Abstract
Background
This study explored the influence of type 2 diabetes and impaired glucose tolerance (IGT) on the risk of the multimorbidity cluster of cardiovascular disease (CVD) and cancer.
Methods
A total of 1629 participants in the Da Qing Diabetes Prevention Outcome Study were recruited in the present analysis, including normal glucose tolerance (NGT, N = 492), IGT (N = 540), and newly diagnosed type 2 diabetes (N = 597) groups. Cox proportional hazards analyses were performed to assess the relationship between NGT, IGT, and newly diagnosed type 2 diabetes and the risk of the multimorbidity cluster of CVD and cancer.
Results
The incidence rates for multimorbidity cluster CVD and cancer were 1.25, 3.17, and 3.23 per 1000 person-years in people with NGT, IGT, and the newly diagnosed type 2 diabetes groups, respectively, over 34-year follow-up. Cox analysis revealed that diabetes status (as time-dependent variable) was significantly associated with the subsequent increased risk of multimorbidity cluster of CVD and cancer compared with non-diabetes (hazard ratios [HR] = 2.55, 95% confidence interval [CI] 1.51–4.31) after adjustment of potential confounders. Similar analysis showed that this risk was significantly higher in the IGT and newly diagnosed type 2 diabetes groups compared with NGT, with HR of 3.28 (95% CI 1.83–5.87) and HR of 3.90 (95% CI 2.14–7.09), respectively. Whereas compared diabetes with IGT group, this risk was not significantly different.
Conclusions
Similar to newly diagnosed type 2 diabetes, IGT is significantly associated with an increased risk of the multimorbidity cluster of CVD and cancer compared with NGT. This finding highlights the urgent need for an active detection of IGT and effective prevention and management of diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03749-6.
Keywords: Impaired glucose tolerance, Type 2 diabetes, Multimorbidity, Cardiovascular disease, Cancer
Background
Advancements in medical science have significantly extended human longevity and concurrently increased the probability of individuals developing multiple chronic conditions, a phenomenon termed multimorbidity [1]. Among these, cardiovascular disease (CVD) previously stood as the predominant cause of premature mortality [2, 3]. Since 1990, the prevalence of CVD has doubled, reaching nearly 94 million in 2016 and the annual number of CVD mortality increased from 2.51 million to 3.97 million between 1990 and 2016 in China [4]. Concurrently, cancer has emerged as a leading cause of mortality, with an estimated 4.8 million new cases and 3.2 million cancer deaths in 2022 in China [5, 6]. The co-occurrence of CVD and cancer significantly exacerbates healthcare challenges by diminishing the quality of life [7], increasing healthcare resource utilisation [8, 9], contributing to increased mortality rates, and underscoring the necessity for global health initiatives focused on multimorbidity prevention.
In China, the prevalence of diabetes dramatically increased from less than 1% in the 1980s to 12.4% in 2018 [10–14], affecting approximately one-eighth of the adult population, and type 2 diabetes accounting for 90% of these cases. Recent studies have highlighted that individuals with type 2 diabetes exhibit an elevated risk of developing cancer [15–17] or CVD [18, 19], and the cluster of different cardiovascular diseases such as myocardial infarction and stroke compared with their counterparts without diabetes. These findings emphasise the role of type 2 diabetes faced an exacerbating risk of a single disease or cluster of the diseases. To our knowledge, studies focus on investigating the role of type 2 diabetes in multimorbidity cluster of CVD and cancer were relatively few. Concurrently, the prevalence of prediabetes in China has increased dramatically, from less than 1% in the 1980s to 38.1% in 2018 [10, 14], which is three times the number of diabetes cases. A significant proportion of prediabetic cases exhibit impaired glucose tolerance (IGT), a condition characterised by abnormal blood glucose levels postprandially. This presents a potential risk factor, the impact of which on the development of CVD and cancer remained to be fully elucidated. The present study aimed to investigate the association between type 2 diabetes and IGT, and the multimorbidity cluster of CVD and cancer.
Methods
Study design and participants
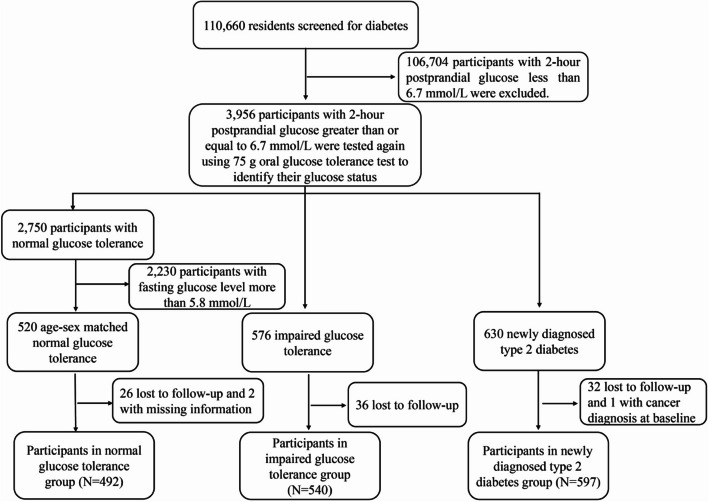
The present study included 1629 participants (492, 540, and 597 in the normal glucose tolerance [NGT], IGT, and newly diagnosed type 2 diabetes groups, respectively) from the original Da Qing Diabetes Prevention Outcome Study (DQDPOS); the design, methods, and population of the study have been previously reported [20]. Briefly, 110,660 residents between 25 and 74 years of age in Da Qing, China, were screened for diabetes in 1986, and 106,704 participants with a 2-h postprandial glucose < 6.7 mmol/L were excluded since they were very likely not diabetes or IGT. Other 3956 residents with a 2-h postprandial glucose ≥ 6.7 mmol/L were tested again using a 75 g oral glucose tolerance test (OGTT) to identify their glucose status. Based on the World Health Organization (WHO) criteria of 1985 [21], 576 participants (312 males and 264 females) were identified as having IGT (impaired fasting glucose [IFG] was not yet defined in 1986) and 630 participants were newly diagnosed type 2 diabetes. In addition, 520 age-sex matched participants with NGT were included as controls. These participants formed the DQDPS. A total of 26 participants in the NGT group were excluded due to loss of follow-up, and 2 participants in NGT group were excluded due to missing baseline information. In the IGT group, 36 participants were excluded due to loss of follow-up. In the newly diagnosed type 2 diabetes group, 32 participants were excluded due to loss of follow-up, and 1 participant was excluded due to a cancer diagnosis at baseline. Thus, the study comprised 1629 participants categorised into NGT (N = 492), IGT (N = 540), and newly diagnosed type 2 diabetes (N = 597) groups (Fig. 1). The DQDPOS is a longitudinal cohort study representing a 34-year follow-up of the original Da Qing Diabetes Study. The primary objective was to explore the effects of IGT and newly diagnosed type 2 diabetes on long-term adverse clinical outcomes, including diabetes-related microvascular and macrovascular complications, osteoporosis, and cancer.
Fig. 1.
Flowchart of the study participants
This study was approved by the institutional review boards of the Fuwai Hospital, Chinese Academy of Medical Science, and all surviving participants and proxies for the deceased participants provided written informed consent.
Assessment of outcome
The primary outcome of interest was the multimorbidity cluster of CVD and cancer over the 34-year follow-up period. Multimorbidity cluster of CVD and cancer was defined as the coexistence of CVD and cancer in an individual. The onset-time of multimorbidity cluster of CVD and cancer was determined as the date when the second disease was diagnosed [22]. CVD events included nonfatal or fatal myocardial infarction or sudden death (I21), hospital admission for heart failure (I50), and nonfatal or fatal stroke (I60–I66) [22]. Cancer (C00–C97) included any site-specific cancer diagnosis [23]. The diagnosis of cancer, site-specific cancer, and CVD was based on the International Classification of Diseases-10 codes. Follow-up interviews conducted at 20, 23, 30, and 34 years post-baseline (1986) enabled for the living participants, proxies’ interview was completed at the same round of follow-up for the deceased participants, then the collected outcomes were further confirmed with medical records. Our follow-up spanned 34 years, the outcomes were independently adjudicated by two professional follow-up physicians, who were blind to the participants’ glycaemic status, with any disagreements resolved by a third senior physician.
Assessment of covariates
We collected data on demographics and lifestyle from participants through standardised interviews at baseline (1986). Clinical examinations included measurements of blood pressure, height, weight, total cholesterol, and other relevant health indicators. Glycaemic status was assessed using plasma glucose levels at fasting and 2 h after a OGTT at baseline according to the 1985 WHO criteria of diabetes. Use of hypoglycaemic, lipid-lowering, and antihypertensive drugs was documented to evaluate their association with the multimorbidity cluster of CVD and cancer.
Statistical analyses
All participants were stratified into four groups based on the occurrence of CVD alone, cancer alone, both CVD and cancer, and those who were free of both these outcomes. The baseline characteristics of the groups were presented as means and standard deviations for continuous variables, and as frequencies and percentages for categorical variables. Continuous variables were assessed for statistical differences using the Student’s t-test, and categorical variables using the test. The information on all variables was available for each participant. Incidence rate was defined as the number of events divided by the number of person-years. Person-years were defined as the time from enrolment to the onset of multimorbidity, death, or 31 December 2020, whichever occurred first. Kaplan–Meier survival curves were generated to determine the time-to-event survival for multimorbidity, and log-rank tests were used to compare difference among groups. The proportionality of hazards assumption of Cox regression model was tested by Schoenfeld residual method, confirming adherence to the proportional hazard’s assumption [24].
Cox proportional hazards analyses were used to calculate hazard ratios (HRs) to quantify the effect of glucose status on the multimorbidity cluster of CVD and cancer. Covariates, such as age, sex, smoking status, obesity status, hypertension status, and the use of lipid-lowering, hypoglycaemic, and antihypertensive drugs, were included in the model. The covariates included in the analysis were selected based on the relevant literature and the availability. Given that IGT and NGT may progress to diabetes during the follow-up, we treated diabetes as a time-dependent variable in one of the Cox proportional hazard analyses. Furthermore, we used the fasting and 1-h/2-h plasma glucose level in the OGTT as a continuous variable to explore its influence on the multimorbidity cluster of CVD and cancer. To enhance the robustness of our findings, we performed sensitivity analyses excluding participants with follow-up durations shorter than 5 years. Furthermore, subgroup analyses were conducted to compare outcomes between older and younger individuals, segmented at a 45-year age threshold. Analyses were also stratified by sex to assess potential differences between male and female participants. All analyses were performed using R software (version 4.2.0). All statistical tests were two-tailed, and statistical significance was set at a P value of less than 0.05.
Results
The mean age of the participants was 46.0 (standard deviation: 9.1) years. Of the participants, 52.8% (860/1629) were male. The baseline characteristics of the participants according to the disease status at follow-up are shown in Table 1. All baseline characteristics in participants with multimorbidity cluster of CVD and cancer group were significantly higher than those participants without CVD or cancer, except for fasting plasma glucose and total cholesterol. The baseline characteristics of the participants with multimorbidity cluster of CVD and cancer were significantly higher than those of participants with cancer alone in terms of glucose status after Bonferroni-adjusted. However, there were no significant differences between participants with CVD alone and those with multimorbidity cluster of CVD and cancer after Bonferroni-adjusted. We also showed the baseline characteristics of the participants according to the glycaemic status at baseline shown in Additional file 1: Table S1.
Table 1.
Baseline characteristic of participants categorised by incident disease status at follow-up
| Variable | Participants without CVD or cancer | Participants with cancer | Participants with CVD | Participants with CVD and cancer | P† | P‡ | P§ |
|---|---|---|---|---|---|---|---|
| Number of participants | 499 | 209 | 817 | 104 | |||
| Age (years) | 42.8 (9.9) | 46.9 (8.9) | 47.4 (8.3) | 48.2 (7.7) | < 0.001 | 0.21 | 0.37 |
| Sex, n (%) | < 0.001 | 0.070 | 0.015 | ||||
| Male | 219 (43.9) | 119 (56.9) | 451 (55.2) | 71 (68.3) | |||
| Female | 280 (56.1) | 90 (43.1) | 366 (44.8) | 33 (31.7) | |||
| Smoking, n (%) | 0.0037 | 0.076 | 0.026 | ||||
| Male | 126 (25.3) | 85 (40.7) | 277 (33.9) | 46 (44.2) | |||
| Female | 41 (8.2) | 16 (7.7) | 61 (7.5) | 2 (1.9) | |||
| BMI (kg/m2) | 24.3 (3.5) | 24.7 (4.0) | 25.5 (3.8) | 25.9 (3.6) | < 0.001 | 0.011 | 0.35 |
| Obesity status, n (%) | 69 (13.83) | 42 (20.1) | 218 (26.7) | 32 (30.8) | < 0.001 | 0.051 | 0.44 |
| Glucose status, n (%) | < 0.001 | < 0.001 | 0.071 | ||||
| Normal glucose tolerance | 194 (38.9) | 83 (39.7) | 197 (24.1) | 18 (17.3) | |||
| Impaired glucose tolerance | 164 (32.9) | 65 (31.1) | 266 (32.6) | 45 (43.3) | |||
| Type 2 diabetes | 141 (28.3) | 61 (29.2) | 354 (43.3) | 41 (39.4) | |||
| Fasting plasma glucose (mmol/L) | 6.0 (2.3) | 6.0 (2.4) | 6.8 (2.7) | 6.4 (2.3) | 0.11 | 0.20 | 0.13 |
| 1-h plasma glucose (mmol/L) | 10.6 (4.3) | 10.9 (4.6) | 12.4 (4.7) | 12.1 (4.0) | 0.0020 | 0.030 | 0.50 |
| 2-h plasma glucose (mmol/L) | 9.1 (4.5) | 9.3 (4.8) | 10.9 (5.0) | 10.5 (4.3) | 0.0030 | 0.038 | 0.44 |
| Systolic blood pressure (mm Hg) | 124 (20.7) | 128 (21.5) | 135 (25.4) | 136 (24.5) | < 0.001 | 0.0050 | 0.64 |
| Diastolic blood pressure (mm Hg) | 82 (12.4) | 85 (13.8) | 88 (15.3) | 89 (15.0) | < 0.001 | 0.012 | 0.52 |
| Hypertension status, n (%) | 185 (37.1) | 93 (44.5) | 455 (55.7) | 58 (55.8) | 0.0010 | 0.078 | 1 |
| Plasma total cholesterol (mmol/L) | 4.9 (1.3) | 5.1 (1.4) | 5.2 (1.5) | 5.2 (1.1) | 0.069 | 0.64 | 1 |
Data are presented as means (standard deviations) for continuous variables and numbers (percentages) for categorical variables. P values comparing means were based on analysis of variance for continuous variables and the chi-squared test for categorical variables. †Participants without cancer or CVD vs. participants with cancer and CVD; ‡participants with cancer vs. participants with cancer and CVD; §participants with CVD vs. participants with cancer and CVD. Bold font indicates significant variables in the comparison
CVD, cardiovascular disease; BMI, body mass index
A Bonferroni-adjusted criterion of P < 0.05/13 = 0.0038 was used
The 34-year follow-up data revealed the incidence rates for multimorbidity of CVD and cancer were 1.25, 3.17, and 3.23 per 1000 person-years in the NGT, IGT, and newly diagnosed diabetes, respectively. However, the incidence rates between the IGT and newly diagnosed type 2 diabetes groups were no significant difference (Table 2).
Table 2.
Association between patients with different glycaemic status at baseline or time-dependent diabetes during follow-up and risk of subsequent multimorbidity cluster of cardiovascular disease and cancera
| Variable | Cases/person-years | Incidence per 1000 person-years | Crude hazard ratio | 95% confidence interval | P value | Adjusted hazard ratio | 95% confidence interval | P value |
|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||
| Normal glucose tolerance | 18/14,394 | 1.25 (0.67–1.83) | Reference | Reference | ||||
| Impaired glucose tolerance | 45/14,174 | 3.17 (2.25–4.10) | 2.7 | 1.60–4.78 | < 0.001 | 3.28 | 1.83–5.87 | < 0.001 |
| Newly diagnosed type 2 diabetes | 41/12,686 | 3.23 (2.24–4.22) | 3.56 | 2.04–6.21 | < 0.001 | 3.90 | 2.14–7.09 | < 0.001 |
| Model 2 | ||||||||
| Diabetes status (time-dependent) | ||||||||
| Non-diabetes status | 14/10,144 | 1.38 (0.66–2.10) | Reference | Reference | ||||
| Diabetes status | 90/31,110 | 2.89 (2.30–3.49) | 2.37 | 1.46–3.83 | < 0.001 | 2.55 | 1.51–4.31 | < 0.001 |
Adjusted for age, sex, smoking status, obesity status, hypertension status, hypoglycaemic drugs, lipid-lowering drugs, and antihypertensive drugs
aAdditional model analysis which took IGT as reference group showed that the risk of multimorbidity cluster of CVD and cancer in diabetes group was not different with IGT group (HR = 1.21, 95% CI 0.78–1.87, P = 0.40) after a same adjustment
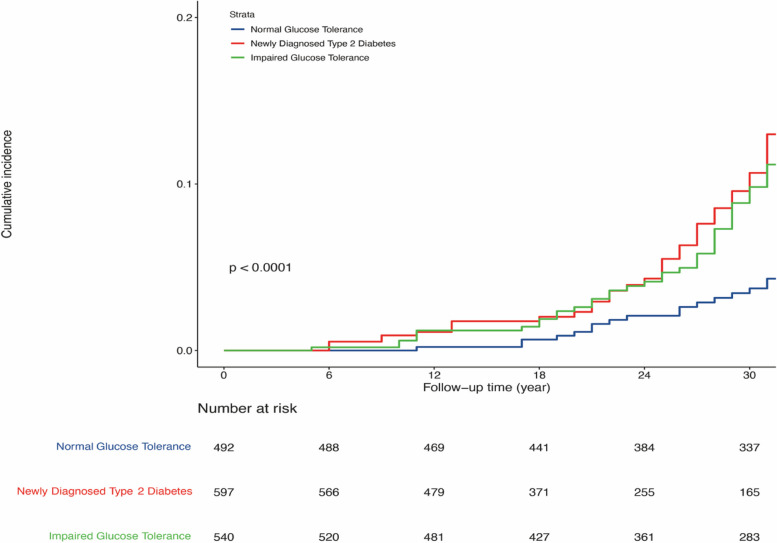
Cox proportional hazards analyses found that, after adjusting for age, sex, smoking, obesity status, hypertension status, and the use of lipid-lowering, hypoglycaemic, and antihypertensive drugs, the HRs for the multimorbidity cluster of CVD and cancer in the IGT and newly diagnosed type 2 diabetes groups were 3.28 (95% confidence interval [CI] 1.83–5.87) and 3.90 (95% CI 2.14–7.09), compared with the NGT group, respectively. However, the difference between newly diagnosed type 2 diabetes and IGT group was not significant (the hazard ratios of confounders were shown in Additional file 1: Table S2). Kaplan–Meier plots showed the cumulative incidence of CVD and cancer multimorbidity in the IGT and newly diagnosed type 2 diabetes groups were significantly higher than those in the NGT group (Fig. 2). When diabetes was treated as a time-dependent variable, the results shown that patients with diabetes (prevalent plus incident diabetes) were significantly associated with an increased risk of the multimorbidity cluster of CVD and cancer compared with those remaining non-diabetes status during the follow-up (HR 2.55, 95% CI 1.51–4.31) after the same adjustment of confounders (Additional file 1: Table S3). Moreover, a significant higher risk of develop CVD only was found in both of the newly diagnosed type 2 diabetes group (HR 2.38, 95% CI 1.96–2.87) and the IGT group (HR 1.58, 95% CI 1.30–1.92). However, neither the newly diagnosed type 2 diabetes group (HR 0.93, 95% CI 0.65–1.33) nor the IGT group (HR 1.06, 95% CI 0.75–1.49) was associated with an increased risk of cancer alone compared with NGT group, after the same adjustment.
Fig. 2.
Kaplan–Meier plots of the cumulative incidence of the multimorbidity cluster of cardiovascular disease and cancer during the 34-year follow-up
In the sex-subgroup analysis, the HR for multimorbidity cluster of CVD and cancer in the IGT and newly diagnosed type 2 diabetes were consistent with the main result in male participants (Table 3). In the female participants, newly diagnosed type 2 diabetes was associated with the increased risk of the multimorbidity cluster of CVD and cancer. However, IGT participants did not show a significant different association (Table 4). The subgroup analyses in older and younger individuals (stratified by 45 years of age) were also performed. Compared with NGT, among older participants, the IGT and newly diagnosed type 2 diabetes groups were associated with an increased risk of the cluster of CVD and cancer, respectively, with HR of 3.53 (95% CI 1.69–7.36) for diabetes group and HR of 3.52 (95% CI 1.73–7.16) for IGT group (Additional file 1: Table S4). While among younger participants, newly diagnosed type 2 diabetes group was associated with an increased risk of multimorbidity cluster of CVD and cancer, but the IGT group was not, although the HR was as high as 2.35 (95% CI 0.83–6.60) (Additional file 1: Table S5). In the sensitivity analyses that excluded participants with follow-up durations shorter than 5 years, it was found that the HRs for multimorbidity cluster of CVD and cancer in the IGT and newly diagnosed type 2 diabetes groups were 3.18 (95% CI 1.77–5.72) and 3.90 (95% CI 2.14–7.11), respectively (Additional file 1: Table S6).
Table 3.
Association between glycaemic status at baseline and risk of subsequent multimorbidity cluster of cardiovascular disease and cancer in male participants
| Variable | Hazard ratios | 95% confidence interval | P value |
|---|---|---|---|
| Age (years) | 1.09 | 1.05–1.12 | < 0.001 |
| Smoking (yes = 1) | 1.57 | 0.95–2.58 | 0.076 |
| Obesity status (yes = 1) | 1.54 | 0.89–2.65 | 0.12 |
| Hypertension status (yes = 1) | 1.07 | 0.65–1.76 | 0.80 |
| Hypoglycaemic drugs (yes = 1) | 0.84 | 0.39–1.79 | 0.64 |
| Lipid-lowering drugs (yes = 1) | 0.19 | 0.08–0.46 | < 0.001 |
| Antihypertensive drugs (yes = 1) | 0.98 | 0.47–2.04 | 0.96 |
| Glucose status at baseline | |||
| Normal glucose tolerance | Reference | ||
| Impaired glucose tolerance | 3.25 | 1.62–6.51 | < 0.001 |
| Newly diagnosed type 2 diabetes | 3.89 | 1.89–7.98 | < 0.001 |
Table 4.
Association between glycaemic status at baseline and risk of subsequent multimorbidity cluster of cardiovascular disease and cancer in female participants
| Variable | Hazard ratios | 95% confidence interval | P value |
|---|---|---|---|
| Age (years) | 1.04 | 0.99–1.10 | 0.087 |
| Smoking (yes = 1) | 0.44 | 0.10–1.86 | 0.26 |
| Obesity status (yes = 1) | 1.56 | 0.74–3.28 | 0.24 |
| Hypertension status (yes = 1) | 1.40 | 0.67–2.94 | 0.36 |
| Hypoglycaemic drugs (yes = 1) | 0.66 | 0.27–1.64 | 0.37 |
| Lipid-lowering drugs (yes = 1) | 1.45 | 0.59–3.6 | 0.41 |
| Antihypertensive drugs (yes = 1) | 0.92 | 0.38–2.24 | 0.85 |
| Glucose status at baseline | |||
| Normal glucose tolerance | Reference | ||
| Impaired glucose tolerance | 2.52 | 0.82–7.75 | 0.11 |
| Newly diagnosed type 2 diabetes | 3.95 | 1.29–12.07 | 0.016 |
We further investigate the influence of plasma glucose levels on the occurrence of CVD and cancer multimorbidity. We found that fasting and 1-h and 2-h post-glucose load glucose levels all significantly associated with increased the risk of multimorbidity cluster of CVD and cancer (Additional file 1: Table S7).
Discussion
In our investigation, utilising 34-year follow-up data, our study revealed a significantly higher risk of the multimorbidity cluster of CVD and cancer in the IGT and newly diagnosed type 2 diabetes groups than in the NGT group, even after adjusting for confounding factors. Sex-subgroup analysis revealed a consistent result, especially among male participants. These findings highlight the urgent need for effective interventions to address the increasing prevalence of type 2 diabetes and IGT.
The multimorbidity cluster of CVD and cancer presents notable health challenges. Previous studies have revealed that type 2 diabetes is associated with CVD [25, 26] or cancer [27], or CVD mortality [28, 29], or cancer mortality [30], and that it increased the risk of CVD in cancer survivors [31] or increased the risk of cancer in CVD survivors, compared with normoglycaemic individuals. Moreover, a study revealed that the consumption of ultra-processed foods was associated with a higher incidence of multimorbidity, defined as the co-occurrence of at least two chronic diseases in an individual among first cancer at any site, cardiovascular disease, and type 2 diabetes [32]. Our study added the evidence between newly diagnosed type 2 diabetes and multimorbidity cluster of CVD and cancer from a more than 30 years long-term follow-up in the Chinese population. This underscores the importance of monitoring and managing the multimorbidity cluster of CVD and cancer risk in patients with type 2 diabetes. In our study, among 114 cases with cluster of CVD and cancer, 78 participants were diagnosed with CVD first, followed by cancer, while another 26 participants were diagnosed with cancer first, followed by CVD. The results highlighted the impact of newly diagnosed type 2 diabetes on the multimorbidity cluster of CVD and cancer, extending the evidence of previous studies.
We also explored the influence of IGT on the multimorbidity cluster of CVD and cancer. Interestingly, we found that the HR of IGT for this multimorbidity was similar to that of newly diagnosed type 2 diabetes, especially among male participants. These results not only served as a reminder to pay attention to the multimorbidity cluster of CVD and cancer among individuals with IGT, which addressed the importance of detection of IGT and prevention of type 2 diabetes in the future. China has a large population with IGT [14]. Moreover, the progression from IGT to type 2 diabetes is rapid, at approximately 6 years [33, 34]. Therefore, it is not surprising that IGT and newly diagnosed type 2 diabetes have similar influence on multimorbidity cluster of CVD and cancer during long-term follow-up. Previous studies have only revealed that IGT affects the occurrence of CVD [35, 36] or cancer [37]. However, our study revealed that IGT was strongly associated with the risk of on the multimorbidity cluster of CVD and cancer, particularly among male participants. The results may draw attention to the prevention of type 2 diabetes and IGT.
The present study also demonstrated that age, sex, obesity status, and the use of lipid-lowering drugs were factors potentially related to the multimorbidity cluster of CVD and cancer. Lipid-lowering drugs, such as statins, are known to reduce cholesterol levels and reduce inflammation, which can decrease the risk of cardiovascular events [38]. Additionally, these drugs may have a role in reducing cancer risk by inhibiting cellular proliferation and angiogenesis [39].
We also explored the relationship between IGT and newly diagnosed type 2 diabetes and CVD and cancer alone. The analysis revealed that newly diagnosed type 2 diabetes and IGT were associated with CVD only, but not with cancer only. As our previous reported, newly diagnosed type 2 diabetes was significantly associated with the risk of cancer (including any cancer occurred in the same cohort). These results suggested that the association between diabetes and cancer risk was largely explained by the cluster of CVD and cancer. We also conducted sensitivity and subgroup analyses, which were consistent with the main findings, especially among male participants and older participants.
The precise mechanisms by which type 2 diabetes and IGT contribute to the multimorbidity cluster of CVD and cancer remain incompletely understood [40, 41]. Type 2 diabetes and IGT affect several diseases, including CVD and cancer, through several mechanisms, including hyperinsulinaemia, insulin resistance, and inflammation. Insulin resistance or hyperinsulinaemia may precede type 2 diabetes and IGT can alter systemic lipid metabolism, leading to the development of dyslipidaemia and the well-known lipid triad: high levels of plasma triglycerides, low levels of high-density lipoproteins, and the appearance of small dense low-density lipoproteins. This triad, along with endothelial dysfunction, contribute to CVD [42–44]. Most cancer cells express insulin and insulin-like growth factor (IGF)-1 receptors, and multiple signalling pathways are activated after insulin receptors or IGF-1 receptors interact with their ligands. These signalling pathways may stimulate multiple cancer phenotypes, including proliferation, protection from apoptotic stimuli, invasion, and metastasis, potentially enhancing the promotion and progression of many types of cancer cells [45]. Moreover, IGT and type 2 diabetes have long been considered a state of chronic, low-level inflammation with signalling pathways including tumour necrosis factor-, interleukin 1 , interleukin 6, and plasminogen activator inhibitor 1. Activation of these signalling pathways is increased in muscle cells, leading to vascular fibrosis, calcification, inflammation, prothrombotic effects, and vascular damage. Each of these inflammatory factors may play an etiological role in the regulation of malignant transformation or cancer progression [45].
Our study has several strengths. First, it drew from a population-based cohort study of Chinese adults with newly diagnosed type 2 diabetes and IGT, utilising a rigorously selected sample from the Da Qing city populace. Second, the diagnoses of type 2 diabetes and IGT adhered to the WHO’s 1985 criteria, employing systematic OGTT, to minimise the common selection biases associated with hospital and clinic-based studies. Third, the study benefited from a homogenous participant demographic in terms of ethnicity, with the majority residing in the same geographic area throughout the observation period. Fourth, a control group with well-established NGT was included for accurate comparisons. Fifth, the medical care received by participants was indicative of the standard provided across China, with the majority accessing treatment from the same healthcare system over the course of the study. Sixth, the verification of cardiovascular and cancer events was primarily achieved through medical record reviews for most participants. Seventh, an extensive follow-up duration of up to 34 years was maintained, offering ample opportunity to observe the emergence of multimorbidity.
Our study also has some limitations. First, there was a lack of systematic re-examinations at predetermined intervals during the follow-up, limiting our capacity to account for time-varying confounders, despite the initial and final evaluations being systematically conducted. Second, the reliance on OGTT results for type 2 diabetes diagnosis implied that some participants may have developed diabetes prior to its detection in our study, potentially leading to an underestimation of the actual duration of diabetes. Third, we cannot rule out the impact of other unmeasured confounders that might explain the association between newly diagnosed type 2 diabetes and IGT and the multimorbidity clustering of CVD and cancer. Fourth, the study had not included patient with IFG because the concept of IFG was introduced many years later after the original Da Qing study, therefore, we are unable to put them in the current post hoc analysis. It is well known that, in addition to IGT, IFG is another subset of prediabetes. The blood glucose and insulin levels in IFG are not as high as in the IGT population. Whether the patients with IFG have a higher aggregated risk of the clustering CVD and cancer as that in IGT population remains to be investigated in further study. Lastly, we have to face the fact that people with T2D are seeing a physician more often than healthy people, and thus they may have a higher likelihood for other illness to be diagnosed (earlier).
Conclusions
Our study found that, similar to newly diagnosed type 2 diabetes, IGT has a significant increased risk of the multimorbidity cluster of CVD and cancer compared with NGT. This finding highlights the urgent need for an active detection of IGT and effective prevention and management of diabetes.
Supplementary Information
Additional file 1: Tables S1–7. Table S1 Baseline characteristic of participants in NGT, IGT, and newly diagnosed type 2 diabetes groups; Table S2 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer; Table S3 Association between time-dependent diabetes during flow-up and the risk of multimorbidity clustering of cardiovascular disease and cancer; Table S4 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer in older participants (≥45 years); Table S5 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer in younger participants (<45 years); Table S6 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer (excluding first 5 years of follow-up); Table S7 Association between fasting, 1-h, and 2-h plasma glucose at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer.
Acknowledgements
The authors thank all the participants in the Da Qing Diabetes Prevention Outcome Study and their relatives who provided information that made the current study possible.
Abbreviations
- CI
Confidence interval
- CVD
Cardiovascular disease
- DQDPOS
Da Qing Diabetes Prevention Outcome Study
- HRs
Hazard ratios
- IGF
Insulin-like growth factor
- IGT
Impaired glucose tolerance
- NGT
Normal glucose tolerance
- OGTT
Oral glucose tolerance test
- WHO
World Health Organization
Authors’ contributions
FC, JPW, BZ, and GWL conceived and designed the study. FC analysed the data and wrote the first draft of the manuscript. JPW, SYH, YFH, YLA, QHG, XPC, YS, XW, and YYC contributed to the data collection and the critical revision of the manuscript. BZ and GWL are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the accuracy of the data analysis. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: CYan1314 (Fei Chen).
Funding
This study was supported by National High Level Hospital Clinical Research Funding (2022-NHLHCRF-YS-01) and National Key Research and Development Program of China (2018YFC1313902).
Data availability
The datasets analysed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review boards of the Fuwai Hospital, Chinese Academy of Medical Science (number of the ethics approval, 2015 − 733). All surviving participants and proxies for the deceased participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Chen and Jinping Wang contributed equally to this work.
Contributor Information
Bo Zhang, Email: drbozhang@yahoo.com.
Guangwei Li, Email: guangwei_li45@126.com.
References
- 1.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205–e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors. J Am Coll Cardiol. 2019;74:2529–32. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, et al. Burden of cardiovascular diseases in China, 1990–2016: findings from the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019;4:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He S, Xia C, Li H, Cao M, Yang F, Yan X, et al. Cancer profiles in China and comparisons with the USA: a comprehensive analysis in the incidence, mortality, survival, staging, and attribution to risk factors. Sci China Life Sci. 2024;67:122–31. [DOI] [PubMed] [Google Scholar]
- 7.Low LL, Kwan YH, Ko MSM, Yeam CT, Lee VSY, Tan WB, et al. Epidemiologic characteristics of multimorbidity and sociodemographic factors associated with multimorbidity in a rapidly aging Asian country. JAMA Netw Open. 2019;2: e1915245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269. [DOI] [PubMed] [Google Scholar]
- 9.Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61:e12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan X-R, Yang W-Y, Li G-W, Liu J, National Diabetes Prevention and Control Cooperative Group. Prevalence of diabetes and its risk factors in China, 1994. Diabetes Care. 1997;20:1664–9. [DOI] [PubMed]
- 11.Wenying Y, Juming L, Jianping W, Weiping J, Linong J, Jianzhong X, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. 2021;326:2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballotari P, Vicentini M, Manicardi V, Gallo M, Chiatamone Ranieri S, Greci M, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer. 2017;17:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350: g7607. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. [DOI] [PubMed] [Google Scholar]
- 18.Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Zhang P, Wang J, Gong Q, An Y, Qian X, et al. Associations of progression to diabetes and regression to normal glucose tolerance with development of cardiovascular and microvascular disease among people with impaired glucose tolerance: a secondary analysis of the 30 year Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2021;64:1279–87. [DOI] [PubMed] [Google Scholar]
- 20.An Y, Zhang P, Wang J, Gong Q, Gregg EW, Yang W, et al. Cardiovascular and all-cause mortality over a 23-year period among chinese with newly diagnosed diabetes in the Da Qing IGT and Diabetes Study. Diabetes Care. 2015;38:1365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Consultation. diabetes_mellitus_1985.pdf. WHO. 1985.
- 22.Freisling H, Viallon V, Lennon H, Bagnardi V, Ricci C, Butterworth AS, et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang W-C, Hsieh T-C, Hsu W-L, Chang F-L, Tsai H-R, He M-S. Diabetes and further risk of cancer: a nationwide population-based study. BMC Med. 2024;22:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 25.Ma C-X, Ma X-N, Guan C-H, Li Y-D, Mauricio D, Fu S-B. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685–95. [DOI] [PubMed] [Google Scholar]
- 27.Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. 2021;30:1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The DECODE Study Group, on behalf of the European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–96. [DOI] [PubMed]
- 29.Nakagami T, Qiao Q, Tuomilehto J, Balkau B, Tajima N, Hu G, et al. Screen-detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: the DECODA study. Eur J Cardiovasc Prev Rehabil. 2006;13:555–61. [DOI] [PubMed] [Google Scholar]
- 30.for the DECODE Study Group, Zhou XH, Qiao Q, Zethelius B, Pyörälä K, Söderberg S, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867–76. [DOI] [PubMed]
- 31.Oh S, Lee J, Hong YS, Kim K. Increased risk of cardiovascular disease associated with diabetes among adult cancer survivors: a population-based matched cohort study. Eur J Prev Cardiol. 2023;30:zwad046. [DOI] [PubMed]
- 32.Cordova R, Viallon V, Fontvieille E, Peruchet-Noray L, Jansana A, Wagner K-H, et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. Lancet Reg Health - Eur. 2023;35: 100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan KMV, Kondal D, Chang HH, Mohan D, Gujral UP, Anjana RM, et al. Natural history of type 2 diabetes in Indians: time to progression. Diabetes Care. 2024;47:858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Zhang Y, Shen S, Wang X, Dong L, Li Q, et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: a multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2023;11:567–77. [DOI] [PubMed] [Google Scholar]
- 35.Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease. Endocrinol Metab Clin North Am. 2018;47:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57:2261–9. [DOI] [PubMed] [Google Scholar]
- 38.Lim SY. Role of statins in coronary artery disease. Chonnam Med J. 2013;49:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin F, Fathi F, Reiner Ž, Banach M, Sahebkar A. The role of statins in lung cancer. Arch Med Sci. 2021. 10.5114/aoms/123225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Boer RA, Meijers WC, Van Der Meer P, Van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21:1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr. 2008;21:160–5. [Google Scholar]
- 44.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins KK. The diabetes-cancer link. Diabetes Spectr. 2014;27:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Tables S1–7. Table S1 Baseline characteristic of participants in NGT, IGT, and newly diagnosed type 2 diabetes groups; Table S2 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer; Table S3 Association between time-dependent diabetes during flow-up and the risk of multimorbidity clustering of cardiovascular disease and cancer; Table S4 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer in older participants (≥45 years); Table S5 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer in younger participants (<45 years); Table S6 Association between glycaemic status at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer (excluding first 5 years of follow-up); Table S7 Association between fasting, 1-h, and 2-h plasma glucose at baseline and risk of multimorbidity cluster of cardiovascular disease and cancer.
Data Availability Statement
The datasets analysed in the current study are available from the corresponding author upon reasonable request.