Abstract
Background
Tuberculosis is a highly prevalent disease in India, while Histoplasmosis, an emerging disease, is often underreported due to limited resources in developing countries. Coinfection with both these organisms is rarely documented in immunocompetent host. Due to overlapping symptoms, it can be easily missed and treatment delays are not uncommon.
Case Presentation
Here, we report a case of a 62-year-old male with a chronic history of intermittent fever and dry cough, splenomegaly, lymphadenopathy, and persistent pancytopenia. He was diagnosed with tuberculosis with cartridge-based nucleic acid amplification test (CBNAAT) positivity from a paratracheal lymph node biopsy. Simultaneously, a bone marrow biopsy revealed Histoplasmosis and the patient was started on dual treatment (Itraconazole and antitubercular drugs). After an initial response, the patient developed new space-occupying cerebral lesions. CSF histoplasma antigen was also positive. The reason for treatment failure was likely to be drug interaction (suboptimal levels of itraconazole due to rifampicin). The patient received liposomal amphotericin and subsequently put on a modified antitubercular treatment regimen to avoid interaction with itraconazole. At 2-month follow-up, the patient’s condition significantly improved with a substantial resolution in CNS lesions.
Conclusions
Histoplasmosis and tuberculosis have overlapping symptoms, diagnosing one does not preclude the possibility of other, even in non-HIV patients. Clinicians should also be vigilant about potential drug interactions.
Keywords: Tuberculosis, CNS histoplasmosis, Coinfection, Immunocompetent, Brain abscess
Introduction
Tuberculosis is a widespread disease in India with a myriad of presentations. Global estimates indicate 10.6 million cases in 2022, with more than two-thirds of these cases found in Southeast Asian countries. India accounts for 27% of these cases, followed by Indonesia at 10% [1]. Diagnostically proven tuberculosis cases are seldom explored for the coexistence of other infections, especially in an immunocompetent host [2]. Histoplasmosis is an emerging systemic fungal infection that primarily affects immunocompromised patients. Due to a similarity in symptoms, patients with histoplasmosis are often initially diagnosed and treated for tuberculosis [3], which can lead to treatment failure. The incidence of Histoplasmosis in India is increasing, possibly due to new highly sensitive assays for diagnosis. Common symptoms include fever, weight loss, night sweats, fatigue, oropharyngeal ulcers, and skin lesions present in one-third of patients, whereas hepatosplenomegaly, granulomatous lesions, lymphadenopathy, and adrenal involvement are most commonly observed in immunocompromised patients [4]. This report aims to highlight a case of concurrent central nervous system histoplasmosis and tuberculosis and discuss the various challenges in the diagnosis and management of such co-infections.
Case presentation
A 68-year-old male, from the Western Rajasthan, presented to the Department of Medicine with complaints of fever and dry cough for 12 months. His comorbities were diabetes, and hypertension. His diabetes was well controlled in past few years (last HbA1c: 7.3%, 2 months before admission). He also had a history of bilateral Meniere’s disease and smoking from last 25 years. The patient also mentioned a strong interest in frequent gardening, feeding pigeons, and recent pilgrimage in the Gangetic plains, including Nepal. Before the current visit, he had multiple hospital admissions with similar complaints and was evaluated extensively. Aetiology was not identified, but the patient had acute kidney injury (AKI) with a serum creatinine of 1.87 mg/dl, normal 0.7–1.3), persistent pancytopenia (Hb: 9.7 g/dL, normal 14-16.5; total leukocyte count: 3700/uL, normal 4000–11000; platelet count: 1,02,000/uL, normal 1,50,000–3,50,000), and splenomegaly. He was managed conservatively with multiple antibiotics. Infective workup in last admission (1 month before the current admission) because of persistent fever, was negative for dengue (negative NS1 Ag and IgM), malaria (negative peripheral blood film and rapid antigen test), leptospirosis (negative Leptospira IgM), scrub typhus (negative IgM), brucellosis (negative standard agglutination test and culture), and leishmaniasis (negative recombinant K39 antigen). Blood and urine cultures were sterile. Autoimmune workup was negative. 2D echocardiogram did not reveal any vegetation. He remained asymptomatic for one month; then, he was readmitted with complaints of high-grade fever, dry cough, and altered behaviour. On evaluation, CEMRI Brain and cerebrospinal fluid (CSF) analysis were unremarkable (cells: 3, protein: 27 mg/dl, glucose: 65 mg/dl, negative culture for tuberculosis and fungi). He was found to have hypercalcemia (PTH independent) with high angiotensin-convertase enzyme (ACE) level (129 mcg/l, normal < 65), hyperferritinemia (1368 ng/ml, normal 24–336) and mediastinal lymphadenopathy (pre tracheal, para tracheal, bilateral hilar 1*1 cm in CT thorax). He was diagnosed with sarcoidosis and started on prednisolone (1 mg/kg). After five days of asymptomatic period, he again developed high-grade fever and dry cough. On examination, he was febrile (103 °F), tachypneic (RR 24/min) and tachycardia was also present (PR-129/min). Blood investigations revealed pancytopenia (Hb: 8.4 g/dL; total leukocyte count: 2300/uL; platelet count: 92,000/uL), renal dysfunction (creatinine: 1.9 mg/dl, normal 0.7–1.3), hyperferritinemia (ferritin: 2100 ng/ml, normal 30–400), and low reticulocyte counts (0.2%, normal 0.5–2.5). His HIV status was negative. Immunocompromised status was highly suspected, so a CD4 count for idiopathic CD4 lymphocytopenia was sent, which was within normal limits (679 cells/mm3).
CECT thorax showed right necrotizing pneumonia with cavitation and mediastinal lymphadenopathy (1.3 cm in subcarinal region, Fig. 1a), for which BAL (bronchoalveolar lavage) followed by TBLB (transbronchial lung biopsy) and EBUS-TBNA (Endobronchial Ultrasound-guided Transbronchial Needle Aspiration) was performed. CT abdomen revealed mild hepatosplenomegaly with a small hypodense lesion in segment V of the liver, likely a calcified granuloma, and no involvement of mesenteric, periportal, or paraaortic lymph nodes or adrenals. Due to persistent pancytopenia, bone marrow aspiration and biopsy were performed. FNA of the mediastinal lymph node revealed positive CBNAAT (Rifampicin resistance not detected). The patient was put on a drug-sensitive ATT regimen (HRZE) on day 7 of admission. Bone marrow aspiration and biopsy showed the presence of numerous yeast-like inclusions suggestive of Histoplasmosis (Fig. 2a and b). Urine Histoplasma antigen was also elevated (31 ng/ml, normal < 1). On day 8, The patient was started on Liposomal Amphotericin B (3 mg/kg) for 14 days, followed by itraconazole (200 mg PO BD).
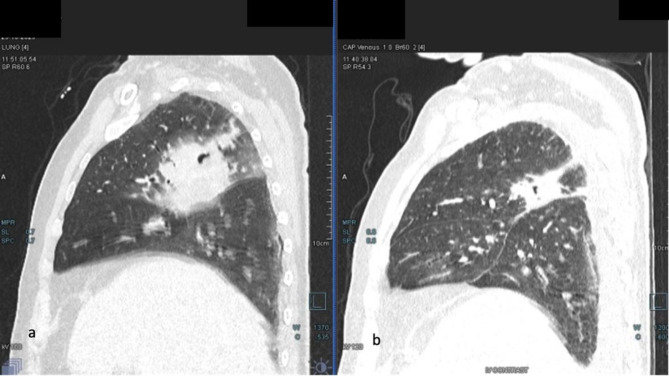
Fig. 1.
a CT thorax showing an ill-defined patch of consolidation seen in posterior segment of right upper lobe with areas of necrosis and cavitation within. Few adjacent patchy areas of consolidation with surrounding GGOs (ground glass opacities) are seen in the right upper lobe, b Significant reduction of the previous cavitary lesion with surrounding fibrosis in right upper lobe of lung
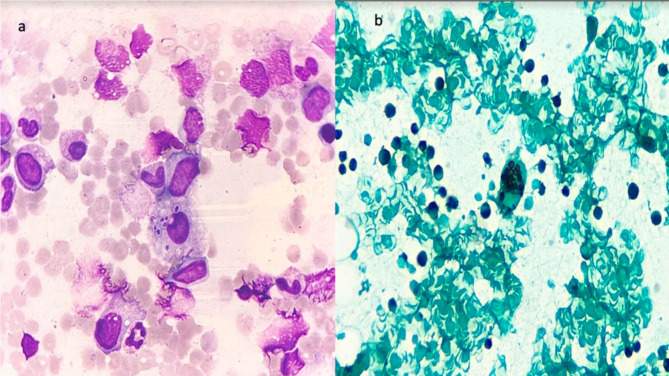
Fig. 2.
a Giemsa stained bone marrow aspirate smear showing a histiocyte with numerous intracellular yeast like organisms; morphologically suggestive of Histoplasma spp.b: Gomori Methanamine silver stained bone marrow aspirate smear showing a histiocyte with numerous intracellular yeast like organisms; morphologically suggestive of Histoplasma spp
After the initiation of dual therapy, the patient became afebrile. However, he experienced a recurrence of fever along with episodes of irrelevant speech and abnormal mental status on day 30. Possibilities of therapy failure (Itraconazole interaction with rifampicin) and TB paradoxical IRIS (immune reconstitution inflammatory syndrome) were considered. A repeat CT chest showed a significant resolution in pneumonia and mediastinal lymphadenopathy after antitubercular therapy (ATT), (Fig. 1b), suggesting an adequate tubercular response. Other causes of altered sensorium were ruled out (normal sodium, calcium and blood glucose levels). His blood cultures were sterile, and the procalcitonin level was within normal range. A CEMRI Brain revealed multiple ring-enhancing lesions with perilesional edema in the left parieto-occipital lobe (Fig. 3a and b). The repeat lumber puncture was unremarkable except a mild increase in CSF protein (78 mg/dl). MRS (Spectroscopy) revealed an absent lipid peak. Additionally, CSF CBNAAT and CSF galactomannan tests were negative. Therapeutic drug monitoring showed low levels of itraconazole (0.34 mg/L, target trough range 0.5-3). We suspected CNS histoplasmosis due to inadequate treatment, further consolidated by a repeat positive urine Histoplasma antigen (29 ng/ml) and CSF histoplasma antigen test. On day 35, Amphotericin was restarted with a higher dose (5 mg/kg) for an additional 14 days with continuation of ATT. Neurosurgery consultation was requested, and it was determined that the brain abscesses were inoperable due to their small size. The patient’s fever resolved after one week of Amphotericin B initiation, and follow-up imaging at day 42 showed a decrease in the abscess size (Fig. 3c). On day 49, The patient was discharged on itraconazole and a modified ATT regimen (Levofloxacin, isoniazid, ethambutol, pyrazinamide) and scheduled for follow-up at the Infectious Diseases OPD. After three months of follow-up, the patient remains afebrile and has no recurrence of previous symptoms.
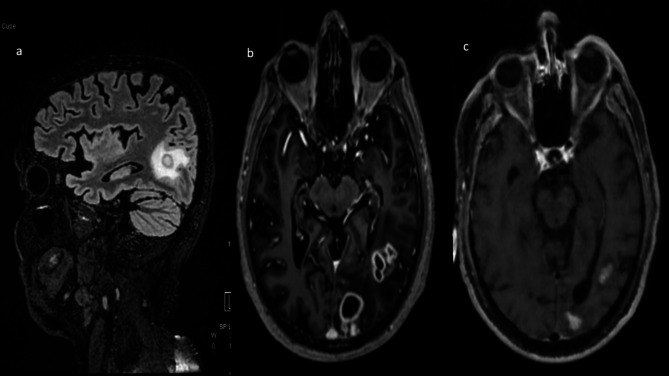
Fig. 3.
MRI Brain - T1 Post-contrast axial (a) and sagittal (b) sections showing few conglomerated thick-walled peripherally enhancing lesions with central diffusion restriction in left temporo-occipital region, Fig. c: T1 Post contrast axial section showing few small residual enhancing lesions with central diffusion restriction in left temporo-occipital region, decreased in size compared to previous study
Discussion
Histoplasmosis is a systemic fungal disease caused by the dimorphic fungus Histoplasma capsulatum. It is acquired through inhalation of microconidia or hyphae in soil contaminated with bird and bat droppings [5, 6]. Historically, histoplasmosis was known to be endemic in the North America, (Ohio and Mississippi river valleys), and the South American countries like Brazil [7]. However, cases have been reported from every continent, with the majority of cases coming from the Africa and Asia [5, 8]. The incidence of histoplasmosis in humid tropics ranges from 10 to 100 cases per 100,000, with over 100 cases per 100,000 in high-risk groups and during outbreaks [9]. In the Indian subcontinent, only 426 cases of histoplasmosis were reported from 1954 to 2018. In contrast, 207 cases were reported from 2018 to 2020. Most cases are found in the regions along the Ganga, Yamuna, and Brahmaputra River basins.
Histoplasmosis can manifest as a wide range of systemic manifestations affecting various organs such as the lungs, lymph nodes, adrenal glands, bone marrow, central nervous system, liver, and spleen. The severity and form of the disease depend on the patient’s immune status. While primary infection can be asymptomatic and self-limiting, it can also progress to disseminated disease. Disseminated histoplasmosis is particularly common in individuals with immunodeficiency, such as those with HIV, but cases have been reported in immunocompetent individuals as well. It is important to note that distinguishing between histoplasmosis and tuberculosis can be challenging, especially in regions where tuberculosis is endemic. Both conditions are associated with defective cell-mediated immunity and present similar clinical features such as prolonged fever, lymphadenopathy, pancytopenia, and granulomatous disseminated disease. In areas like India, where tuberculosis is prevalent and the incidence of Histoplasmosis is rising among immunocompromised individuals, clinical differentiation becomes even more complex. Recent studies have shown that up to 50% of histoplasmosis cases occur in people living with HIV, with a co-occurrence of histoplasmosis and tuberculosis ranging from 2 to 35% in the Latin American countries [10]. For example, a study in Panama found that 15.4% of histoplasmosis cases in AIDS patients were coinfected with tuberculosis [11]. In comparison, a retrospective study in French Guyana reported a coinfection frequency of 8% in people living with HIV [12]. These findings highlight the importance of differentiating between these conditions, especially in immunocompromised individuals.
Adenis et al. discussed whether it is possible to differentiate between histoplasmosis and TB in HIV-infected patients by examining epidemiological, clinical, biological, and radiological characteristics. They concluded that symptoms related to the lungs and chest were more common in individuals with TB; while abnormal imaging in the abdominal area was more frequent in those with histoplasmosis [13]. There is scanty literature on the simultaneous occurrence of histoplasmosis and tuberculosis in immunocompetent individuals [14]. In the present case, the patient tested negative for HIV, had not undergone any immunosuppressive therapy, and had not experienced any significant illness or frequent recurrent infections since childhood until last year. Further tests for idiopathic CD4 lymphocytopenia and immunoglobulin deficiency also came back negative. The use of steroids for only five days did not indicate an immunocompromised state. Although, patient was diabetic which itself is an immunodeficient state and can predisposes to tuberculosis and other opportunistic infections. It is unclear whether tuberculosis was the initial infection or if histoplasmosis led to other infections.
This case of CNS Histoplasmosis and TB coinfection posed a dual challenge for diagnosis and treatment due to overlapping symptoms and drug interactions. Rifampicin induces itraconazole metabolism through CYP3A4 activation, resulting in decreased therapeutic concentrations as seen in our case. Therefore, therapeutic strategies must be continuously adjusted based on the patient’s clinical assessment. The poor understanding of histoplasmosis prevalence and the lack of disease awareness among clinicians directly contribute to clinical confusion between histoplasmosis and tuberculosis [3]. Patients with histoplasmosis are often initially misdiagnosed and treated for TB, leading to treatment failure. The main limitation of this study is its lack of generalizability, as it is based on a single case report. We emphasize the need for larger, more comprehensive studies in non-HIV patients with tuberculosis to better determine the incidence of histoplasmosis co-infection and to gain a deeper understanding of the associated clinical outcomes.
Conclusion
This case highlights the importance of considering TB-Histoplasmosis co-infection even in HIV-negative individuals. The overlapping clinical manifestations of these infections can complicate diagnosis; therefore, tuberculosis should not be the sole focus in such cases. A comprehensive diagnostic approach, including the possibility of co-infections, is crucial for achieving favorable outcomes. Additionally, careful monitoring of drug interactions between antitubercular drugs and azoles is essential for optimizing treatment efficacy and minimizing adverse effects.
Acknowledgements
None.
Abbreviations
- CBNAAT
Cartridge-based nucleic acid amplification test
- AKI
Acute kidney injury
- CSF
Cerebrospinal fluid
- ACE
Angiotensin-convertase Convertase
- BAL
Bronchoalveolar lavage
- TBLB
Transbronchial lung biopsy
- EBUS-TBNA
Endobronchial Ultrasound-guided Transbronchial Needle Aspiration
- IRIS
Immune reconstitution inflammatory syndrome
Author contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by BK, DA and DSM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study did not require ethical approval.
Consent for publication
Written informed consent was obtained from the patient included in this report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Global Tuberculosis Report. https://www.who.int/tb/publications/global_report/en/. 2022.
- 2.Yan H, Guo L, Pang Y, Liu F, Liu T, Gao M. Clinical characteristics and predictive model of pulmonary tuberculosis patients with pulmonary fungal coinfection. BMC Pulm Med. 2023;23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekeng BE, Oladele RO, Emanghe UE, Ochang EA, Mirabeau TY. Prevalence of histoplasmosis and molecular characterization of Histoplasma species in patients with presumptive pulmonary tuberculosis in Calabar, Nigeria. Open Forum Infect Dis. 2022;9:ofac368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Abraham OC, Rupali P, Zachariah A, Mathews MS, Mathai D. Disseminated histoplasmosis. J Assoc Physicians India. 2005;53:185–9. [PubMed] [Google Scholar]
- 5.Staffolani S, Buonfrate D, Angheben A, Gobbi F, Giorli G, Guerriero M, et al. Acute histoplasmosis in immunocompetent travelers: a systematic review of literature. BMC Infect Dis. 2018;18:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida MA, Almeida-Silva F, Guimarães AJ, Almeida-Paes R, Zancopé-Oliveira RM. The occurrence of histoplasmosis in Brazil: a systematic review. Int J Infect Dis. 2019;86:147–56. [DOI] [PubMed] [Google Scholar]
- 7.Guerra BT, Almeida-Silva F, Almeida-Paes R, Basso RP, Bernardes JPRA, Almeida MA, et al. Histoplasmosis outbreaks in Brazil: lessons to learn about preventing exposure. Mycopathologia. 2020;185:881–92. [DOI] [PubMed] [Google Scholar]
- 8.Oladele RO, Osaigbovo II, Akanmu AS, Adekanmbi OA, Ekeng BE, Mohammed Y, et al. Prevalence of histoplasmosis among persons with Advanced HIV Disease, Nigeria. Emerg Infect Dis. 2022;28:2261–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues AM, Beale MA, Hagen F, Fisher MC, Terra PPD, de Hoog S, et al. The global epidemiology of emerging Histoplasma species in recent years. Stud Mycol. 2020;97:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cáceres DH, Gómez BL, Tobón ÁM, Restrepo Á, Chiller T, Lindsley MD, et al. Tackling histoplasmosis infection in People Living with HIV from Latin America: From Diagnostic Strategy to Public Health Solutions. J Fungi (Basel). 2023;9:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez ME, Canton A, Sosa N, Puga E, Talavera L. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clin Infect Dis. 2005;40:1199–202. [DOI] [PubMed] [Google Scholar]
- 12.Huber F, Nacher M, Aznar C, Pierre-Demar M, El Guedj M, Vaz T, et al. AIDS-related Histoplasma capsulatum var. Capsulatum infection: 25 years experience of French Guiana. AIDS. 2008;22:1047–53. [DOI] [PubMed] [Google Scholar]
- 13.Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: a comparative study. Am J Trop Med Hyg. 2014;90:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhury AK, Mishra AK, Gautam DK, Tilak R, Tilak V, Gambhir IS, et al. Case Report: Histoplasmosis Accompanying disseminated tuberculosis in an Immunocompetent adolescent boy. Am J Trop Med Hyg. 2020;102:352–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.