Abstract
Background
Nasal continuous positive airway pressure (NCPAP) is widely used for premature infants with respiratory distress syndrome (RDS). A high-flow nasal cannula (HFNC) provides positive end-expiratory pressure using high-flow oxygen; however, the variability in distending pressure is a primary concern. This study evaluated the feasibility and safety of a newly designed protocol for NCPAP weaning with cyclic HFNC use for premature infants.
Methods
Premature infants with RDS using NCPAP support who were ready for weaning were enrolled. The weaning protocol used cyclic NCPAP with HFNC every 3 h for 3 days in the neonatal intensive care unit. The heart rate (HR), respiratory rate (RR), pulse oximetry (SpO2), transcutaneous carbon dioxide (PtcCO2), and cerebral tissue oxygen saturation (StO2) at the end of NCPAP with HFNC support were recorded once daily for 3 days.
Results
From June 2019 to April 2021, 46 premature infants (27 male, 19 female) were enrolled. The mean gestational age and birth body weight were 28.7 ± 2.6 weeks and 1181 ± 354 g, respectively. No statistically significant differences in the HR, RR, SpO2, and cerebral StO2 during NCPAP weaning with HFNC were observed. However, the mean PtcCO2 with NCPAP was statistically significantly lower than that with HFNC (46.9 ± 6.0 mmHg vs. 47.9 ± 6.4 mmHg, P = 0.02).
Conclusions
The feasibility and safety of the NCPAP weaning protocol with cyclic HFNC for premature infants are acceptable in this preliminary study. Due to the limited number of participants, further studies are required for more comprehensive analysis.
Trial registration
This prospective observational case study was approved by the Human Experiment and Ethics Committee of our hospital (approval number: KMUHIRB-SV(I)-20180059; approval date: January 11, 2019).
Keywords: Nasal continuous positive airway pressure, High-flow nasal cannula, Premature infant
Background
Respiratory distress syndrome (RDS) is a common cause of neonatal intensive care unit (NICU) admission for premature infants [1–3]. Non-invasive respiratory support for premature infants includes nasal intermittent positive pressure ventilation, nasal continuous positive airway pressure (NCPAP), and high-flow nasal cannula (HFNC) use. NCPAP has been widely used to treat RDS in premature infants by providing continuous positive pressure to prevent alveolar prolapse and stabilize functional residual capacity [1–3]. However, the need for prongs that completely fit the nostrils may damage the nasal mucosa and septum, and the higher positive airway pressure may also induce complications such as pneumothorax, pneumomediastinum, and abdominal distension [1, 2].
Conversely, the HFNC provides positive end-expiratory pressure (PEEP) using high-flow oxygen (2–8 L/min) for infants [1, 2, 4, 5]. The advantages of the HFNC include a lower risk of injury to the nares, increased comfort, and ease of use. However, the variability in PEEP is one of the primary concerns associated with HFNC use [1, 2, 4, 5]. The HFNC has been considered an NCPAP weaning device (step-down) for premature infants [1, 2, 4, 5]. This study aimed to design and evaluate the feasibility and safety of a new protocol involving cyclic HFNC use for NCPAP weaning among premature infants.
Methods
Study population
We designed a prospective observational study involving premature infants (gestational age [GA] < 37 weeks) with RDS using NCPAP support who were ready for weaning. Participants were enrolled between June 6, 2019 and April 13, 2021. The evaluation criteria for NCPAP weaning included a fraction of inspired oxygen (FiO2) of 0.21, PEEP of 4–5 cm H2O, with relatively stable vital signs, and no episodes of apnea while supported by NCPAP support for 3 days. The exclusion criteria were term infants and preterm infants with major birth defect. The major birth defect included chromosome anomalies, congenital heart defects, neural tube defects, and congenital gastrointestinal defects (such as gastroschisis or omphalocele).
Ethics
This prospective observational case study was approved by the Human Experiment and Ethics Committee of our hospital (approval number: KMUHIRB-SV(I)-20180059; approval date: January 11, 2019). All experiments were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from the parents of the included premature infants.
Study design
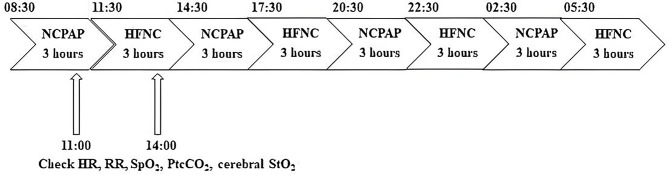
The 3-day weaning protocol comprised alternating 3-hour sessions of NCPAP and cyclic HFNC in each participant in the NICU at our hospital, beginning from 08:30 AM with NCPAP. The flow rate of HFNC was adjusted to match the pressure levels during its use, as measured by a GiO Digital Pressure Gauge (GIO 6, GaleMed Corporation, Taipei, Taiwan) at the distal end of the nasal cannula. The pressure was set to correspond with the PEEP used during the preceding NCPAP treatment. The flow rate of HFNC was maintained at 4–6 L per minute throughout the study. The heart rate (HR), respiratory rate (RR), pulse oximetry (SpO2), transcutaneous carbon dioxide (PtcCO2), and cerebral tissue oxygen saturation (StO2) were recorded for 0.5 h. Then, the respiratory support was changed to HFNC use for 3 h starting at 11:30 AM. We again recorded the HR, RR, SpO2, PtcCO2, and cerebral StO2 for 0.5 h (Fig. 1). The primary outcomes were the differences in the mean values of HR, RR, SpO2, PtcCO2, and cerebral StO2 recorded during NCPAP and HFNC. After cyclic use for 3 days, neonatologists in the NICU evaluated the clinical condition of the infants and assessed if they could tolerate continuous HFNC support until complete weaning was achieved.
Fig. 1.
Daily weaning protocol for 3 days
This cyclic approach was chosen in line with the standard nursing care schedule in our NICU, which follows a 3-hour cycle. We also believed that providing respiratory support via HFNC in 3-hour intervals would offer more consistent support to preterm infants than the shorter cycles would [6]. The initiation of cyclic HFNC use for 3 days in enrolled preterm infants was based on the inclusion criterion of no apnea occurrences while on NCPAP support for 3 consecutive days. We monitored the frequency of apnea during the cyclic use of NCPAP and HFNC. The absence of apnea during the 3-day cyclic period was considered indicative of successful weaning from NCPAP to HFNC.
However, some infants could not successfully wean from NCPAP using the HFNC during the protocol due to observed respiratory distress, which included a heart rate (HR) > 160 beats per min, a respiratory rate (RR) over 60 cycles per min, an oxygen saturation (SpO2) below 90%, or PtcCO2 above 60 mmHg during the 3-day weaning period. Therefore, we further divided the study participants into those successfully weaned from NCPAP using the HFNC within 3 days (success group) and those weaned after > 3 days (failure group).
Data on the sex, GA, birth body weight (BW), delivery mode, Neonatal Therapeutic Intervention Scoring System score, Apgar score, medication used to treat apnea, respiratory therapy condition, post-menstrual age (PMA), BW when starting weaning from NCPAP, apnea frequency, vital sign changes, and possible adverse effects associated with the HFNC of all enrolled infants were collected.
Study devices
This study used the Optiflow System HFNC (Fisher & Paykel Optiflow System Healthcare, Auckland, New Zealand); short binasal prongs with different sizes were chosen based on the infant’s BW. NCPAP was administered using the Babi.Plus® Bubble CPAP system (GaleMed Corporation, Taipei, Taiwan). The SenTec Digital Monitoring System (SenTec AG, Therwil, Switzerland) was used to measure PtcCO2. The FORE-SIGHT Oximeter MC-2000 Series Cerebral Oximeter (CAS Medical Systems, Inc., Branford, CT, USA) was used to measure cerebral StO2.
Statistics
Data recording and evaluation were performed using JMP 10 software (SAS Institute Inc., Cary, NC, USA). The rank sum test was performed to compare numerical variables of primary outcomes during NCPAP and HFNC and the characteristics and clinical outcomes in the success and failure groups. Univariate regression analyses were performed to analyze the factors associated with the PtcCO2 of preterm infants using NCPAP and HFNC. The chi-square test was performed to compare categorical variables in the success and failure groups. In contrast, the rank sum test was performed to compare numerical variables of primary outcomes, respiratory outcomes, and prognoses in the success and failure groups.
Results
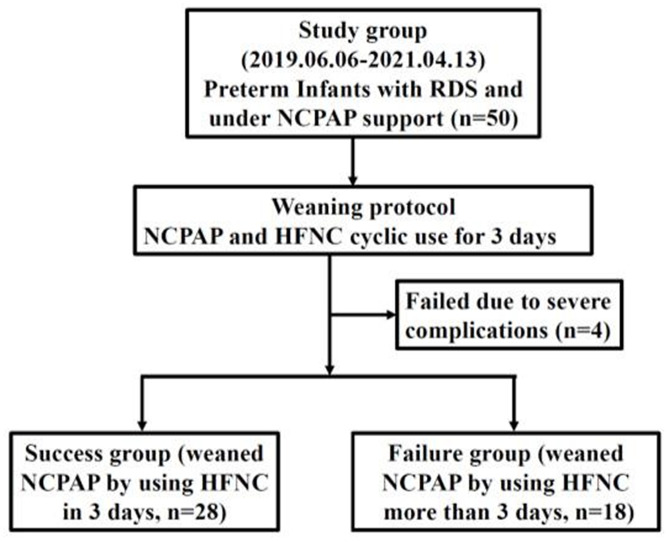
Fifty premature infants admitted to the NICU at our university hospital who met the inclusion criteria underwent the weaning protocol after written informed consent was received from their parents. Four participants were excluded due to severe complications, such as severe pulmonary hypertension or hydrocephalus (Fig. 2). Therefore, 46 participants were included in this study. No adverse effects were observed among the remaining 46 infants. The mean GA was 28.7 weeks (standard deviation (SD), ± 2.6 weeks), whereas the mean birth BW was 1181 g (SD, ± 354 g). The characteristics and clinical outcomes of the 46 infants are shown in Table 1.
Fig. 2.
Flow chart of the study design
Table 1.
Characteristics and clinical outcomes of infants
| Item | N | % |
|---|---|---|
| Premature | 46 | 100 |
| GA (weeks) | ||
| < 28 | 18 | 39 |
| 28 to < 32 | 22 | 48 |
| Birth BW (g) | ||
| ≤ 1000 | 16 | 35 |
| 1001–1499 | 20 | 43 |
| PMA (weeks) when involved | ||
| < 37 | 13 | 28 |
| Male | 27 | 59 |
| VD | 29 | 6 |
| NTISS score ≥ 15 | 42 | 91 |
| 5-min Apgar score ≤ 7 | 18 | 39 |
| Respiratory distress syndrome | ||
| Grade 2 | 11 | 24 |
| Grade 3 | 26 | 57 |
| Grade 4 | 9 | 19 |
| Surfactant usage | 23 | 50 |
| Intubation after birth | 20 | 43 |
| Medicine for apnea | ||
| Theophylline | 20 | 43 |
| Caffeine | 16 | 35 |
| Packed RBC transfusion | 36 | 78 |
| Moderate-to-severe BPD | 20 | 43 |
| PDA | 30 | 65 |
| Without treatment | 6 | 13 |
| With conservative treatment | 24 | 52 |
| Late-onset sepsis | 16 | 35 |
| Blood culture proved | 9 | 20 |
| Clinical diagnosis | 7 | 15 |
| Hypotension | 12 | 26 |
| IVH grade ≥ III | 3 | 7 |
| ROP stage ≥ III | 7 | 15 |
| NEC stage ≥ II | 1 | 2 |
GA, gestational age; BW, birth body weight; PMA, post-menstrual age; VD, vaginal delivery; NTISS, Neonatal Therapeutic Intervention Scoring System; RBC, red blood cell; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis
The mean PMA during NCPAP weaning was 35.6 weeks (SD, ± 2.3 weeks), and the mean BW was 1990 g (SD, ± 527 g). The mean apnea frequency during the 3-day cyclic use of NCPAP and HFNC was less than once daily. The primary outcomes (mean ± SD) of 46 enrolled infants, in whom NCPAP and HFNC support were administered, revealed no statistical differences in the HR (162 ± 13 beats per min under NCPAP, 163 ± 13 beats per min under HFNC, P = 0.34), RR (46 ± 12 cycles per min under NCPAP, 46 ± 13 cycles per min under HFNC, P = 0.71), SpO2 (97 ± 3% under NCPAP, 97 ± 3% under HFNC, P = 0.46), and cerebral StO2 (75.3 ± 5.8% under NCPAP, 74.9 ± 5.2% under HFNC, P = 0.58) during NCPAP weaning with HFNC. The median of SpO2 and cerebral StO2 under NCPAP and HFNC were 97% vs. 97% and 75.8% vs. 75.5%. The PtcCO2 was higher in this population with HFNC support (47.9 ± 6.4 mmHg) than with NCPAP support (46.9 ± 6.0 mmHg; P = 0.02).
Table 2 shows the associated factors influencing the differences in PtcCO2 with cyclic use of NCPAP and HFNC on univariate regression analysis. None of these factors affected the differences in PtcCO2.
Table 2.
Factors influencing differences in PtcCO2 with the use of NCPAP and HFNC support
| Differences in PtcCO2 (HFNC and NCPAP) | ||||
|---|---|---|---|---|
| Item | Regression coefficient | Lower 95% CI | Upper 95% CI |
P-value |
| GA | -0.104 | -0.424 | 0.215 | 0.52 |
| BBW | -0.001 | -0.003 | 0.002 | 0.52 |
| Female-male* | 1.083 | -0.556 | 2.722 | 0.19 |
| CS-VD† | -0.634 | -2.313 | 1.044 | 0.46 |
| NTISS | -0.002 | -0.208 | 0.204 | 0.98 |
| 1-min Apgar score | 0.032 | -0.358 | 0.422 | 0.87 |
| 5-min Apgar score | 0.023 | -0.329 | 0.375 | 0.89 |
| RDS grade | 0.918 | -0.306 | 2.142 | 0.14 |
| Surfactant usage | -1.017 | -2.632 | 0.597 | 0.22 |
| Intubation after birth | -0.624 | -2.424 | 1.176 | 0.49 |
| Apnea medication | ||||
| Used-not used‡ | -1.582 | -3.533 | 0.368 | 0.11 |
| Caffeine-theophylline§ | -0.508 | -2.296 | 1.280 | 0.58 |
| Packed RBC transfusion | 1.054 | -0.906 | 3.014 | 0.29 |
| Moderate-to-severe BPD | 0.289 | -1.348 | 1.926 | 0.73 |
| PDA | ||||
| Without Tx | -0.933 | -3.339 | 1.472 | 0.44 |
| With conservative Tx | -0.001 | -1.626 | 1.624 | 0.99 |
| Late-onset sepsis | 0.886 | -0.812 | 2.584 | 0.30 |
| Blood culture-proven | 0.147 | -1.899 | 2.193 | 0.89 |
| Clinical diagnosis | 1.548 | -0.697 | 3.793 | 0.18 |
| Hypotension | 0.202 | -1.646 | 2.051 | 0.83 |
| IVH grade ≥ III | -0.226 | -3.515 | 3.062 | 0.89 |
| ROP stage ≥ III | 0.728 | -1.529 | 2.985 | 0.53 |
| NEC stage ≥ II | 0.601 | -4.965 | 6.168 | 0.83 |
*Female infants compared with male ones
†CS compared with VD
‡Used compared with not used
§Caffeine compared with aminophylline
NCPAP, nasal continuous positive airway pressure; HFNC, high-flow nasal cannula; PtcCO2, transcutaneous carbon dioxide; CI, confidence interval; GA, gestational age; BBW, birth body weight; CS, cesarean section; VD, vaginal delivery; NTISS, Neonatal Therapeutic Intervention Scoring System; RDS, respiratory distress syndrome; RBC, red blood cell; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; Tx, treatment; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis
Eighteen premature infants could not be completely weaned from NCPAP to HFNC after 3 days of protocol and required an extended period of weaning (defined as the failure group). There were no statistical differences in infants’ characteristics and clinical outcomes in the success and failure groups (Table 3). The mean apnea frequency during the 3-day course of cyclic use of NCPAP and HFNC in both groups was less than once daily.
Table 3.
Characteristics and clinical outcomes of enrolled infants in the success and failure groups
| Item | *Success group (n = 28) | †Failure group (n = 18) | P-value |
|---|---|---|---|
| GA (weeks) (mean ± SD) | 29.2 ± 2.4 | 27.9 ± 2.6 | 0.16 |
| Birth BW (g) (mean ± SD) | 1201 ± 317 | 1150 ± 412 | 0.44 |
| PMA at the beginning of weaning (weeks) (mean ± SD) | 35.3 ± 2.4 | 35.9 ± 2.2 | 0.33 |
| Male, n (%) | 17 (61) | 10 (56) | 0.73 |
| Vaginal delivery, n (%) | 17 (61) | 13 (72) | 0.68 |
| NTISS (mean ± SD) | 18 ± 4 | 20 ± 4 | 0.14 |
| 1-min Apgar score (mean ± SD) | 4 ± 2 | 4 ± 2 | 0.91 |
| 5-min Apgar score (mean ± SD) | 6 ± 2 | 6 ± 2 | 0.74 |
| RDS grade (mean ± SD) | 3 ± 0 | 3 ± 1 | 0.35 |
| Surfactant usage | 13 (46) | 10 (56) | 0.55 |
| Intubation after birth | 12 (43) | 8 (44) | 0.92 |
| Medicine for apnea, n (%) | |||
| Theophylline | 11 (39) | 9 (50) | 0.47 |
| Caffeine | 11 (39) | 5 (28) | 0.42 |
| Packed RBC transfusion | 21 (58) | 15 (42) | 0.50 |
| Moderate-to-severe BPD, n (%) | 10 (36) | 10 (56) | 0.19 |
| PDA, n (%) | 17 (61) | 13 (72) | 0.42 |
| Without Tx | 3 (11) | 3 (17) | 0.56 |
| With conservative Tx | 14 (50) | 10 (55) | 0.71 |
| Late-onset sepsis, n (%) | 7 (25) | 9 (50) | 0.08 |
| Blood culture proved | 3 (11) | 6 (33) | 0.059 |
| Clinical diagnosis | 4 (14) | 3 (17) | 0.83 |
| Hypotension, n (%) | 7 (25) | 5 (28) | 0.83 |
| IVH grade ≥ III, n (%) | 2 (7) | 1 (6) | 0.83 |
| ROP stage ≥ III, n (%) | 5 (18) | 2 (11) | 0.53 |
| NEC stage ≥ II, n (%) | 1 (4) | 0 (0) | 0.42 |
*Success group: infants weaned from NCPAP successfully using the HFNC for 3 days
†Failure group: infants weaned from NCPAP using the HFNC for > 3 days
GA, gestational age; SD, standard deviation; BW, body weight; PMA, post-menstrual age; NTISS, Neonatal Therapeutic Intervention Scoring System; RDS, respiratory distress syndrome; RBC, red blood cell; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; Tx, treatment; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis
Table 4 presents the primary outcomes of infants in the success and failure groups with NCPAP or HFNC support. The median of SpO2 under NCPAP or HFNC in the success and failure groups were 98% vs. 97% and 98% vs. 97%, respectively. The median of cerebral StO2 under NCPAP or HFNC in the success and failure groups were 76.0% vs. 75.7% and 75.9% vs. 74.6%, respectively. The cerebral StO2 with HFNC support was lower for infants in the failure group than in the other groups.
Table 4.
Primary outcomes of enrolled infants in the success and failure groups
| Respiratory support | Item | Mean ± SD | P-value | |
|
*Success group (n = 28) |
†Failure group (n = 18) | |||
| NCPAP | HR (bpm) | 165 ± 12 | 158 ± 13 | < 0.001* |
| RR (cpm) | 46 ± 13 | 45 ± 11 | 0.75 | |
| SpO2 (%) | 97 ± 2 | 97 ± 3 | 0.84 | |
| PtcCO2 (mmHg) | 46.6 ± 6.0 | 47.4 ± 6.1 | 0.89 | |
| Cerebral StO2 (%) | 75.8 ± 5.2 | 75.2 ± 5.7 | 0.40 | |
| HFNC | HR (bpm) | 166 ± 12 | 159 ± 14 | 0.008* |
| RR (cpm) | 44 ± 12 | 47 ± 13 | 0.25 | |
| SpO2 (%) | 97 ± 2 | 96 ± 3 | 0.13 | |
| PtcCO2 (mmHg) | 47.2 ± 6.3 | 49.1 ± 6.3 | 0.09 | |
| Cerebral StO2 (%) | 75.7 ± 5.6 | 73.9 ± 4.4 | 0.023* | |
*Success group: infants weaned from NCPAP successfully using the HFNC for 3 days
†Failure group: infants weaned from NCPAP using the HFNC for > 3 days
NCPAP, nasal continuous positive airway pressure; HFNC, high-flow nasal cannula; SD, standard deviation; HR, heart rate; bpm, beats per minute; RR, respiratory rate; cpm, cycles per minute; SpO2, pulse oximetry; PtcCO2, transcutaneous carbon dioxide; StO2, tissue oxygen saturation
Table 5 shows infants’ respiratory outcomes and prognoses in the success and failure groups. The failure group required more NCPAP weaning days, HFNC usage days, and total days of respiratory therapy. The PMA at discontinuation of NCPAP and HFNC was greater in the failure group than in the success group.
Table 5.
Respiratory outcomes and prognoses of enrolled infants in the success and failure groups
| Mean ± SD | |||
|---|---|---|---|
| Item | *Success group (n = 28) | †Failure group (n = 18) | P-value |
| PMA at the beginning of weaning (weeks) | 35.3 ± 2.4 | 35.9 ± 2.2 | 0.33 |
| BW at the beginning of weaning (g) | 1907 ± 500 | 2121 ± 564 | 0.13 |
| NCPAP weaning days‡ | 3 ± 0 | 13 ± 14 | < 0.001* |
| Total days of NCPAP usage | 30 ± 16 | 39 ± 18 | 0.09 |
| PMA at discontinuation of NCPAP (weeks) | 35.7 ± 2.4 | 37.9 ± 3.3 | 0.04* |
| HFNC usage days§ | 24 ± 16 | 34 ± 22 | 0.03* |
| PMA at discontinuation of HFNC (weeks) | 38.8 ± 3.8 | 41.6 ± 4.7 | 0.03* |
| Length of hospital stay (days) | 88 ± 30 | 110 ± 46 | 0.07 |
| PMA at discharge (weeks) | 41.7 ± 3.5 | 43.5 ± 4.8 | 0.15 |
| Total days of respiratory therapy‖ | 69 ± 28 | 96 ± 44 | 0.03* |
*Success group: infants weaned from NCPAP successfully using the HFNC for 3 days
†Failure group: infants weaned from NCPAP using the HFNC for > 3 days
‡Duration between the start and end of weaning from NCPAP (d)
§Total days of HFNC usage after weaning from NCPAP (d)
‖Total days of respiratory therapy support (including ventilator, nasal intermittent positive pressure ventilation, NCPAP, and HFNC) during admission
SD, standard deviation; PMA, post-menstrual age; BW, body weight; NCPAP, nasal continuous positive airway pressure; HFNC, high-flow nasal cannula
Discussion
Our study investigated the feasibility and safety of the NCPAP weaning protocol with the cyclic use of an HFNC. This protocol showed no differences in HR, RR, SpO2, and cerebral StO2; however, the PtcCO2 was higher during HFNC use than during NCPAP use. No adverse effects were observed during this study.
The differences in PtcCO2 among premature infants during NCPAP and HFNC use in our study may be associated with lower respiratory tract support pressure stability with the HFNC than with NCPAP [4, 5]. Although the difference in PtcCO2 levels between HFNC and NCPAP support was statistically significant—with PtcCO2 being slightly higher in HFNC support (47.9 ± 6.4 mmHg) compared to NCPAP (46.9 ± 6.0 mmHg; P = 0.02)—the clinical relevance of this difference may be considered minor. This suggests that while NCPAP provides more stable expiratory pressure and thus more effective CO2 washout, HFNC still serves as a viable option for weaning from NCPAP due to its sufficient support capabilities. Notably, most studies have focused on comparing NCPAP and HFNC support as the main respiratory therapy for premature infants after birth. Lampland et al. found no differences in the HR and arterial oxygen saturation during NCPAP and HFNC use in premature infants, but the respiratory rate was higher with the HFNC [7]. However, Taha et al. reported that HFNC use resulted in higher mortality and bronchopulmonary dysplasia rates, prolonged hospital stay, and longer time for oral feeding than NCPAP use among newborns [8]. Therefore, the American Academy of Pediatrics suggested HFNC support as an alternative respiratory therapy for infants after extubation rather than the main respiratory therapy for premature infants after birth [9, 10]. As previously mentioned, HFNC support has been suggested as an accompanying respiratory therapy for NCPAP weaning in premature infants because of the variable stability of the airway support pressure [1–3, 5, 11–15]. Consequently, we inferred that the PtcCO2 was higher with HFNC support than with NCPAP support, possibly due to the greater stability of the airway support pressure of NCPAP.
There were no obvious differences in cerebral StO2 with NCPAP or HFNC support among the 46 premature infants in our study. Sett et al. found no obvious differences in cerebral StO2 when performing NCPAP weaning with HFNC use in premature infants [16]. Bemdich et al. found no influence of cerebral StO2 and cerebral blood flow with PEEP of 3–8 cm H2O [17]. Combining our findings with those of the literature mentioned above, it is evident that using the HFNC for NCPAP weaning did not influence cerebral StO2. Therefore, the SpO2 and cerebral StO2 did not negatively influence premature infants when the HFNC was used during the NCPAP weaning process, which indicates the safety of the cyclic use of HFNC for NCPAP weaning.
Our study design involved the cyclic use of NCPAP and HFNC every 3 h for NCPAP weaning for premature infants with RDS; however, 18 (39%) premature infants required cyclic use for > 3 days to achieve complete NCPAP weaning (failure group). We inferred that lower GA, lower BW, higher NTISS, and late-onset sepsis may be associated with a higher failure rate due to the unstable condition of premature infants. However, the results showed no significant differences in clinical outcomes and complications, which may have been influenced by the small sample size (Table 3). Additionally, we were unable to measure lung function directly in these preterm infants, some of whom might inherently have delayed lung maturation. A lower cerebral StO2 was observed in the failure group during NCPAP weaning using the HFNC for 3 days, indicating more unstable oxygenation with HFNC support (Table 4); therefore, the infants required more number of days to achieve successful NCPAP weaning (Table 5). Sett et al. found no obvious differences in the cerebral StO2 when performing NCPAP weaning with 6 cm H2O using the HFNC at 8 L/min; however, they adjusted the fraction of inspired oxygen for the HFNC to maintain the SpO2 at approximately 92–95%, which could be why their results were different from ours [16]. The PMA at discontinuation of NCPAP or HFNC use was older, and the total number of days of respiratory therapy was longer in the failure group (Table 5). The length of hospitalization and PMA at discharge were higher in the failure group, and the longer respiratory therapy course might influence both groups. However, the statistical results showed no obvious difference between the success and failure groups, which might be associated with the small sample size in our study (Table 5).
This study had some limitations. The small sample size might have influenced the analysis results. In this prospective study, the HFNC devices were funded by Kaohsiung Medical University Hospital. Due to the limitations of the grant, we were only able to purchase approximately 50 devices. Additionally, this study is structured similarly to a cross-over study, rather than a comparative study, which precludes calculating the difference in treatment effects. As a result, this is a preliminary study. Previously, using HFNC was self-paid in Taiwan; however, because the health insurance of Taiwan began covering the use of the HFNC in 2022, further analyses with larger sample sizes can be considered in the future.
Conclusions
The feasibility and safety of the weaning protocol from NCPAP using cyclic HFNC support for premature infants are acceptable. During the weaning process, there were no significant changes in HR, RR, SpO2, or StO2, although PtcCO2 levels were higher with HFNC support. No adverse effects related to HFNC use were observed, and there was no increase in apnea frequency. However, due to the limited number of participants, further studies with larger samples are required for more comprehensive analysis.
Acknowledgements
Not applicable.
Abbreviations
- RDS
Respiratory distress syndrome
- NICU
Neonatal intensive care unit
- NCPAP
Nasal continuous positive airway pressure
- HFNC
High-flow nasal cannula
- PEEP
Positive end-expiratory pressure
- GA
Gestational age
- FiO2
Fraction of inspired oxygen
- HR
heart rate
- RR
Respiratory rate
- SpO2
Pulse oximetry
- PtcCO2
Transcutaneous carbon dioxide
- StO2
Cerebral tissue oxygen saturation
- BW
Body weight
- PMA
Post-menstrual age
- SD
Standard deviation
Author contributions
S.-T.Y. and H.-L.C conceived and designed of the study. S.-T.Y. and H.-W.C acquired the data of the study. S.-T.Y. and H.-L.C analyzed and interpreted the data of the study. S.-T.Y. drafted the manuscript. H.-L.C revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was supported by grants from our hospital in 2018 (KMUH107-7M26 and KMUH107-7R44).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This prospective observational case study was approved by the Human Experiment and Ethics Committee of our hospital (approval number: KMUHIRB-SV(I)-20180059; approval date: January 11, 2019). All experiments were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from the parents of the included premature infants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manley BJ, Owen LS. High-flow nasal cannula: mechanisms, evidence and recommendations. Semin Fetal Neonatal Med. 2016;21:139–45. [DOI] [PubMed] [Google Scholar]
- 2.Al-Alaiyan S, Dawoud M, Al-Hazzani F. Positive distending pressure produced by heated, humidified high flow nasal cannula as compared to nasal continuous positive airway pressure in premature infants. J Neonatal Perinat Med. 2014;7:119–24. [DOI] [PubMed] [Google Scholar]
- 3.Aly S, El-Dib M, Lu Z, El Tatawy S, Mohamed M, Aly H. Factors affecting cerebrovascular reactivity to CO2 in premature infants. J Perinat Med. 2019;47:979–85. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2016;2:CD006405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liew Z, Fenton AC, Harigopal S, Gopalakaje S, Brodlie M, O’Brien CJ. Physiological effects of high-flow nasal cannula therapy in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2020;105:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S-T, Chung H-W, Chen H-L. Comparison of two methods for weaning from nasal continuous positive Airway pressure via the cyclic use of High-Flow Nasal Cannula or Room Air in Preterm infants. Children (Basel). 2024. Mar 15;11(3):351. [DOI] [PMC free article] [PubMed]
- 7.Lampland AL, Plumm B, Meyers PA, Worwa CT, Mammel MC. Observational study of humidified high-flow nasal cannula compared with nasal continuous positive airway pressure. J Pediatr. 2009;154:177–82. [DOI] [PubMed] [Google Scholar]
- 8.Taha DK, Kornhauser M, Greenspan JS, Dysart KC, Aghai ZH. High flow nasal cannula use is associated with increased morbidity and length of hospitalization in extremely low birth weight infants. J Pediatr. 2016;173:50 – 5.e1. [DOI] [PMC free article] [PubMed]
- 9.Ramaswamy VV, More K, Roehr CC, Bandiya P, Nangia S. Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: systematic review and network meta-analysis. Pediatr Pulmonol. 2020;55:2940–63. [DOI] [PubMed] [Google Scholar]
- 10.Badiee Z, Eshghi A, Mohammadizadeh M. High flow nasal cannula as a method for rapid weaning from nasal continuous positive airway pressure. Int J Prev Med. 2015;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dani C. Nasal continuous positive airway pressure and high-flow nasal cannula today. Clin Perinatol. 2021;48:711–24. [DOI] [PubMed] [Google Scholar]
- 12.Murki S, Singh J, Khant C, Kumar Dash S, Oleti TP, Joy P, et al. High-flow nasal cannula versus nasal continuous positive airway pressure for primary respiratory support in preterm infants with respiratory distress: a randomized controlled trial. Neonatology. 2018;113:235–41. [DOI] [PubMed] [Google Scholar]
- 13.Shin J, Park K, Lee EH, Choi BM. Humidified high flow nasal cannula versus nasal continuous positive airway pressure as an initial respiratory support in preterm infants with respiratory distress: a randomized, controlled non-inferiority trial. J Korean Med Sci. 2017;32:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotecha SJ, Adappa R, Gupta N, Watkins WJ, Kotecha S, Chakraborty M. Safety and efficacy of high-flow nasal cannula therapy in preterm infants: a meta-analysis. Pediatrics. 2015;136:542–53. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama A, Okazaki K, Kondo M, Oka S, Motojima Y, Namba F, et al. Randomized controlled trial of high-flow nasal cannula in preterm infants after extubation. Pediatrics. 2020;146:e20201101. [DOI] [PubMed] [Google Scholar]
- 16.Sett A, Noble EJ, Forster DE, Collins CL. Cerebral oxygenation is stable in preterm infants transitioning to heated humidified high-flow nasal cannula therapy. Acta Paediatr. 2021;110:2059–64. [DOI] [PubMed] [Google Scholar]
- 17.Bembich S, Travan L, Cont G, Bua J, Strajn T, Demarini S. Cerebral oxygenation with different nasal continuous positive airway pressure levels in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2015;100:F165–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.