30–1.86) | 0.92 (0.76–1.12) | 0.40 | |
| Breast cancer that developed after 50 years of age | |||||||||||
| 1944 or before | 38,518 | 0.85 (0.58–1.24) | 0.94 (0.74–1.20) | 1 (ref.) | 1.03 (0.81–1.31) | 1.26 (0.97–1.64) | 1.33 (0.93–1.90) | 1.18 (0.69–2.01) | 1.15 (1.02–1.30) | 0.03 | 0.98 |
| 1945–1952 | 41,962 | 1.02 (0.65–1.60) | 0.81 (0.63–1.05) | 1 (ref.) | 1.10 (0.88–1.36) | 1.11 (0.88–1.41) | 1.09 (0.79–1.51) | 1.05 (0.67–1.64) | 1.10 (0.97–1.24) | 0.13 | |
| 1953 or after | 30,060 | 0.74 (0.40–1.38) | 0.92 (0.68–1.23) | 1 (ref.) | 0.94 (0.70–1.24) | 1.03 (0.75–1.41) | 0.99 (0.63–1.57) | 0.98 (0.49–1.93) | 1.08 (0.91–1.28) | 0.39 | |
| Postmenopausal women at baseline | |||||||||||
| 1928 or earlier | 68,886 | 0.66 (0.48–0.92) | 0.68 (0.53–0.87) | 1 (ref.) | 1.17 (0.94–1.46) | 1.23 (0.97–1.55) | 1.69 (1.27–2.24) | 1.81(1.24-.62) | 1.44(1.31–1.58) | < 0.001 | 0.24 |
| 1929–1936 | 71,600 | 0.99 (0.67–1.46) | 0.79 (0.62–1.01) | 1 (ref.) | 1.32 (1.10–1.59) | 1.26 (1.04–1.53) | 1.23 (0.96–1.56) | 1.45 (1.07- | 1.23 (1.12–1.35) | < 0.001 | |
| 1937 or later | 59,917 | 0.80 (0.51–1.27) | 1.17 (0.93–1.46) | 1 (ref.) | 1.31 (1.08–1.59) | 1.42 (1.16–1.73) | 1.55 (1.21–1.99) | 2.03 (1.52–2.72) | 1.30 (1.19–1.43) | < 0.001 | |
| Country | |||||||||||
| Premenopausal women at baseline | |||||||||||
| Breast cancer that developed before 50 years of age | |||||||||||
| Japan | 63,588 | 0.86 (0.48–1.55) | 1.10 (0.81–1.50) | 1 (ref.) | 0.97 (0.70–1.35) | 1.36 (0.95–1.94) | 1.08 (0.61–1.91) | 1.81 (0.94–3.49) | 1.17 (0.97–1.41) | 0.09 | 0.04 |
| Korea | 10,229 | 1.14 (0.46–2.80) | 1.43 (0.85–2.39) | 1 (ref.) | 1.04 (0.57–1.88) | 0.78 (0.36–1.67) | 0.21 (0.03–1.58) | 0.74 (0.17–3.13) | 0.63 (0.43–0.92) | 0.02 | |
| China | 32,976 | 0.67 (0.27–1.69) | 1.09 (0.73–1.63) | 1 (ref.) | 0.95 (0.63–1.42) | 1.16 (0.75–1.80) | 1.01 (0.53–1.94) | 0.67 (0.21–2.13) | 1.02 (0.80–1.29) | 0.88 | |
| Breast cancer that developed after 50 years of age | |||||||||||
| Japan | 67,282 | 0.96 (0.70–1.32) | 0.93 (0.76–1.12) | 1 (ref.) | 1.12 (0.92–1.35) | 1.19 (0.96–1.47) | 1.45 (1.10–1.92) | 1.19 (0.77–1.83) | 1.16 (1.05–1.28) | 0.005 | 0.82 |
| Korea | 6794 | 0.61 (0.08–4.60) | 0.63 (0.28–1.45) | 1 (ref.) | 0.92 (0.46–1.83) | 0.61 (0.25–1.47) | 0.45 (0.10–1.99) | 0.40 (0.05–3.05) | 0.86 (0.53–1.39) | 0.53 | |
| China | 36,464 | 0.77 (0.47–1.27) | 0.89 (0.70–1.13) | 1 (ref.) | 0.96 (0.77–1.19) | 1.13 (0.90–1.42) | 0.90 (0.64–1.26) | 1.04 (0.66–1.62) | 1.08 (0.95–1.22) | 0.25 | |
| Postmenopausal women at baseline | |||||||||||
| Japan | 141,822 | 0.74 (0.57–0.94) | 0.76 (0.64–0.90) | 1 (ref.) | 1.27 (1.11–1.45) | 1.22 (1.05–1.41) | 1.42 (1.18–1.71) | 1.45 (1.11–1.88) | 1.33 (1.24–1.42) | < 0.001 | 0.15 |
| Korea | 22,050 | 0.59 (0.14–2.45) | 1.43 (0.89–2.31) | 1 (ref.) | 1.30 (0.85–1.99) | 0.98 (0.60–1.59) | 1.10 (0.58–2.09) | 1.95 (0.96–3.94) | 1.05 (0.82–1.34) | 0.70 | |
| China | 36,531 | 1.11 (0.69–1.80) | 1.12 (0.83–1.51) | 1 (ref.) | 1.31 (1.03–1.67) | 1.60 (1.27–2.03) | 1.61 (1.23–2.12) | 2.17 (1.61–2.92) | 1.33 (1.21–1.47) | < 0.001 | |
HR hazard ratio, CI confident interval
Estimated hazard ratio after adjustments for age at enrollment (continuous), study (13 cohorts), smoking status (never, former, current smokers), alcohol consumption (non-drinkers or current drinkers), age at menarche (≤ 10 y, 11–12 y, 13–14 y, 15–16 y, ≥ 17 y), nulliparity (yes, no), age at first delivery (≤ 20 y, 21–25 y, 26–30 y, ≥ 31 y), and age at menopause (≤ 44 y, 45–49 y, 50–54 y, ≥ 55 y)(postmenopausal women only)
Discussion
This pooled analysis, including 13 ongoing prospective cohorts in East Asia, showed that BMI was positively associated with subsequent risk of breast cancer in both premenopausal and postmenopausal women.
A 2021 pooled analysis of 20 prospective studies, consisting mainly of Western studies and one Japanese study, reported a nonlinear positive association with a plateau in increasing postmenopausal breast cancer risk at a BMI > 30 kg/cm2 [28]. The present pooled analysis of Asians revealed a steady increase in breast cancer risk with increasing BMI in postmenopausal women, but the slope of the increase in risk appeared to decrease at a BMI of approximately 26–28 kg/m2. Although nonlinear, the overall risk increase of 32% per 5-kg/m2 increase in BMI was compatible with the results of previous meta-analyses in Asians [26, 27, 30]. As has been suggested, the magnitude of risk increase may be greater in Asian women than in Western women [27, 30]. One reason may be that Asian women are less likely to use hormone replacement therapy (HRT) than Western women are. It was reported that only approximately 3% of Asian women had received HRT [42], whereas approximately 30% of European women had [44]. The positive association between BMI and breast cancer in postmenopausal women has been reported to be stronger among never HRT users than ever HRT users [22, 23, 25, 27, 28].
The potential biological mechanism underlying this association may be related to estrogen. After estrogen production in the ovary decreases, adipose tissue becomes the primary source of estrogen in postmenopausal women [29]. The positive association between BMI and breast cancer risk in postmenopausal women has been shown to be more pronounced for estrogen receptor-positive (ER +) and progesterone receptor-positive (PR +) tumors [16, 24, 25, 28].
In contrast with the inverse association between BMI and breast cancer risk observed mainly in premenopausal Western women [34], this pooled analysis of premenopausal Asian women revealed a positive association, similar to the results of a previous pooled analysis in Japan (eight studies in our analyses overlapped with the pooled analysis) [26]. This positive association was attributed to an increase in the risk of breast cancer that developed after 50 years of age, suggesting that this may be due primarily to the effect of BMI carried over after menopause. The positive association with BMI was weaker compared with postmenopausal women. This may be because the postmenopausal period before breast cancer diagnosis was shorter in premenopausal women (average age at diagnosis: 60 years) than in postmenopausal women (average age at diagnosis: 70 years). The association between BMI and breast cancer risk has been reported to become stronger with increasing age [27, 45].
There was no significant association between BMI and breast cancer that developed before 50 years of age. The reason for the lack of an inverse association in Asian women is unclear. One possible explanation may be the lower prevalence of overweight or obese Asian women [46, 47], especially severely obese women, who are more prone to anovulation and result in lower exposure to estrogen [48, 49]. However, in Western women, the inverse association was observed even in the range of BMI less than 30 kg/cm2. The difference in risk between Western and Asian women with the same BMI may be due to BMI in young adulthood in the twenties or earlier. Lower early-adult BMI has been reported to be associated with an increased risk of breast cancer in Asian and Western women [17, 28, 33, 34, 50]. It is likely that the Asian women included in this pooled analysis had much lower early-adult BMI than Western women. Another possibility is that Asian women might have a lower incidence of ER + or PR + breast cancer [51], which has been implicated in the reduced risk associated with a higher BMI in premenopausal women [16, 25, 28, 33, 51]. Other factors might include race, ethnicity, and obesity-related lifestyles.
Moreover, the current pooled analysis suggested a positive association between BMI and breast cancer in premenopausal women among Japanese and an inverse association among Koreans. All the Japanese cohorts were population-based studies, whereas the Koreans included one hospital-based study. The Japanese cohorts had a baseline in the 1980s and the early 1990s, whereas the Korean cohorts included data of the 2000s or later. In addition, two larger-scale retrospective cohort studies in Japan and Korea, conducted in the 2000s to the 2010s with shorter follow-up periods and likely composed of health-conscious participants, suggested an inverse association [14, 35]. Furthermore, the current study revealed that a positive association between BMI and the risk of developing breast cancer after 50 years of age was more pronounced in the older birth cohort, and the association between BMI and the risk of developing breast cancer before 50 years of age changed from positive to inverse as the birth cohort became younger. Although we were unable to distinguish the effect by birth cohort or country, the association between BMI and breast cancer in premenopausal Asian women might be moving towards an inverse association like that in Western women, given that newer Asian generations show a greater prevalence of obesity starting in childhood with Westernization. Further investigations will be needed in the form of prospective Asian studies of these newer generations with longer follow-up periods.
The strengths of this study include the large number of participants from Japan, Korea, and China. Each study had a long follow-up period as well as information on several confounders. Because only cohort studies were included in the pooled analysis, the recall bias of exposure should be minimal. Unlike with meta-analyses of published studies, common approaches for exposures, outcomes, covariates, and statistical models were applied to the pooled data, and publication bias is considered limited because of the inclusion of studies that have not previously been published on the association. Although self-reported height and weight data were used in the Japanese cohort studies, the validity was high, and Japanese people are suggested to be more accurate in reporting their weight and height compared with other populations [52]. Although BMI at baseline might have changed due to preclinical signs, the exclusion of patients during the first three years of follow-up did not change the results.
Several limitations should be considered. None of the analyzed studies obtained information on menopausal status after the start of follow-up. Although 50 years of age was used as a proxy cutoff point, some misclassification might have occurred. However, sensitivity analyses using another cutoff point did not substantially change the results. Because information on exposures and confounders was obtained only at baseline, changes during follow-up were not considered. The lack of information on tumor subtypes, including ER, PR, and human epidermal growth factor receptor 2 (HER2), as well as experience with HRT, was also a limitation because these factors might modify the association between BMI and breast cancer risk. The possibility of residual confounding could not be fully ruled out even after accounting for several factors.
Conclusions
This pooled analysis of prospective studies in Japan, Korea, and China confirmed increased risks of breast cancer among postmenopausal women with higher BMIs. In premenopausal women, the association between BMI and the risk of breast cancer tended to be positive in the older birth cohorts and inverse in the younger cohorts. In Asia, the role of BMI in breast cancer in premenopausal women may be changing along with the increase in obesity and breast cancer. New-generation prospective Asian studies with longer follow-up periods warrant further investigation. Understanding changes in the association over time will help to elucidate the mechanism by which obesity contributes to the etiology of breast cancer.
Supplementary Information
Acknowledgements
We appreciate all participants of the Asia Cohort Consortium and the staff of the Coordinating Center.
Abbreviations
- BMI
Body mass index
- ACC
The Asia Cohort Consortium
- ICD-O
The International Classification of Diseases for Oncology
- ICD-10
The International Classification of Diseases and Health Related Problems, 10th Revision
- HR
Hazard ratio
- CI
Confidence interval
- HRT
Hormone replacement therapy
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
Author contributions
KW designed the research, analyzed and interpreted the data, drafted the original manuscript, and took responsibility for the integrity of the data and the accuracy of the data analysis; KK analyzed and interpreted the data and critically revised the draft; CN supervised the study, assisted in data interpretation, and critically revised the draft; NS, AT, XOS, RS, AH, SK, HI, YS, SKP, SK, AO, TK, WW, IO, and MHS provided data and critically reviewed the draft; SKA, MSR, MRI, and ES provided administrative, technical, or material support and critically reviewed the draft; AS, JK, JEL, KM, NR, YLQ, WZ, PB, and MI. supervised the study and critically reviewed the draft.
Funding
The Asia Cohort Consortium Coordinating Center, National Cancer Center Japan Research and Development Fund; Japan Public Health Center-Based Prospective Study (1 and 2), National Cancer Center Japan Research and Development Fund (since 2011) and a grant-in-aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010); the Japan Collaborative Cohort Study, National Cancer Center Japan Research and Development Fund, grants for health service and comprehensive research on cardiovascular and lifestyle related diseases from the Ministry of Health, Labour and Welfare, Japan, and a grant for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Life Span Study Cohort, Radiation Effects Research Foundation, the Japanese Ministry of Health, Labour and Welfare and the US Department of Energy; Miyagi Cohort Study, National Cancer Center Japan Research and Development Fund; Ohsaki National Health Insurance Cohort Study, National Cancer Center Japan Research and Development Fund; Three Prefecture Cohort Study Aichi, The Japanese Ministry of the Environment (former Environment Agency); Three Prefecture Cohort Study Miyagi, National Cancer Center Japan Research and Development Fund; Takayama Study, National Cancer Center Japan Research and Development Fund; Korea Multi-Center Cancer Cohort Study, the Frontier Functional Human Genome Project from the Ministry of Science and Technology of Korea, the National Research Foundation of Korea grant funded by the Korea government (MSIP), the Korean Foundation for Cancer Research, a research grant from the National Cancer Center, Korea, partly by grant from the Seoul National University Hospital Research Fund; Korea National Cancer Center Cohort, National Cancer Center Korea Research Grant; The Namwon Study, Chonnam National University Hwasun Hospital Research grant; The Shanghai Women’s Health Study, the US National Cancer Institute. The study funders had no role in the design or execution of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. Each participating study was approved by the relevant institutional ethical review boards. The protocol for the ACC analysis was approved by the institutional review board of the National Cancer Center, Japan. The requirement for consent from several cohort studies was waived due to the justification for lack of consent at the time of the survey, the difficulty in obtaining new consent, and the public necessity of the research.
Consent for publication
All authors reviewed and approved the final version for submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fitzmaurice C, Abate D, Abbasi N, et al. Global, Regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mubarik S, Cao J, Wang F, et al. Lifestyle and socioeconomic transition and health consequences of breast cancer in the East Asia Region, From 1990 to 2019. Front Nutr. 2022;9:817836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu N, Yang DW, Wu YX, et al. Burden, trends, and risk factors for breast cancer in China from 1990 to 2019 and its predictions until 2034: an up-to-date overview and comparison with those in Japan and South Korea. BMC Cancer. 2022;22(1):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005–systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23(5):1362–9. [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi M, Abe SK, Sawada N, et al. Burden of cancer attributable to excess bodyweight and physical inactivity in Japan in 2015. GHM Open. 2021;1(2):56–62. [Google Scholar]
- 6.Wang D, Zheng W, Wang SM, et al. Estimation of cancer incidence and mortality attributable to overweight, obesity, and physical inactivity in China. Nutr Cancer. 2012;64(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Kim Y, Shin HR, et al. Population-attributable causes of cancer in Korea: obesity and physical inactivity. PLoS ONE. 2014;9(4):e90871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol. 2008;26(20):3395–402. [DOI] [PubMed] [Google Scholar]
- 9.Chen MJ, Wu WY, Yen AM, et al. Body mass index and breast cancer: analysis of a nation-wide population-based prospective cohort study on 1 393 985 Taiwanese women. Int J Obes (Lond). 2016;40(3):524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KR, Hwang IC, Han KD, et al. Waist circumference and risk of breast cancer in Korean women: a nationwide cohort study. Int J Cancer. 2018;142(8):1554–9. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Jin G, Yu C, et al. Cancer incidence in relation to body fatness among 0.5 million men and women: Findings from the China Kadoorie Biobank. Int J Cancer. 2020;146(4):987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho PJ, Lau HSH, Ho WK, et al. Incidence of breast cancer attributable to breast density, modifiable and non-modifiable breast cancer risk factors in Singapore. Sci Rep. 2020;10(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran TXM, Kim S, Song H, et al. Mammographic breast density, body mass index and risk of breast cancer in Korean women aged 75 years and older. Int J Cancer. 2022;151(6):869–77. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Han K, Shin DW, et al. Obesity and breast cancer risk for pre- and postmenopausal women among over 6 million Korean women. Breast Cancer Res Treat. 2021;185(2):495–506. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama S, Tsubono Y, Hozawa A, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113(1):148–57. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki M, Otani T, Inoue M, et al. Body size and risk for breast cancer in relation to estrogen and progesterone receptor status in Japan. Ann Epidemiol. 2007;17(4):304–12. [DOI] [PubMed] [Google Scholar]
- 17.Kawai M, Minami Y, Kuriyama S, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer. 2010;103(9):1443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Kojima M, Tokudome S, et al. Obesity/weight gain and breast cancer risk: findings from the Japan collaborative cohort study for the evaluation of cancer risk. J Epidemiol. 2013;23(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki Y, Tsunoda H, Kimura T, et al. BMI change and abdominal circumference are risk factors for breast cancer, even in Asian women. Breast Cancer Res Treat. 2017;166(3):919–25. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Kitamura Y, Kitamura T, et al. Reproductive and lifestyle factors related to breast cancer among Japanese women: an observational cohort study. Medicine (Baltimore). 2019;98(51):e18315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tehard B, Lahmann PH, Riboli E, et al. Anthropometry, breast cancer and menopausal status: use of repeated measurements over 10 years of follow-up-results of the French E3N women’s cohort study. Int J Cancer. 2004;111(2):264–9. [DOI] [PubMed] [Google Scholar]
- 22.Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(3):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White KK, Park SY, Kolonel LN, et al. Body size and breast cancer risk: the Multiethnic Cohort. Int J Cancer. 2012;131(5):E705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet MM, Carter BD, Patel AV, et al. Waist circumference, body mass index, and postmenopausal breast cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Cancer Causes Control. 2014;25(6):737–45. [DOI] [PubMed] [Google Scholar]
- 25.Munsell MF, Sprague BL, Berry DA, et al. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada K, Nagata C, Tamakoshi A, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol. 2014;25(2):519–24. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Liu L, Zhou Q, et al. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: a dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. 2017;17(1):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Brandt PA, Ziegler RG, Wang M, et al. Body size and weight change over adulthood and risk of breast cancer by menopausal and hormone receptor status: a pooled analysis of 20 prospective cohort studies. Eur J Epidemiol. 2021;36(1):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 31.Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–78. [DOI] [PubMed] [Google Scholar]
- 32.Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1121–7. [PubMed] [Google Scholar]
- 33.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21):2395–402. [DOI] [PubMed] [Google Scholar]
- 34.Schoemaker MJ, Nichols HB, Wright LB, et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018;4(11):e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konishi T, Fujiogi M, Michihata N, et al. Association between body mass index and incidence of breast cancer in premenopausal women: a Japanese nationwide database study. Breast Cancer Res Treat. 2022;194(2):315–25. [DOI] [PubMed] [Google Scholar]
- 36.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song M, Rolland B, Potter JD, et al. Asia Cohort Consortium: challenges for collaborative research. J Epidemiol. 2012;22(4):287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue M, Sobue T, Tsugane S. Impact of body mass index on the risk of total cancer incidence and mortality among middle-aged Japanese: data from a large-scale population-based cohort study–the JPHC study. Cancer Causes Control. 2004;15(7):671–80. [DOI] [PubMed] [Google Scholar]
- 39.Kuriyama S, Ohmori K, Miura C, et al. Body mass index and mortality in Japan: the Miyagi Cohort Study. J Epidemiol. 2004;14(Suppl 1):S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu N, Nagata C, Shimizu H, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. Br J Cancer. 2003;88(7):1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363(9403):157–63. [DOI] [PubMed]
- 42.Katagiri R, Iwasaki M, Abe SK, et al. Reproductive Factors and Endometrial Cancer Risk Among Women. JAMA Netw Open. 2023;6(9): e2332296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 44.Ellingjord-Dale M, Christakoudi S, Weiderpass E, et al. Long-term weight change and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Epidemiol. 2022;50(6):1914–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirose K, Tajima K, Hamajima N, et al. Effect of body size on breast-cancer risk among Japanese women. Int J Cancer. 1999;80(3):349–55. [DOI] [PubMed] [Google Scholar]
- 46.Yoshiike N, Seino F, Tajima S, et al. Twenty-year changes in the prevalence of overweight in Japanese adults: the National Nutrition Survey 1976–95. Obes Rev. 2002;3(3):183–90. [DOI] [PubMed] [Google Scholar]
- 47.Funatogawa I, Funatogawa T, Nakao M, et al. Changes in body mass index by birth cohort in Japanese adults: results from the National Nutrition Survey of Japan 1956–2005. Int J Epidemiol. 2009;38(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potischman N, Swanson CA, Siiteri P, et al. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1996;88(11):756–8. [DOI] [PubMed] [Google Scholar]
- 49.Tworoger SS, Eliassen AH, Missmer SA, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2494–501. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki R, Iwasaki M, Inoue M, et al. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status–the Japan public health center-based prospective study. Int J Cancer. 2011;129(5):1214–24. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki R, Orsini N, Saji S, et al. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124(3):698–712. [DOI] [PubMed] [Google Scholar]
- 52.Wada K, Tamakoshi K, Tsunekawa T, et al. Validity of self-reported height and weight in a Japanese workplace population. Int J Obes (Lond). 2005;29(9):1093–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


