Abstract
Objective
Type 2 diabetes mellitus (T2DM) poses a substantial global health concern. Statins are widely used among T2DM patients for managing dyslipidemia, preventing cardiovascular disease (CVD), and offering renal protection. However, the extent to which their renal protective effects contribute to reducing the incidence of severe renal complications, including chronic kidney disease (CKD) and renal failure, is not well-defined.
Methods
This investigation scrutinizes the impact of simvastatin versus placebo on renal outcomes among T2DM patients utilizing data from the ACCORD trial. It encompasses incidence rate comparisons, Kaplan-Meier estimates, Cox proportional hazards models, and mediation analyses.
Results
The study consisted of 3,619 individuals diagnosed with T2DM, among which 2,753 were treated routinely with simvastatin, while 866 did not receive any statin therapy. After adjusting for baseline characteristics and time-dependent covariates, simvastatin treatment was associated with a 71% reduction in the risk of CKD (HR 0.29, 95% CI 0.27–0.31, p < 0.01) and a 47% reduction in the risk of renal failure (HR 0.53, 95% CI 0.44–0.65, p < 0.01) compared to the statin-free group. Further subgroup analysis, accounting for the initial lipid and kidney profiles, indicated variable impacts of simvastatin on CKD and renal failure outcomes. Nevertheless, a consistent reduction in CKD risk was observed across all subgroups within the statin-treated population. Additional mediation analysis revealed that the reduction in low-density lipoprotein cholesterol (LDL-C) may be a potential mediator in the association between simvastatin and CKD, with a mediation effect of 14.9%, (95% CI 0.11–0.19, p < 0.01).
Conclusion
Administering statins, specifically simvastatin, to T2DM patients at elevated risk for CVD, is likely to offer augmented renal advantages, notably in lowering the occurrence of CKD and renal failure. This protective effect against CKD manifests regardless of initial lipid profiles, albuminuria, and estimated glomerular filtration rate (eGFR) levels. The association between simvastatin and CKD may be partially mediated by LDL-C reduction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-024-01514-6.
Keywords: Simvastatin, Chronic kidney disease, Renal failure, Type 2 diabetes, ACCORD
Introduction
Type 2 diabetes mellitus (T2DM) poses a significant concern globally, exerting a considerable economic strain on individuals and society at large. Patients with T2DM frequently exhibit lipid abnormalities, notably dyslipidemia, which predisposes them to lipid accumulation in the glomeruli and tubules, thereby accelerating the progression of diabetic nephropathy [1]. Concurrently, hyperlipidemia-induced atherosclerosis stands as a primary contributor to cardiovascular disease (CVD) [2], with serum low-density lipoprotein cholesterol (LDL-C) being a critical determinant for CVD onset [3].
Statins, as cholesterol-lowering agents that inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), are the cornerstone for reducing LDL-C levels. Consequently, current medical guidelines advocate for statin therapy as an essential preventive measure against CVD in T2DM patients [4].
Intriguingly, both diabetic nephropathy and CVD frequently co-occur in individuals suffering from T2DM, a phenomenon attributed to overlapping lipid metabolism-related pathophysiological pathways. While prior studies have demonstrated that statin therapy can mitigate proteinuria and decelerate the reduction of estimated glomerular filtration rate (eGFR), research on the effect of statins on severe renal outcomes such as chronic kidney disease (CKD) or renal failure in T2DM patients remains conspicuously scarce [5, 6]. Considering the routine administration of statins in T2DM management, comprehending their effect on renal events emerges as both a clinical necessity and a significant research interest. However, ethical constraints in conducting placebo-controlled clinical trials—stemming from the established cardiovascular benefits of statins in diabetes patients—complicate the singular evaluation of statins’ effects [7]. Hence, large-scale cohort studies offer a viable alternative to further investigate this domain.
This research leverages data from the ACCORD trial, provided by the National Heart, Lung, and Blood Institute (NHLBI), to examine the impact of simvastatin versus placebo on a spectrum of renal outcomes, particularly CKD and renal failure, among T2DM patients at elevated risk of CVD.
Research design and methods
Study design
Details of the ACCORD trial design has been reported elsewhere [8]. ACCORD was a randomized, multicenter, double 2 × 2 factorial designs in 10,251 type 2 diabetes patients at high risk of CVD. In addition to fulfilling the overarching glycemia trial entry criteria, patients also needed to fulfill the entry criteria for either the lipid or blood pressure components of the trial. All patients in the ACCORD study were diagnosed to be type 2 diabetes and a glycated hemoglobin level of 7.5% or higher. If patients had evidence of clinical cardiovascular disease, age of the patients is limited to 40 ~ 79 years old; if they had evidence of subclinical cardiovascular disease or at least two additional cardiovascular risk factors, the age range is limited to 55 ~ 79 years old. Patients were specifically eligible to participate in the lipid trial if they also had the following criteria: an LDL-C level of 60 to 180 mg/dl, an High-density lipoprotein cholesterol (HDL-C) level below 55 mg/dl for women and blacks or below 50 mg/dl for all other groups, and a triglyceride level below 750 mg/dl if they were not receiving lipid therapy or below 400 mg/dl if they were receiving lipid therapy. Key exclusion criteria were frequent or recent serious hypoglycemic events, unwillingness to do home glucose monitoring or inject insulin, a body mass index of more than 45 kg/m2, a serum creatinine level of more than 1.5 mg/dl (132.6 umol/l), or other serious illness.
All 10,251 patients were randomly assigned to receive comprehensive intensive therapy targeting a glycated hemoglobin (HbA1c) level of < 6.0% or to receive standard therapy targeting a level of 7.0 to 7.9%. A double 2 × 2 factorial design was used to further randomize participants; 4733 patients were randomly assigned to lower their blood pressure by receiving either intensive therapy or standard therapy. In addition, 5518 patients were randomly assigned to receive either fenofibrate or placebo while maintaining good control of LDL-C with simvastatin. The starting dose of open-labeled simvastatin was determined by presence of cardiovascular disease by randomization. Primary prevention participants started at a simvastatin dose of 20 mg/d. Secondary prevention participants started at a simvastatin dose of 40 mg/d. For participants starting at 20 mg/d of simvastatin, if the LDL-C is greater than 100 mg/dl (2.59 mmol/l), the daily dose of simvastatin was increased to 40 mg. Additionally, if a cardiovascular event occurs during follow-up, simvastatin dose for the participants will be increased to 40 mg/d.
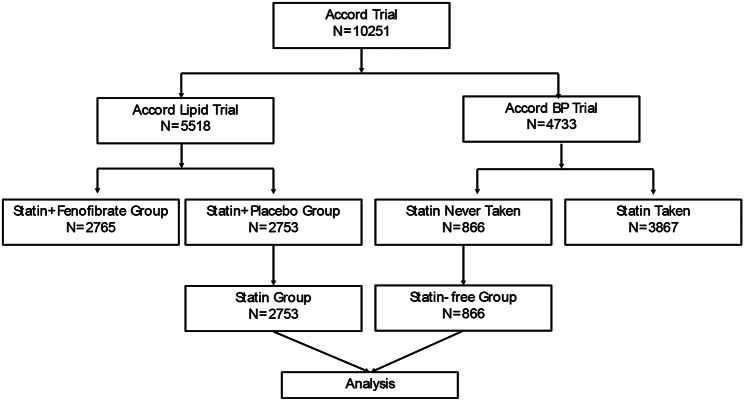
The ACCORD trial dataset examined is accessible from the NHLBI (https://biolincc.nhlbi.nih.gov/studies/accord/) upon formal request. This investigation received approval from Xiangya Hospital, Central South University. Based on initial and yearly reports on the usage of statins, individuals not on any statin therapy were categorized into the statin-free group (n = 866), whereas participants randomly designated to receive simvastatin alongside placebo within the lipid component of ACCORD were identified as the statin group (n = 2753). For a visual comparison between the statin and statin-free groups, refer to Fig. 1.
Fig. 1.
Study profile
Outcomes
The primary outcomes were development of CKD (steadily eGFR < 60 ml/min/1.73m2) and development of renal failure (initiation of dialysis or ESRD, or renal transplantation, or rise of serum creatinine > 291.72 umol/l in absence of an acute reversible cause). The secondary outcomes were development of renal function decline (doubling of baseline serum creatinine or more than 20 mL/min/1.73 m2 decrease in eGFR), development of microalbuminuria (urine albumin/creatinine ratio ≥ 30 mg/g), development of macroalbuminuria (urine albumin/creatinine ratio ≥ 300 mg /g).
Statistical analysis
Continuous variables were presented as means and standard deviations (SD). For distributions that were notably skewed, the median and interquartile range (IQR) were reported. Categorical variables were summarized by counts and percentages. The baseline characteristics of the statin and statin-free groups were compared utilizing two-sample t-tests, two-sample Wilcoxon tests, and chi-squared tests for contingency tables.
Incidence rates (per 100 person-years) were calculated for each group, factoring in the censoring of follow-up data. Kaplan-Meier estimates were employed to delineate cumulative incidence risk. The impact of simvastatin on primary and secondary outcomes was assessed using Cox proportional hazards models, presented as hazard ratios (HR) with 95% confidence intervals (CI). Initial analyses were unadjusted, while subsequent analyses adjusted for covariates. The covariates included all baseline variables that significantly differed between the statin and statin-free groups, excluding serum creatinine due to its relationship with the calculation of eGFR. Subsequently, covariates were filtered using LASSO regression (10-fold cross-validation) to retain only those with non-zero effects. The final analyses also accounted for differences in antihypertensive therapy, specifically the use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB), and changes in blood levels as time-dependent covariates, based on follow-up data.
The effect of simvastatin on primary outcomes within subgroups defined by baseline lipid profiles and renal disease status was examined by analyzing the interaction between subgroups and statin treatment in the most fully adjusted model (excluding covariates correlated with the grouping variable). Subgroups were segmented based on the tertiles of lipid indices to most effectively differentiate within subgroups. The urine albumin-to-creatinine ratio (UACR) was categorized by values of 30, 300 mg/g, and eGFR was grouped by 60, 90, 120 ml/min/1.73 m2.
The mediating effect of LDL-C reduction in the association between simvastatin and CKD was evaluated through mediation analysis. LDL-C reduction was represented by the baseline LDL-C level minus the time-weighted average of LDL-C. The proportion mediated (PM) was estimated, and the non-parametric bootstrap method (1000 draws) was used to calculate 95% CIs of the PM. All p values reported were two-sided, with a nominal significance threshold set at 0.05. Data analysis was performed using R software (version 4.2.1).
Results
Clinical characteristics of patients
The baseline characteristics of the patient cohort are delineated in Table 1, encompassing a total of 3619 individuals diagnosed with T2DM, of which 2753 were routinely administered simvastatin, while the remaining 866 did not undergo any statin therapy. The median duration of follow-up extended to 4.9 years (IQR 4.0-5.7), corresponding to 17,324 person-years. On average, participants had been living with diabetes for 9 years (IQR 5–15) and experienced hyperlipidemia for a duration of 4 years. The median eGFR was calculated to be 89.9 ml/min/1.73 m² (IQR 76.3-105.2).
Table 1.
Baseline characteristics of patients with and without simvastatin treatment
| Statin (n = 2753) | Statin-free (n = 866) | p | |
|---|---|---|---|
| Age, y | 62.8 ± 6.7 | 62.9 ± 6.4 | 0.79 |
| Sex, n (%) | < 0.01 | ||
| Female | 843(30.6) | 482(55.7) | |
| Male | 1910(69.4) | 384(44.3) | |
| Race, n (%) | < 0.01 | ||
| White | 1789(65.0) | 466(53.8) | |
| Black | 438(15.9) | 246(28.4) | |
| Hispanic | 194(7.0) | 54(6.2) | |
| Other | 332(12.1) | 100(11.5) | |
| BMI, kg/m2 | 32.4 ± 5.4 | 32.2 ± 6.0 | 0.45 |
| Duration of diabetes, y | 9(5,15) | 9(5,15) | 0.84 |
| HbA1c, % | 8.1(7.5,8.8) | 8.1(7.6,9.0) | 0.01 |
| Fasting plasma glucose, mg/dL | 169(139,204) | 170(138,209) | 0.27 |
| Duration of hypertension, y | 7(3,15) | 7(2,16) | 0.22 |
| SBP(BL), mmHg | 134.0 ± 17.9 | 140.5 ± 16.8 | < 0.01 |
| DBP(BL), mmHg | 74.0 ± 10.9 | 77.4 ± 10.7 | < 0.01 |
| Duration of hyperlipidemia, y | 4(2,8) | 4(1,9) | 0.86 |
| Triglycerides, mg/dL | 160(113,227) | 140(94,219) | < 0.01 |
| Total cholesterol, mg/dL | 175.7 ± 37.9 | 197.6 ± 46.0 | < 0.01 |
| HDL-C, mg/dL | 38.2 ± 7.8 | 47.6 ± 15.6 | < 0.01 |
| LDL-C, mg/dL | 101.1 ± 30.9 | 114.4 ± 39.0 | < 0.01 |
| VLDL-C, mg/dL | 32(23,45) | 28(19,44) | < 0.01 |
| Retinopathy, n (%) | 279(11.7) | 84(11.2) | 0.79 |
| UACR, mg/g | 14(7,44) | 15(7,49) | 0.38 |
| Serum creatinine, mg/dL | 0.9(0.8,1.0) | 0.8(0.7,1.0) | < 0.01 |
| eGFR, ml/min/1.73 m2 | 89.6(75.4,104.7) | 90.4(77.8,107.5) | < 0.01 |
| intensive glycemic control, n (%) | 1383(50.2) | 414(47.8) | 0.23 |
| ACEi/ARB, y | 4(2,5) | 4(1,5) | 0.34 |
| SBP(EXIT), mmHg | 130.2 ± 17.7 | 130.3 ± 18.7 | 0.82 |
| DBP(EXIT), mmHg | 68.9 ± 10.6 | 69.5 ± 10.9 | 0.15 |
BMI Body Mass Index; HbA1c Glycosylated Hemoglobin, Type A1c; SBP(BL) Systolic Blood Pressure (Baseline); DBP(BL) Diastolic Blood Pressure (Baseline); HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; VLDL-C Very low-density lipoprotein cholesterol; UACR Urine Albumin-to-Creatinine Ratio; eGFR estimated glomerular filtration rate; ACEi Angiotensin-converting enzyme inhibitor; ARB angiotensin II receptor blocker
Renal outcomes
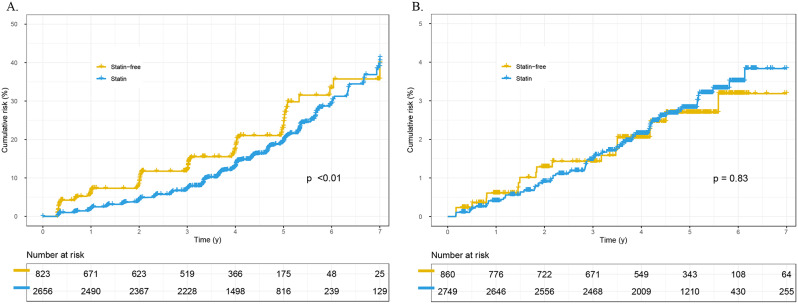
The impact of simvastatin on renal outcomes is detailed in Table 2. For CKD, the simvastatin group had an incidence rate of 3.9 per 100 person-years, compared to 4.9 in the statin-free group. The administration of simvastatin was associated with a significantly reduced risk of developing CKD, as evidenced in the unadjusted analysis (HR 0.75, 95% CI 0.62–0.91, p < 0.01) (Fig. 2A). This beneficial effect persisted after adjustment for baseline variables (HR 0.54, 95% CI 0.43–0.67, p < 0.01) and remained consistent upon full adjustment, including baseline variables plus time-dependent covariates (HR 0.29, 95% CI 0.27–0.31, p < 0.01). Regarding the other primary outcome, renal failure, the incidence rates were 0.56 per 100 person-years in the statin group and 0.53 per 100 person-years in the statin-free group, with no significant difference observed in the unadjusted analysis (HR 1.06, 95% CI 0.64–1.75, p = 0.83) (Fig. 2B). However, a significantly decreased risk of renal failure was noted in the statin group in the fully adjusted analysis (HR 0.53, 95% CI 0.44–0.65, p < 0.01). Additionally, statistical differences in risks of renal function decline and developing microalbuminuria were observed between the two groups, yet no statistical differences in the risk of developing macroalbuminuria were found after comprehensive adjustment.
Table 2.
Treatment effect of simvastatin versus placebo on primary and secondary outcomes
| Statin, n = 2753 | Statin-free, n = 866 | Statin vs. Statin-free | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events, n(%) | Annual rate, %/y | Events, n(%) | Annual rate, %/y | Unadjusted | Adjusted* | Adjusted# | ||||
| Hazard Ratio, (95%CI) | p | Hazard Ratio, (95%CI) | p | Hazard Ratio, (95%CI) | p | |||||
| Chronic kidney disease | 433(15.7) | 3.9 | 134(15.5) | 4.9 | 0.75 (0.62,0.91) | < 0.01 | 0.54 (0.43,0.67) | < 0.01 | 0.29(0.27,0.31) | < 0.01 |
| Renal failure | 72(2.6) | 0.56 | 19(2.2) | 0.53 | 1.06 (0.64,1.75) | 0.83 | 1.14 (0.65,2.01) | 0.65 | 0.53(0.44,0.65) | < 0.01 |
| Renal function decline | 1322(48) | 15.5 | 416(48) | 19.5 | 0.82 (0.73,0.91) | < 0.01 | 0.87 (0.79,0.99) | 0.04 | 0.54(0.52,0.56) | < 0.01 |
| Microalbuminuria | 456(16.6) | 6.1 | 87(10.0) | 4.4 | 1.40 (1.11,1.76) | < 0.01 | 1.31 (1.02,1.68) | 0.04 | 0.81(0.73,0.89) | < 0.01 |
| Macroalbuminuria | 189(6.9) | 1.7 | 38(4.4) | 1.3 | 1.37 (0.97,1.94) | 0.08 | 1.25 (0.85,1.83) | 0.26 | 1.05(0.91,1.22) | 0.49 |
* Adjusted for sex, race, HbA1c, SBP(BL), DBP(BL), HDL cholesterol, LDL cholesterol, VLDL cholesterol and eGFR
# Adjusted for sex, race, HbA1c, HDL cholesterol, LDL cholesterol, VLDL cholesterol, eGFR, SBP, DBP and ACEi/ARB, where SBP, DBP, and ACEI/ARB were treated as time-dependent covariates
Fig. 2.
Cumulative incidence of primary outcomes for patients with and without simvastatin treatment. (A) chronic kidney disease; (B) renal failure
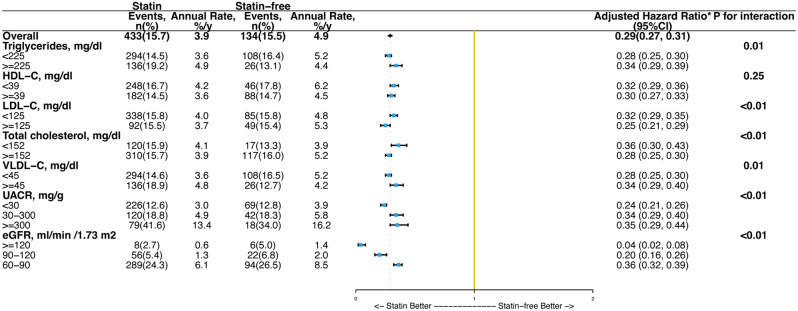
The effects of simvastatin on the primary outcome of CKD in subgroups defined by baseline lipid and kidney profiles are depicted in Fig. 3, and for the other primary outcome, renal failure, in Figure S1 (in the Supplementary Material). The relative effects of simvastatin on CKD and renal failure differed significantly across the majority of the profile. Nonetheless, the absolute effects of simvastatin on CKD were consistently reduced across all subgroups, including those categorized by triglycerides (HR 0.28 vs. 0.34, P for interaction = 0.01), HDL-C (HR 0.32 vs. 0.30, P for interaction = 0.25), or LDL-C (HR 0.32 vs. 0.25, P for interaction < 0.01).
Fig. 3.
Treatment effects of simvastatin versus placebo on chronic kidney disease in subgroups
The mediating effect of LDL-C reduction in the association between simvastatin and CKD is shown in Figure S2 (in the Supplementary Material). The reduction in LDL-C appears to be a potential mediator (PM 14.9%, 95% CI 0.11–0.19, p < 0.01).
Discussion
Our analyses demonstrated that administering simvastatin at dosages of 20 to 40 mg/day, adjusted based on cardiovascular disease presence and follow-up LDL-C levels, for an average duration of 4.9 years, significantly reduced the risk of CKD and renal failure compared to not using statin therapy in T2DM patients at elevated risk of cardiovascular disease, with a baseline median eGFR of 89.9 ml/min/1.73 m2. Furthermore, the analysis revealed significant variability in the treatment effect on CKD and renal failure based on baseline lipid levels, albuminuria, or eGFR levels, yet the risk of CKD consistently decreased across all subgroups. Additionally, LDL-C reduction appears to be a potential mediator of simvastatin’s protective effect on CKD.
This study represents a post hoc analysis of the ACCORD Trial. Uniquely, our analysis included a statin-free control group, a rarity in contemporary intervention studies due to the well-documented cardiovascular benefits of statin therapy in diabetes patients [9]. The absence of a placebo control group challenges the reliability and validity of findings. Furthermore, our study benefited from a large sample size and an extensive, nearly complete follow-up period.
Our findings suggest simvastatin’s beneficial effects on kidney function, particularly in reducing CKD and renal failure among T2DM patients. This study concentrated on the impact on clinically relevant renal events rather than solely on eGFR decline. Our results align with those from the Heart Protection Study (HPS), which found that simvastatin significantly mitigated the decrease in eGFR during follow-up in diabetic and non-diabetic individuals alike [10]. Similarly, the Scandinavian Simvastatin Survival Study (4 S) showed that simvastatin significantly reduced the frequency of a ≥ 25% decline in kidney function. However, it did not significantly affect kidney function in participants who developed CKD by the end of the follow-up [11]. Statins, including atorvastatin, pravastatin, and simvastatin, displayed a trend toward reducing the risk of kidney failure, with a combined risk reduction for renal failure of 6% (95% CI, 1–12%), whereas other statins did not exhibit similar effects [5]. Therefore, we advocate for the use of statins for their renal benefits, emphasizing the reduction of clinically relevant renal events and the thoughtful selection of statin type in T2DM patients.
Our subgroup analysis revealed that the protective effect of simvastatin on CKD and renal failure in patients with T2DM varies across different baseline lipid and renal profiles. Existing evidence indicates that diabetes and CKD are often associated with an altered lipid profile, typically characterized by low HDL-C and elevated triglyceride levels [12, 13]. Although in patients with typical diabetic or CKD lipid profiles—low HDL-C and high triglycerides—the protective effect of simvastatin on CKD appeared slightly less pronounced, its effect remained consistently beneficial across all subgroups. This finding supports the use of simvastatin to reduce the risk of CKD in T2DM patients, regardless of their lipid profile or renal function status.
Our mediator analyses suggest that LDL-C reduction mediates the protective effect of simvastatin on CKD. Indeed, high LDL-C levels have been both observationally and genetically associated with increased risks of CKD, indicating a potential causal role of LDL-C in the pathogenesis of the disease [14]. The mechanisms behind lipid-induced renal damage are centered on lipotoxicity, where excessive lipid accumulation leads to cellular dysfunction and injury, particularly affecting renal tubular epithelial cells, renal interstitial cells, and glomerular podocytes. This process is closely linked to the progression of diabetic nephropathy [15]. However, as previously mentioned, not all LDL-C -lowering statins offer renal protection. Our study shows that LDL-C reduction only partially mediates this protective effect, suggesting that simvastatin may have renal benefits independent of LDL-C reduction. Recent studies have demonstrated the senolytic activity of lipophilic statins—specifically simvastatin, lovastatin, and atorvastatin—on cultured human endothelial cells [16]. Further research is needed to explore the underlying mechanisms of simvastatin’s potential renal protective effects.
The limitation of our analysis is that most ACCORD participants had normal kidney function at baseline, leading to a limited number of renal failure events. Nevertheless, the significant effect of simvastatin on this outcome, even after comprehensive adjustments, warrants further investigation to confirm its robustness. Additionally, unlike the original ACCORD trial design, this study focused on assessing the renal effects of simvastatin, excluding patients on fenofibrate to avoid potential synergistic or confounding effects. While this approach allows for a more accurate evaluation of simvastatin’s impact, it also reduces the sample size and may introduce selection bias. These limitations should be considered when interpreting the findings.
In conclusion, using statins, particularly simvastatin, in individuals with T2DM at high risk of cardiovascular disease, is likely to offer additional renal benefits, especially in reducing clinically relevant renal events such as CKD and renal failure. These findings have important implications given the burden of diabetes and the urgent need for effective therapies to slow the progression of significant renal endpoints.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful for the contributions BioLINCC and the ACCORD group made in data collection and sharing. We thank Ruiyang Xiao from Institute of Environmental Engineering, School of Metallurgy and Environment at Central South University for priceless comments on the manuscript.
Author contributions
J. P., M. G., Q. Y., L. T., and Z. P. played pivotal roles in conceptualizing and designing the study. J. P. and J. T. were instrumental in data curation and conducting the statistical analysis. The interpretation of the data was a collaborative effort by J. P., P. Y., and Z. P. The initial draft of the manuscript was penned by J. P., enriched with contributions from all co-authors. The manuscript’s final version received approval from J. P., J. Y., and Z. P. As the guarantor, Z. P. ensured comprehensive access to all the data, upholding the data’s integrity and the accuracy of the analysis.
Funding
This study is funded by the Major Program of the National Natural Science Foundation of China (82090024), the General Programs of the National Natural Science Foundation of China (82173877), the Key Research and Development Program of Hunan Province (2021SK2015), the Outstanding Youth Foundation of the Natural Science Foundation of Hunan Province (2022JJ10100), and Hunan Provincial Natural Science Foundation General Program (2024JJ5571). The views expressed are those of the authors.
Data availability
The ACCORD trial dataset examined is accessible from the NHLBI (https://biolincc.nhlbi.nih.gov/studies/accord/) upon formal request.
Declarations
Ethics approval and consent to participate
This investigation received approval from Xiangya Hospital, Central South University (Study number 202210011) and conducted according to the Declaration of Helsinki. Participants had previously given informed consent for the whole project [8].
Consent for publication
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, and have given their approval for this version to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15(7):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, et al. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med. 1998;104(2A):S14–8. [DOI] [PubMed] [Google Scholar]
- 3.Silverman MG, et al. Association between lowering LDL-C and Cardiovascular Risk Reduction among different therapeutic interventions: a systematic review and Meta-analysis. JAMA. 2016;316(12):1289–97. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Professional Practice. 10. Cardiovascular Disease and Risk Management: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144–74. [DOI] [PubMed] [Google Scholar]
- 5.Su X, et al. Effect of statins on kidney Disease outcomes: a systematic review and Meta-analysis. Am J Kidney Dis. 2016;67(6):881–92. [DOI] [PubMed] [Google Scholar]
- 6.Esmeijer K, et al. Effect of different types of statins on kidney function decline and proteinuria: a network meta-analysis. Sci Rep. 2019;9(1):16632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanifer JW, et al. Benefit of Ezetimibe added to simvastatin in reduced kidney function. J Am Soc Nephrol. 2017;28(10):3034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group AS, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Zeeuw D, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3(3):181–90. [DOI] [PubMed] [Google Scholar]
- 10.Collins R, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16. [DOI] [PubMed] [Google Scholar]
- 11.Huskey J, et al. Effect of simvastatin on kidney function loss in patients with coronary heart disease: findings from the scandinavian simvastatin survival study (4S). Atherosclerosis. 2009;205(1):202–6. [DOI] [PubMed] [Google Scholar]
- 12.Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speer T, et al. Lipoproteins in chronic kidney disease: from bench to bedside. Eur Heart J. 2021;42(22):2170–85. [DOI] [PubMed] [Google Scholar]
- 14.Emanuelsson F, et al. Impact of LDL Cholesterol on Microvascular Versus Macrovascular Disease: a mendelian randomization study. J Am Coll Cardiol. 2019;74(11):1465–76. [DOI] [PubMed] [Google Scholar]
- 15.Nishi H, Higashihara T, Inagi R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients, 2019. 11(7). [DOI] [PMC free article] [PubMed]
- 16.Belakova B et al. Lipophilic statins eliminate senescent endothelial cells by inducing Anoikis-related cell death. Cells, 2023. 12(24). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ACCORD trial dataset examined is accessible from the NHLBI (https://biolincc.nhlbi.nih.gov/studies/accord/) upon formal request.