Abstract
Background
Pulmonary cryptococcosis is a fungal infection of the lungs, particularly challenging to treat in patients with multiple comorbidities such as obesity, type 2 diabetes, and cirrhosis. Fluconazole is a first-line medication for the treatment of pulmonary cryptococcosis, but currently there is a lack of clinical medication experience in obese patients with multiple comorbidities, especially in dose adjustment after treatment failure.
Case Introduction
This case report describes the experience of fluconazole in the treatment of pulmonary cryptococcal infection in a 45-year-old Chinese male with obesity, type 2 diabetes, and cirrhosis. The patient had a history of antifungal therapy for two weeks before admission, but the cough and hemoptysis were not improved. The treatment failed. After admission, it was recommended to use a conventional dose of fluconazole as an antifungal regimen according to the guidelines. However, the treatment effect was still unsatisfactory, due to the patients’ cough, hemoptysis, and fever symptoms were not relieved. During this period, it was newly found that the patient had cirrhosis and type 2 diabetes and had not previously controlled blood glucose. Considering the above situation, combined with the pharmacokinetic characteristics of fluconazole and the patient’s weight reaching 113 kg, the team readjusted the fluconazole medication regimen, and ultimately, the pulmonary infection improved without significant adverse reactions.
Results
We found that it was more suitable for patients with obesity to calculate the dose of fluconazole by the lean weight. By estimation, the patient was finally given a loading dose of 800 mg fluconazole, and his condition improved significantly. After two weeks of medication, it was adjusted to a maintenance dose of 600 mg until the pulmonary infection in the patient disappeared.
Conclusion
This case suggests that fluconazole antifungal therapy for pulmonary cryptococcal infection should fully consider the risk of comorbidities in patients. If necessary, medication dosage can be adjusted according to weight, and it is recommended to use lean bodyweight for evaluation and optimization. In addition, close attention should be paid to liver and kidney function.
Keywords: pulmonary cryptococcosis, fluconazole, antifungal therapy, dose optimization, obese, lean bodyweight
Background
Pulmonary cryptococcosis is a fungal infection of the lungs, particularly challenging to treat in patients with multiple comorbidities such as obesity, type 2 diabetes, and cirrhosis, which is mainly caused by Cryptococcus neoformans or C.gattii, and may involve the central nervous system (CNS)1. In the past, immunosuppressed people were the high-risk group of cryptococcal infection, such as HIV-infected people. However, it is worth noting that in recent years, there have been more and more reports of cryptococcal infection in HIV-negative individuals2–5. At present, pulmonary cryptococcosis is the third most common pulmonary fungal infection in China6–8, and its treatment strategy mainly stems from clinical trial inference, case observation, case reports, and expert opinions.9
Fluconazole is a triazole antifungal agent commonly used in critically ill patients10, and is usually recommended for the treatment of simple pulmonary cryptococcosis.11 It has good pharmacokinetics and safety in healthy people and non-critical patients, but this is influenced by the patient’s disease status and obesity level.12,13 Due to the fact that monitoring the blood concentration of fluconazole is not a clinical necessity, there is limited research data on fluconazole use in patients with obesity, while pharmacokinetic data in cryptococcal patients is almost non-existent.14,15
We report a case of a Chinese male patient with pulmonary cryptococcosis with normal immunity, who was morbidly obese with type 2 diabetes and cirrhosis. The initial effect of fluconazole treatment in this case was poor. This article provides a detailed description of the optimization and considerations of the drug treatment plan, which has reference significance for choosing fluconazole treatment for pulmonary cryptococcosis in obese patients.
Case Presentation
Case Information
The patient was a 45-year-old Chinese male, height 180 cm, weighing 113 kg, and having a body mass index (BMI) of 34.88 kg/m2. He was admitted due to “intermittent cough for 6 months and blood in the sputum for 5 months”. Half a month before admission, he received a percutaneous CT-guided lung biopsy in other hospitals. The pathological results showed fungal granulomatous inflammation, and the acid-fast staining test was negative, and he was suspected of C. neoformans infection. He received symptomatic treatment such as anti-infection, hemostasis, and liver protection, but the effect was not satisfactory. He still had cough and blood in sputum, about 7–8 times a day. The patient has a history of fatty liver disease and gallstones. At admission, the body temperature was 36.8 °C, heart rate 92 beats/min, breathing 20 beats/min, and blood pressure 113/65 mmHg. Clear mind, average mental state, rough breathing sounds in both lungs, no obvious dry or wet rales heard, soft abdomen, no tenderness or rebound pain, liver, spleen, subcostal not reached, no percussion pain in both renal areas, and no edema in both lower limbs. The laboratory inspection has been improved, and the results are shown in Table 1. The results of routine blood tests showed that the white blood cell count (WBC) was 6.24×109/L, the percentage of neutrophils (Neut%) was 69.9%, the lymphocyte (Lym) was 1.39×109/L, the red blood cell (RBC) was 4.02×1012/L, and the hemoglobin (HGB) was 110.0 g/L. C-reactive protein (CRP) was 10.4 mg/L, and erythrocyte sedimentation rate (ESR) was 53 mm/h; Glycosylated hemoglobin (HbA1c) was 6.1%, and blood glucose (Glu) was 13.8 mmol/L; The results of liver and kidney function showed that alanine aminotransferase (ALT) was 22 U/L, aspartate aminotransferase (AST) was 42 U/L, globulin (GLO) was 48.9 g/L, albumin (ALB) was 33.6 g/L, total bilirubin (TBIL) was 24.8 μmol/L, and direct bilirubin (DBIL) was 13.2 μmol/L; There was no obvious abnormality in urine routine; Coagulation function showed that prothrombin time (PT) was 15.8 s, prothrombin activity (PTA) was 69.0%, international normalized ratio (INR) was 1.29, and D-D dimer was 0.34 µg/mL FEU; Serum (1,3)-β-D glucan test (G test) was 134.13 pg/mL; Galactomannan test (GM test) was 0.12; Normal oral microbiota in sputum culture; The pathological report of percutaneous lung puncture in the upper right lung of our hospital still shows chronic granulomatous inflammation, which is considered as cryptococcal infection.
Table 1.
The Laboratory Indexes of the Patient on Admission
| Index, Unit | Value | Index, Unit | Value |
|---|---|---|---|
| WBC, ×109/L | 6.24 | GLO, g/L | 48.9↑ |
| Neut, % | 69.9 | ALB, g/L | 33.6 |
| Lym, ×109/L | 1.39 | TBIL, μmol/L | 24.8 |
| RBC, ×1012/L | 4.02↓ | DBIL, μmol/L | 13.2 |
| HGB, g/L | 110.0↓ | PT, s | 15.8↑ |
| CRP, mg/L | 10.4↑ | PTA, % | 69.0↓ |
| ESR, mm/h | 53↑ | INR | 1.29↑ |
| HbA1c, % | 6.1↑ | D-D dimer, µg/mL FEU | 0.34 |
| Glu, mmol/L | 13.8↑ | G test, pg/mL | 134.13 |
| ALT, U/L | 22 | GM test | 0.12 |
| AST, U/L | 42↑ | / | / |
Notes: The normal reference range: White blood cell count (WBC): (3.50–9.50)×109/L; The percentage of Neutrophils (Neut%): 40.0%~75.0%; Lymphocyte (Lym): (1.10–3.20)×109/L; Red blood cell (RBC): (4.30–5.80)×1012/L; Hemoglobin (HGB): 130.0–175.0 g/L; C-reactive protein (CRP): >10 mg/L Suggest possible infection or inflammation; Erythrocyte sedimentation rate (ESR): (0.00–15.00) mm/h; Glycosylated hemoglobin (HbA1c): 4.0%-6.0%; Blood glucose (Glu): (4.4–6.1)mmol/L; Alanine aminotransferase (ALT): (0–41)U/L; Alanine aminotransferase (AST): (0–40)U/L; Globulin (GLO): (20–35) g/L; Albumin (ALB): (35–52)g/L; Total Bilirubin (TBIL): (0–26)μmol/L; Direct Bilirubin (DBIL): (0–8.0)μmol/L; Prothrombin time (PT): 11.5 s-14.5 s; Prothrombin activity (PTA): 75.0%-125.0%; International normalized ratio (INR): 0.80–1.20; D-D dimer: <0.5 µg/mL FEU; Serum (1,3)-β-D glucan test (G test): <70 pg/mL negative, 70–95 pg/mL uncertain, >95 pg/mL positive; Galactomannan test (GM test): <0.5 negative, ≥0.5 positive. ↑: Above the normal reference range. ↓: Below the normal reference range.
Treatment and Outcome
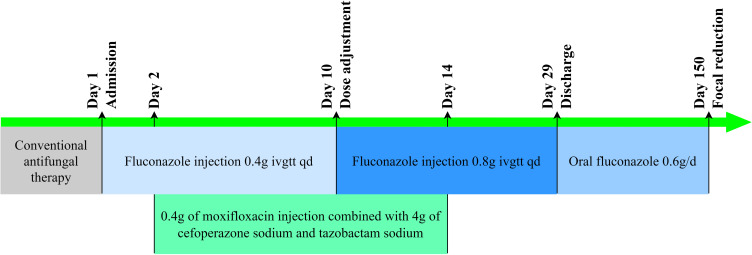
According to the preliminary diagnostic results, upon admission, fluconazole injection 0.4 g ivgtt qd was administered for antifungal treatment, as well as cough and hemostasis related treatments. The time line of antibacterial treatment is shown in Figure 1. At the same time, improve relevant examinations such as bronchoscopy, chest CT, sputum culture, GM test, G-test, acid fast staining, Tuberculous infection of T cell spot test (T-SPOT), oral glucose tolerance test (OGTT), abdominal ultrasound, etc.
Figure 1.
The timeline of the treatment.
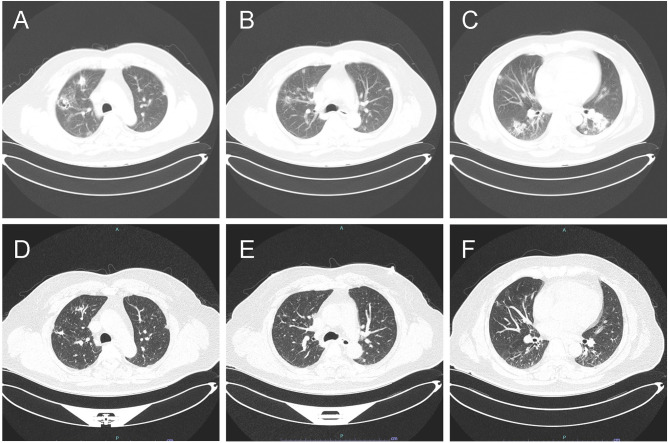
On the 2nd to 14th day after admission, 0.4 g qd of moxifloxacin injection combined with 4 g bid of cefoperazone sodium and tazobactam sodium for injection was added to cover bacterial infection. The patient still presents low fever in the afternoon, cough, expectoration and hemoptysis. Bronchoscopy showed possible bronchopulmonary hemorrhage, with localized protrusion of the mucosa on the posterior wall of the left main bronchus. Chest CT has suggested bilateral lung infection, considering fungal infection. The related images are shown in Figure 2A–C. Cerebrospinal fluid (routine, biochemical, culture) and related immune diseases all showed no abnormalities; Both glycosylated hemoglobin and OGTT supported the diagnosis of type 2 diabetes. Abdominal B-ultrasound showed the thickening of liver parenchyma light spots, multiple gallstones in the gallbladder, and splenomegaly.
Figure 2.
A computed tomography (CT) scan revealed multiple pulmonary nodules, ground-glass opacities in both lungs, and cavity in the right upper lung when the patient just to be hospitalized (A–C); A computed tomography (CT) scan conducted 4 months after discharged from hospital revealed multiple pulmonary nodules, cavity had diminished, and ground-glass opacities had decreased a lot in both lungs (D–F).
On the 9th day, the patient’s symptoms were not relieved, and C. neoformans was detected in sputum and lavage fluid culture of fiberoptic bronchoscope. Drug sensitivity indicated that amphotericin B, 5- fluorocytosine, fluconazole (MIC=2), itraconazole, and voriconazole were all sensitive, cerebrospinal fluid was stained with ink (-), and C. infection was diagnosed.
On the 10th day after admission, the patient’s symptoms were still not significantly relieved. A follow-up chest CT scan showed no significant absorption of infection foci in both lungs, indicating poor anti-infection control. After consultation with clinical pharmacists, combined with the patient’s medical history and related examination results, considering the patient’s high body mass index, the dosage of fluconazole was adjusted to 800 mg ivgtt qd, and the liver and kidney function and adverse drug reactions of the patient were closely monitored. The changes in liver and kidney function indexes are shown in Table 2. Three days after the dosage was adjusted, the patient stopped hemoptysis. Five days later, on the 14th day of admission, the patient’s low fever frequency decreased in the afternoon, and cough and sputum were significantly relieved. There were no obvious abnormalities in the blood routine examination, indicating a low possibility of bacterial infection. Moxifloxacin, and cefoperazone sodium and tazobactam sodium were gradually discontinued. Re-examination of CT liver portal vein imaging showed cirrhosis, splenomegaly, portal hypertension, gastric varicose veins, and open umbilical vein; Gallstones and edema of the gallbladder wall. Considering the liver function was in the normal range, and the chest CT had not shown any improvement, the dose of fluconazole did not adjust immediately. On the 29th day after admission, the patient’s symptoms improved and he was discharged and continued to receive fluconazole 600 mg/d as a maintenance dose.
Table 2.
Changes of Liver and Kidney Function Indexes Before and After Fluconazole Dose Adjustment
| Index, Unit | Timeline of Treatment, Day (Dose, mg) | ||||
|---|---|---|---|---|---|
| 1 (400) | 10 (800) | 16 (800) | 22 (800) | 46 (600) | |
| ALT, U/L | 22 | 34 | 31 | 31 | 29 |
| AST, U/L | 42↑ | 57↑ | 47↑ | 51↑ | 57↑ |
| ALB, g/L | 33.6↓ | 34.9↓ | 32.6↓ | 33.5↓ | 37.4 |
| TBIL, μmol/L | 24.8 | 14.6 | 14.8 | 13.2 | 18.4 |
| DBIL, μmol/L | 13.2↑ | 8.3↑ | 7.5 | 7.3 | 9↑ |
| ALP, U/L | 66 | 62 | 61 | 57 | 59 |
| GGT, U/L | 102↑ | 134↑ | 145↑ | 144↑ | 114↑ |
| LDH, U/L | 164 | 135 | 143 | 165 | 175 |
| Cr, μmol/L | 53↓ | 53↓ | 53↓ | 47↓ | 48↓ |
| eGFR, mL/min/1.73 m2 | 121.5 | 121.5 | 121.5 | 127.6 | / |
Notes: The normal reference range: Alanine aminotransferase (ALT): (0–41) U/L; Alanine aminotransferase (AST): (0–40) U/L; Albumin (ALB): (35–52) g/L; Total Bilirubin (TBIL): (0–26) μmol/L; Direct Bilirubin (DBIL): (0–8.0) μmol/L; Alkaline phosphatase (ALP): (40–130) U/L; Gamma-glutamyl transferase (GGT): (10–71) U/L; Lactate dehydrogenase (LDH): (135–225) U/L; Creatinine (Cr): (59–104) μmol/L; Estimated Glomerular Filtration Rate (eGFR): >90 mL/min/1.73 m2. ↑: Above the normal reference range. ↓: Below the normal reference range.
Four months after discharge, a follow-up chest CT scan at the outpatient department showed a reduction in solid infectious lesions (Figure 2D–F), with no significant abnormalities in blood routine and liver function.
Discussion
There is still a lack of rapid and simple diagnostic methods for cryptococcosis, and its clinical manifestations and imaging examination are not specific, while it is of great significance to find fungi directly through histopathological examination. Finding Cryptococcus from diseased tissues is the current gold standard for diagnosis1,16,17. If the patient has typical imaging manifestations of pulmonary infection, Cryptococcus antigen exists in serum, and sputum and alveolar lavage fluid smear or culture are positive, it can be clinically diagnosed as pulmonary cryptococcosis. According to the clinical manifestations, imaging, microbiological, and histopathological examination results, this case is considered pulmonary cryptococcosis, though this case does not have a clear history of contact with pigeons and other birds as a high-risk factor9. The examination result showed no central nervous system infection and no respiratory failure. Therefore, according to IDSA’s recommendation and a guideline18,19, the patient was treated according to mild to moderate lung infection, starting with a conventional dose of 400 mg/d fluconazole injection for antifungal treatment. Later, type 2 diabetes and cirrhosis were found in the examination, both of which were susceptible factors of cryptococcosis20–23, and were also factors to consider in the adjustment of drug dosage in the later period.
Diabetes mellitus is one of the most common susceptible factors of cryptococcosis in HIV-negative individuals, accounting for 10–20% of the underlying diseases of cryptococcosis patients.24–27 Hyperglycemia can lead to the deficiency of the host immune system, make diabetic patients susceptible to opportunistic infections such as cryptococcosis, and also aggravate the degree of infection and affect the prognosis28,29. A study in the Chinese mainland found that 62% of the infected people had type 2 diabetes before infection20. Another study in Taiwan Province, China, found that type 2 diabetes was related to cryptococcosis and cryptococcosis meningitis in one year and overall mortality28. The patient was newly diagnosed with type 2 diabetes after admission, and his blood sugar level was not controlled before admission, which may be one of the susceptible factors. It may also be one of the reasons for the poor anti-infection effect of fluconazole.
Concomitant liver disease is another susceptible factor for cryptococcosis.9 Liver disease predisposes individuals to invasive fungal infections (IFI), including acute liver failure, severe alcohol-related hepatitis (AH), decompensated cirrhosis, and liver transplantation.30 In patients with decompensated cirrhosis, 10% can be complicated with IFI, while in liver transplant recipients, the proportion can reach 40%.31 The risk of developing IFI in patients with liver cirrhosis is directly proportional to the severity of the liver disease. In compensated cirrhosis, the incidence of IFI is lower than that of bacterial infections. However, in patients with decompensated cirrhosis or critically ill, the incidence of IFI can be as high as 10–14%30, among which Cryptococcus infection ranks third.21,32
Fluconazole has a wide range of doses in clinical application, and the label recommends a commonly used dose range of 50–400 mg/d. When treating cryptococcal infections, the recommended dosage according to relevant guidelines can range from 200 mg/d to 2000 mg/d based on the scope and severity of the infection18. The patient’s risk factors were clear, and based on clinical manifestations, pathology, and imaging results, it was determined to be a pulmonary cryptococcal infection. A half-month course of antifungal treatment was conducted before admission, but there was no significant effect. Later, he was admitted to our hospital for further antifungal treatment. Due to the limited clinical application and research data on fluconazole, a relatively high dosage of 400 mg/d was determined based on other medication experiences, combined with a high level of blood glucose and liver clinical manifestations. Moxifloxacin, and cefoperazone sodium and tazobactam sodium were gradually given as empirical treatments for possible hospital infections. On the 9th day, Cryptococcus neoformans was detected in the sputum and lavage fluid of the fiberoptic bronchoscope, and Cryptococcus infection was confirmed. The MIC of fluconazole was 2. The early treatment direction was correct, but the conventional doses of fluconazole combined with moxifloxacin and cefoperazone sodium tazobactam sodium did not significantly alleviate symptoms.
Further analysis, the underlying reason may also be related to obesity. Obesity can lead to changes in the pharmacokinetics of fluconazole and even seriously affect the clinical efficacy13,33,34. In the Asian population, BMI ≥ 25 Kg/m2 is obesity35, and this case not only has a BMI of 34.88 Kg/m2, which is morbid obesity but also has diabetes, both of which may lead to an insufficient conventional dose of fluconazole. Generally, when calculating the drug dosage, the total weight is used in the normal weight population. However, due to differences in weight composition, cardiac output, and regional blood flow changes on different drugs, especially considering that most blood will flow directly through the lean tissue with rich blood vessels, it is not appropriate to calculate the dosage directly based on total weight in obese patients36. Compared to ideal weight and body surface area, which cannot reflect the weight composition ratio of obese patients, lean bodyweight increases disproportionately with total weight and is related to cardiac output. Therefore, it is more suitable for calculating medication dosage in obese patients. Due to the increase of lean tissue, glomerular filtration and renal tubular secretion in obese patients, the distribution range and volume of fluconazole will increase37. Therefore, compared with normal-weight patients, the steady-state serum concentration of fluconazole in obese patients is low, which may be related to the increase of Vd.37
The Clinical and Laboratory Standards Institute (CLSI) has not yet established the breaking point standard of antifungal drugs against Cryptococcus, therefore, this case mainly refers to the relevant breaking point of anti-Candida. At present, the target parameters of PK/PD for anti-Candida include AUC/MIC > 25 which can produce an antibacterial effect and AUC/MIC > 100 which can achieve a higher cure rate for sensitive strains13. According to a study of fluconazole in obese people, when MIC=2, the target value of AUC/MIC is set to 25, and then the loading dose is 400 mg/d, even if BMI reaches 40, it can reach the standard13. However, the actual situation in this case is that at the initial stage of treatment, the dose of fluconazole 400 mg/d did not achieve the expected effect. Therefore, the team considered adjusting the target value to AUC/MIC > 100. The patient’s BMI was 34.88, and the calculated lean bodyweight was 73.67 kg. According to the commonly used range of 6–12 mg/kg recommended by the guidelines, the dose should be 442.03~884.07 mg, so only an 800 mg loading dose is possible to meet the standard. The treatment experience of Cojutti et al also shows that when the loading dose of fluconazole is 800 mg/d and the maintenance dose is 500 mg/d, after three days, the Cmax is 20.91 mg/L and the AUC0–24/MIC is 1154, which can really achieve the pharmacodynamic target (AUC0–24/MIC > 100).34
Finally, the loading dose of fluconazole was determined to be 800 mg, and the liver and kidney functions were closely monitored. The patient did not have hemoptysis after 3 days of dose adjustment, and his body temperature returned to normal after 5 days. Considering that the patient had concurrent cirrhosis, after half a month of stable condition, according to the study by Alobaid et al13, the maintenance dose was set at 6–9 mg/kg, which was about 600 mg/d. The patient underwent follow-up visits one and four months after discharge, and his condition was stable, so he continued to take medicine until the focuses of infection in the lungs were completely absorbed.
Conclusion
This case suggests that when diagnosing HIV-negative pulmonary cryptococcosis, it is important to carefully investigate the comorbidities in order to fully evaluate the degree and scope of infection and improve the treatment plan. When fluconazole is used in obese patients, there should be accurate and well-based dose adjustment methods. It can be considered to adjust the dosage based on weight, and it is recommended to use lean bodyweight for dose adjustment and optimization. When the conventional dose of fluconazole is not effective in anti-pulmonary cryptococcal infection, we can consider adjusting the loading dose with AUC/MIC > 100 as the target value, and the liver function should also be considered in the subsequent maintenance dose plan. The accurate medication basis of fluconazole in the treatment of cryptococcosis still needs to be obtained through high-quality pharmacokinetics and clinical studies.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was sponsored by Health Commission of Hubei Province WJ2021M132.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Data Sharing Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. This study fully considers the personal privacy protection of patients and does not contain any identifiable personal information. The patient has signed an informed consent form and agreed to participate in this study, allowing the data and images related to this case to be used and published publicly.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019;57(2):133–150. doi: 10.1093/mmy/myy086 [DOI] [PubMed] [Google Scholar]
- 2.Marr KA. Cryptococcus gattii as an important fungal pathogen of western North America. Expert Rev Anti Infect Ther. 2012;10(6):637–643. doi: 10.1586/eri.12.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Wang X, Yu X, et al. Pleural effusion as the initial clinical presentation in disseminated cryptococcosis and fungaemia: an unusual manifestation and a literature review. BMC Infect Dis. 2015;15:385. doi: 10.1186/s12879-015-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong N, Chen M, Fang W, et al. Cryptococcosis in HIV-negative patients with renal dialysis: a retrospective analysis of pooled cases. Mycopathologia. 2017;182(9–10):887–896. doi: 10.1007/s11046-017-0163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yau LF, Chan WH, Li YX, et al. Serum sphingolipids aid in diagnosing adult HIV-negative patients with pulmonary cryptococcosis: a clinical cohort study. J Thorac Dis. 2023;15(10):5534–5548. doi: 10.21037/jtd-23-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng HK, Liu CP, Ho MW, et al. Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS One. 2013;8(4):e61921. doi: 10.1371/journal.pone.0061921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JF, Valencia-Rey PA, Davis WB. Pulmonary cryptococcosis in the immunocompetent patient-many questions, some answers. Open Forum Infect Dis. 2016;3(3):ofw167. doi: 10.1093/ofid/ofw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuchong C, Fubin C, Jianghan C, et al. Cryptococcosis in China (1985–2010): review of cases from Chinese database. Mycopathologia. 2012;173(5–6):329–335. doi: 10.1007/s11046-011-9471-1 [DOI] [PubMed] [Google Scholar]
- 9.Howard-Jones AR, Sparks R, Pham D, Halliday C, Beardsley J, Chen SC. Pulmonary cryptococcosis. J Fungi. 2022;8(11):1156. doi: 10.3390/jof8111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011;86(8):805–817. doi: 10.4065/mcp.2011.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Zhang D, Xue X, Yang L, Chen L, Pan L. Clinical analysis of 16 cases of pulmonary cryptococcosis in patients with normal immune function. Ann Palliat Med. 2020;9(3):1117–1124. doi: 10.21037/apm-20-897 [DOI] [PubMed] [Google Scholar]
- 12.Sinnollareddy M, Peake SL, Roberts MS, Lipman J, Roberts JA. Using pharmacokinetics and pharmacodynamics to optimise dosing of antifungal agents in critically ill patients: a systematic review. Int J Antimicrob Agents. 2012;39(1):1–10. doi: 10.1016/j.ijantimicag.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 13.Alobaid AS, Wallis SC, Jarrett P, et al. Effect of obesity on the population pharmacokinetics of fluconazole in critically ill patients. Antimicrob Agents Chemother. 2016;60(11):6550–6557. doi: 10.1128/AAC.01088-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kably B, Launay M, Derobertmasure A, Lefeuvre S, Dannaoui E, Billaud EM. Antifungal drugs TDM: trends and Update. Ther Drug Monit. 2022;44(1):166–197. doi: 10.1097/FTD.0000000000000952 [DOI] [PubMed] [Google Scholar]
- 15.Gómez-López A. Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin Microbiol Infect. 2020;26(11):1481–1487. doi: 10.1016/j.cmi.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 16.Espinel-Ingroff A, Kidd SE. Current trends in the prevalence of Cryptococcus gattii in the United States and Canada. Infect Drug Resist. 2015;8:89–97. doi: 10.2147/IDR.S57686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng H, Zhang J, Li J, Wang D, Pan L, Xue X. Clinical features and radiological characteristics of pulmonary cryptococcosis. J Int Med Res. 2018;46(7):2687–2695. doi: 10.1177/0300060518769541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CC, Harrison TS, Bicanic TA, et al. Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect Dis. 2024;24(8):e495–e512. doi: 10.1016/S1473-3099(23)00731-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Fang W, Jiang W, et al. Cryptococcosis in patients with diabetes mellitus II in mainland China: 1993–2015. Mycoses. 2017;60(11):706–713. doi: 10.1111/myc.12645 [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Husain S, de Vera M, Gayowski T, Cacciarelli TV. Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine. 2004;83(3):188–192. doi: 10.1097/01.md.0000126760.45299.69 [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Sifri CD, Silveira FP, et al. Cryptococcosis in patients with cirrhosis of the liver and posttransplant outcomes. Transplantation. 2015;99(10):2132–2141. doi: 10.1097/TP.0000000000000690 [DOI] [PubMed] [Google Scholar]
- 23.Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis. 2015;3(1):ofv197. doi: 10.1093/ofid/ofv197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis. 2006;10(1):72–78. doi: 10.1016/j.ijid.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 25.Wu HH, Chen YX, Fang SY. Clinicopathological features of isolated pulmonary cryptococcosis in HIV-negative patients. J Int Med Res. 2020;48(6):300060520927877. doi: 10.1177/0300060520927877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohno S, Kakeya H, Izumikawa K, et al. Clinical features of pulmonary cryptococcosis in non-HIV patients in Japan. J Infect Chemother. 2015;21(1):23–30. doi: 10.1016/j.jiac.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 27.Tsai WC, Lien CY, Lee JJ, et al. The clinical characteristics and therapeutic outcomes of cryptococcal meningitis in elderly patients: a hospital-based study. BMC Geriatr. 2019;19(1):91. doi: 10.1186/s12877-019-1108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KH, Chen CM, Chen TL, et al. Diabetes mellitus is associated with acquisition and increased mortality in HIV-uninfected patients with cryptococcosis: a population-based study. J Infect. 2016;72(5):608–614. doi: 10.1016/j.jinf.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 29.Nsenga L, Kajjimu J, Olum R, et al. Cryptococcosis complicating diabetes mellitus: a scoping review. Ther Adv Infect Dis. 2021;8:20499361211014769. doi: 10.1177/20499361211014769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma N, Singh S, Singh M, et al. Global epidemiological burden of fungal infections in cirrhosis patients: a systematic review with meta-analysis. Mycoses. 2022;65(3):266–284. doi: 10.1111/myc.13387 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Lan C, Qin S, et al. Efficacy of anti-fungal agents for invasive fungal infection prophylaxis in liver transplant recipients: a network meta-analysis. Mycoses. 2022;65(10):906–917. doi: 10.1111/myc.13508 [DOI] [PubMed] [Google Scholar]
- 32.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 33.Srinivas NR. Influence of morbid obesity on the clinical pharmacokinetics of various anti-infective drugs: reappraisal using recent case studies-issues, dosing implications, and considerations. Am J Ther. 2018;25(2):e224–e246. doi: 10.1097/MJT.0000000000000401 [DOI] [PubMed] [Google Scholar]
- 34.Cojutti PG, Carnelutti A, Mattelig S, Sartor A, Pea F. Real-time therapeutic drug monitoring-based pharmacokinetic/pharmacodynamic optimization of complex antimicrobial therapy in a critically ill morbidly obese patient. Grand round/a case study. Ther Drug Monit. 2020;42(3):349–352. doi: 10.1097/FTD.0000000000000740 [DOI] [PubMed] [Google Scholar]
- 35.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. [DOI] [PubMed] [Google Scholar]
- 36.Gouju J, Legeay S. Pharmacokinetics of obese adults: not only an increase in weight. Biomed Pharmacother. 2023;166:115281. doi: 10.1016/j.biopha.2023.115281 [DOI] [PubMed] [Google Scholar]
- 37.Cohen LG, DiBiasio A, Lisco SJ, Hurford WE. Fluconazole serum concentrations and pharmacokinetics in an obese patient. Pharmacotherapy. 1997;17(5):1023–1026. doi: 10.1002/j.1875-9114.1997.tb03793.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.